As a key element of the smooth muscle cell contractile apparatus, the actin cytoskeleton participates in the development of force by acting as a molecular track for the myosin cross bridge motor. At the same time, the actin cytoskeleton must transmit the force developed during contraction to the extracellular matrix and, thus, to neighboring cells. This propagation of force to the cell periphery and beyond is initiated in part on specifically localized cellular cortical actin filaments also involved in mechano-chemical transduction. During the contractile process itself and in response to extracellular structural and chemical alterations, the smooth muscle actin cytoskeletal remodels. This indicates that the cytoskeleton is a dynamic cellular organelle that adapts to the changes in cell shape and chemical cues.
1. Organization of Actin Filaments in the Vascular Smooth Muscle Cell
In striated muscle, the force generated by the cross bridges of the contractile filaments in the sarcomeres is transmitted to tendons
[7][1]. In contrast, smooth muscle lacks clear sarcomeres and how the forces generated by cross bridges in the interior of the cell are transmitted to the transmembrane integrins that communicate with the extracellular matrix and the outside world is a continuing area of investigation.
Here, we review past studies in the area of the composition and function of the cortical cytoskeleton of the mammalian vascular smooth muscle cell and the implications of these studies for the plasticity of the smooth muscle cell. We also include novel, unpublished, images we have obtained with the use of immunoelectron microscopy of metal replicas to directly visualize the cytoskeletal binding proteins and their interactions with actin in the cortex of the cell.In 1994, North et al.
[8][2] presented a novel model for the cytoskeleton of chicken gizzard smooth muscle cells where the contractile filaments were shown to run diagonally in the contractile smooth muscle cells from the dense bodies in the core of the cell to the adhesion plaques (also called dense plaques, or focal adhesions) at the cell surface. In the North et al. work, alpha actin was described as being part of the main contractile unit, while the dense bodies were linked by beta non-muscle actin linearly down the length of the cell. Gamma actin was not imaged due to lack of a specific antibody at that time. Furthermore, at that time, since only static structures were examined, the functional dynamics of the smooth muscle cytoskeleton apparatus could not be related to mechano-transduction principles.
More recently, immunofluorescence studies at the subcellular level in mammalian vascular smooth muscle cells (VSMC) suggest some significant differences from this model. All three actin isoforms present in contractile vascular smooth muscle cells have now been directly immunolocalized with deconvolution microscopy at the light microscope level. Alpha smooth muscle actin is now known to be confined to the contractile filament bundles in the core of the cell and beta actin is associated with dense bodies, consistent with the North model, but the beta actin isoform is also found at the focal adhesions at the cell edge, referred to here as the cell cortex. Gamma non-muscle actin is primarily located in the cortex of the cell, near the cell membrane
[9][3]. Alpha actin as well as beta actin are relatively stable and do not show detectable changes in polymerization with alpha-adrenergic stimulation by an agonist such as phenylephrine (PE). In contrast, PE triggers an increased actin polymerization of gamma non-muscle actin as well as an endosome-dependent recycling of the focal adhesion protein zyxin
[9,10,11][3][4][5]. The agonist stimulation of VSMCs activates a signaling cascade which may increase actomyosin cross-bridge cycling and generate mechanical force. As a consequence, the force increases the tension on non-muscle actin in which some isoforms are associated with focal adhesion proteins, such as focal adhesion kinase (FAK), talin, vinculin and paxillin and to integrins. However, the functional significance of the agonist-induced changes in the structure of the non-muscle actin cytoskeleton in the living smooth muscle cell has not been clear.
Smooth muscle cells contain four isoforms of the actin: alpha smooth muscle actin, beta non-muscle actin, gamma smooth muscle actin and gamma non-muscle actin
[12,13][6][7]. These isoforms are products of the separate genes with different expression patterns and different functions. Surprisingly, they share quite similar protein sequences, almost 93% identical to each other
[13,14][7][8]. While alpha smooth muscle and gamma smooth muscle actin are the predominant isoforms in the contractile filaments of VSMC, the potential roles of beta non-muscle and gamma non-muscle actin are less clearly defined. Previous deconvolution immunofluorescence microscopy of the vascular smooth muscle structure demonstrated the presence of gamma non-muscle actin specifically restricted to the cell cortex
[15][9] though the precise function of the cortical non-muscle actin remains to be defined in detail. Alpha smooth muscle actin, however, clearly appears in the contractile domain of the cytoplasm. Beta actin is found in prominent association with dense bodies as well as focal adhesion proteins. It is suggested that beta actin functions to attach contractile filaments at dense bodies and transmit contractile forces generated in the core of the cell to the focal adhesions at the surface of the cell and then to the inter-cellular space. Non-muscle gamma actin is concentrated in filaments at the cell cortex but its functions are still yet to be clearly defined.
Recently, we have investigated the structural disposition and the possibility of remodeling of VSMC actin filaments and select actin binding proteins was investigated by immunoelectron microscopy (EM) of the smooth muscle cytoskeleton, specifically focusing on the cortex of the cell with the method of metal replica imaging. We did this as a means to test and refine the concept of a dynamic cortical cytoskeleton in the smooth muscle cell. The complexity of the inter-weaving fabric of the smooth muscle cytoskeleton can be appreciated by examination of Figure 1, where the Triton-extracted actin cytoskeleton is shown at sequentially increasing levels of magnification. Despite the complexity of this structure, it is clear that an alpha-adrenergic agonist, such as phenylephrine (PE), produces defined remodeling of the cytoskeletal connections to the alpha actinin-VASP adaptor protein, zyxin. In contrast, no remodeling of the cytoskeletal connections to the focal adhesion protein talin occurs. Thus, the followin this studyg content, agonist-induced remodeling of the contractile smooth muscle cell cytoskeleton is confirmed at the EM level and is shown to be selective and protein-specific. Since contractile smooth muscle cells, unlike migrating, proliferating smooth muscle cells are often assumed to have a fixed cytoskeletal structure, these results are important in that they indicate directly, a dynamic nature of the cortical cytoskeleton of the differentiated, contractile smooth muscle cell.
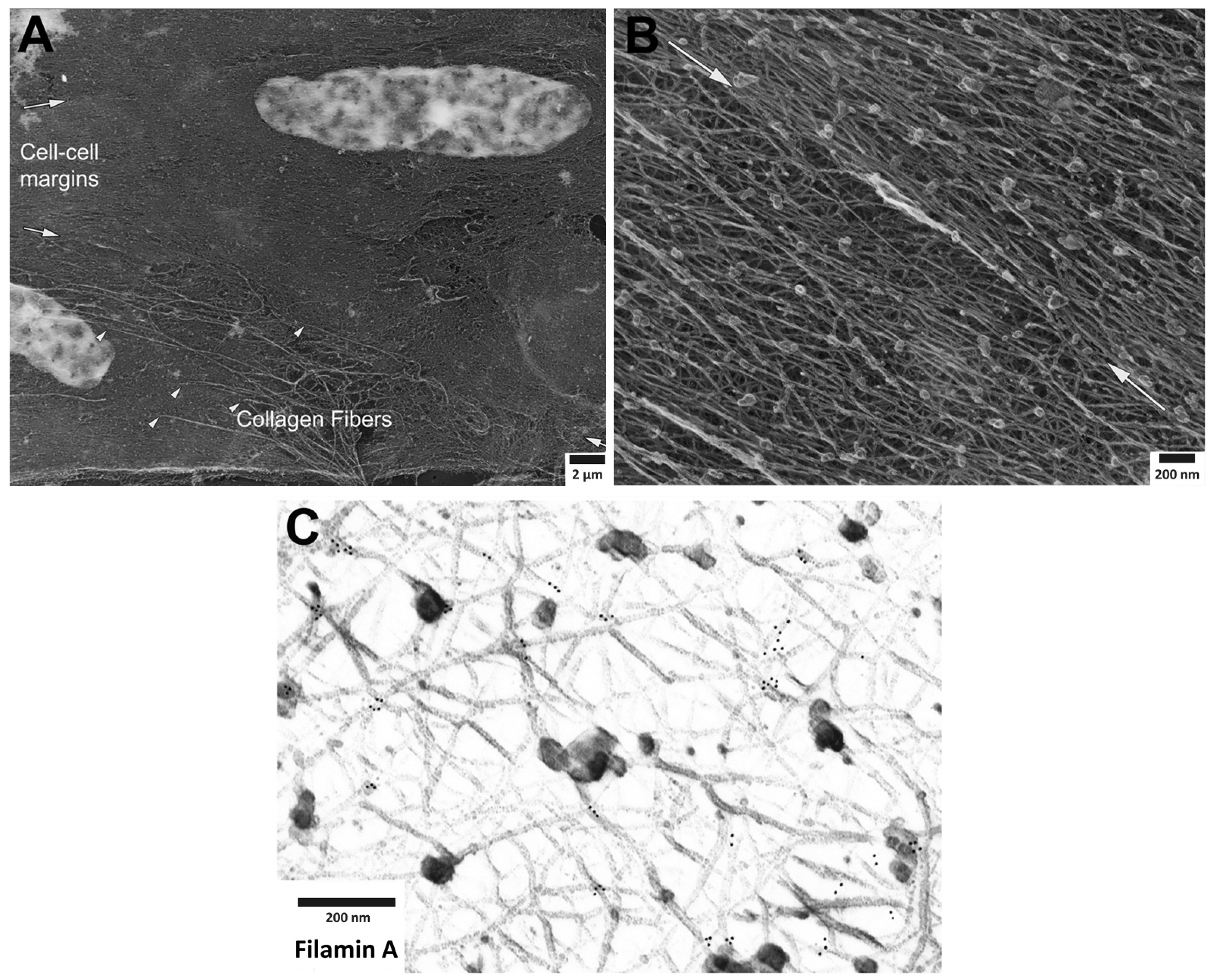
Figure 1. Cortical actin cytoskeleton of vascular smooth muscle at increasing levels of magnification. (
A) Low magnification view of two adherent vascular smooth muscle cells, defined by central nuclei in each cell and collagen fibers (arrowheads), especially prominent at cell-to-cell margins. (
B) Higher magnification of cortical actin cytoskeleton; white arrows denoting cell-to-cell margins of two adherent enzymatically isolated cells. (
C) Further increase in magnification reveals details of a branched, interconnecting actin cytoskeleton. Gold beads mark immunoEM labeling of filamin A at the branch points of actin filaments.
See text for details and Appendix A for materials and methods.
2. Roles of Adhesion Proteins on the Cortical Actin
Cortical actin supports the vascular smooth muscle cell morphology by bridging beta integrin at the cell membrane and focal adhesion proteins to the contractile elements inside the cell
[16,17,18,19][10][11][12][13]. This cell type is highly dynamic and undergoes remodeling by the complex interactions of actin binding proteins and focal adhesion proteins. As is shown in
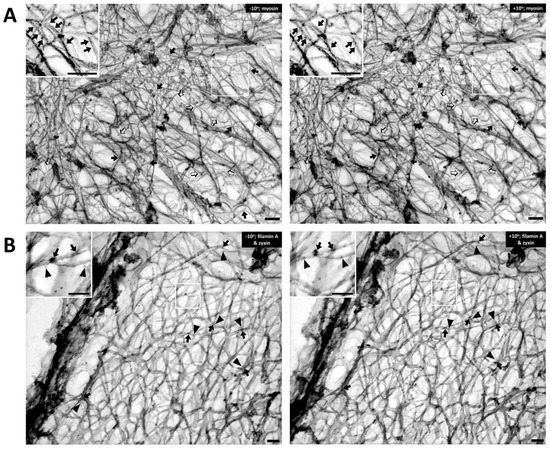
Figure 2A, the Triton-extracted, cortical, actin cytoskeleton of the contractile VSMC is a branched network of groups of linear actin bundles. Apparent 60–65 degree (open arrows) and 90 degree (solid arrows) branches are seen, which suggest linkages to Arp2/3-capped daughter filaments or filamin A cross-connections, respectively
[20,21][14][15]. The presence of orthogonal cross-linking in the cortex suggests an active role of filamin A, possibly in determining the structure and function of the cortical actin cytoskeleton
[22][16]. Although
we did not
observe the classic 70-degree branches associated with Arp2/3
[20was not observed [14][17][18],
23,24], it seems likely that if there are mechanical pushing or pulling forces generated within the cortical cellular cytoskeleton, it is possible that Arp2/3 mechanisms could be associated with angles other than the classic 70 degrees. Further investigations are needed to address questions concerning the dynamic change of the newly branched actin cytoskeleton at the cell cortex when there is intracellular, mechanical force induced by agonist stimulation. With respect to filamin A-induced cross-linking, it is also important to rule out the possibility that these filaments are simply lying on top of each other.
Figure 2. The cortical actin cytoskeleton of contractile VSMC consists of a branched network of groups of linear actin bundles containing filamin A and zyxin. Tilt-paired images of S1-decorated cortical actin (left, −10° tilted image; right, +10° tilted image). (A) Labeling of filaments with myosin S1. Arrows = myosin S1. Bar = 100 nm. Depth estimation is 160–180 nm. Actin filaments are Triton X-100 extracted, labeled with myosin S1 and metal cast. Inset shows S2 myosin “arrowheads” (marked here with arrows). Both orthogonal 90-degree (solid arrows) and 60-degree (open arrows) branches are seen in the rest of the figure, suggesting filamin A-mediated cross-linking and Arp2/3-mediated branching, respectively. (B) Double labeling of filamin A and zyxin (left, −10° tilted image; right, +10° tilted image). Arrows = 10 nm beads of anti-filamin A labeling. Arrowheads = 6-nm beads of anti-zyxin labeling. Bar = 100 nm. Depth estimation is 180–200 nm. Inset shows higher magnification of actin cytoskeleton where filamin A (arrows) binds mostly at the crosslinks but zyxin (arrowheads) is located about 20–30 nm away from the crosslink. Some of the black arrows are on a filament that is out of the major plane of the image.
To test the idea that these orthogonal structures are due to filamin A-induced cross-linking of actin filaments, rather than simply overlying unconnected filaments,
we immunost
ainedhe metal replicas of the cortical cytoskeleton from contractile VSMCs for filamin A
was immunostained (arrows,
Figure 2B). For comparison,
we co-st
ained the same sections with anti-zyxin antibodies
was co-stained in this process (arrowheads,
Figure 2B). Zyxin is an adaptor protein that has been reported to link VASP with alpha-actinin at focal adhesions
[23,25][17][19]. VASP is reported to drive linear actin polymerization in tetrameric bundles and has been shown to be involved in actin polymerization in VSMC
[26,27][20][21]. In the inset of
Figure 2B, filamin A (solid arrows, 10 nm beads) is seen at points of orthogonal branches, as predicted. The fact that zyxin is known to bind proteins other than actin (VASP and alpha-actinin) and, thus, is not expected to directly bind actin, is consistent with the apparent lack of direct association of the zyxin beads (arrowheads) with actin filaments.
Zyxin has the postulated function to serve as a structural protein as well as a modulator to stretch-induced gene expression in VSMC. When exposed to cyclic stretch, zyxin rapidly translocates from the focal adhesion multiprotein complex to the nucleus and alters the expression of mechano-sensitive genes
[28,29][22][23]. This mechanism implies a role for zyxin in transducing mechanical stimuli from the cell membrane to the nucleus of VSMC. Thus, current evidence is consistent to its responsive role to mechanical cues
[28,30][22][24]. Direct observation of zyxin-actin interaction has not been reported. A study of zyxin co-culture with a barbed end-capping protein revealed its recruitment to the free barbed ends suggesting the linkage of zyxin to actin polymerizing and associated proteins, such as VASP. Although zyxin is one of the focal adhesion proteins involved in the remodeling of the actin cytoskeleton, the interaction between zyxin and other proteins and how zyxin contributes to remodeling mechanisms remains unclear.
During contractile activity, the presence of talin in focal adhesions also leads to the recruitment of vinculin, further stabilizing the focal adhesion complex
[31][25]. Fluorescence microscopic analysis has demonstrated the fluctuating length of talin in a range of 100–350 nm in living CV1 cells
[32][26]. In vivo measurement of talin extension with over-expression of the vinculin head revealed a tight binding of the vinculin head that promotes talin stretching. However, the expected amplitude of change is suppressed implying a subsequent relaxation of the force in the talin-mediated force transmission pathway. Stability of vinculin at focal adhesions primarily depends on the binding with talin, whereas the binding to F-actin filaments is also affected by the impact due to the force load
[33][27]. Both a decrease of actomyosin-based force load to focal adhesions or an imposed deficiency in vinculin expression revealed delocalization of vinculin from adhesion sites
[34,35][28][29].
In contractile VSMCs, talin is known to be an abundant cytoskeleton protein associated with focal adhesions and has recently been described to be a “molecular ruler” to define the size of focal adhesions
[36][30]. The cells shown in
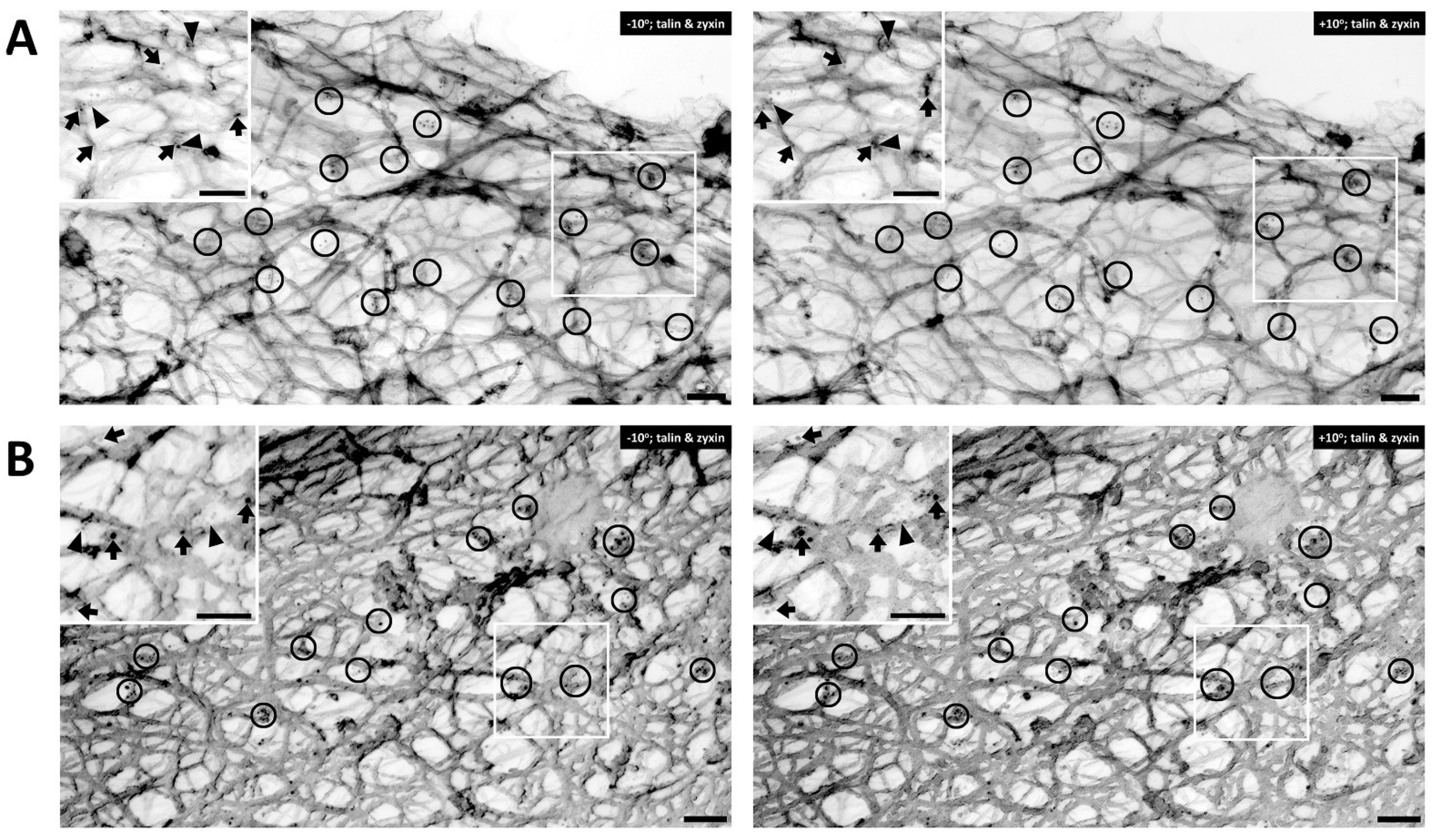
Figure 3 are co-immunostained with talin and zyxin. Similar to filamin A, talin is found mostly in the cortical area of the cell.
Figure 3A displays an unstimulated cell, whereas
Figure 3B shows a cell activated with PE. In both figure panels talin (arrows) and zyxin (arrowheads) are colocalized close to actin filaments. Clusters of talin and zyxin are indicated by circles. The distance between beads was quantified except in areas where superimposed filaments and image depth prevented accurate measurement. In the unstimulated cell shown in
Figure 3A the distance between beads for talin is 120–200 nm and in
Figure 3B, with PE activation, the distance is a very similar (100–200 nm in range), indicating that talin and zyxin are closely interacted. The distance between talin beads is rather in a fixed position and not perturbed by the activation of a vasoconstrictor agonist such PE. In contrast, the distance between zyxin beads in the unstimulated cell shown in
Figure 3A is 300–400 nm but that in the presence of PE is 200–300 nm. These differing results with different proteins suggest that the apparent decreased zyxin–zyxin distance in the presence of PE is likely due to a cytoskeletal remodeling event rather than simply the shortening of the cell. This is confirmed by the statistical analysis shown in
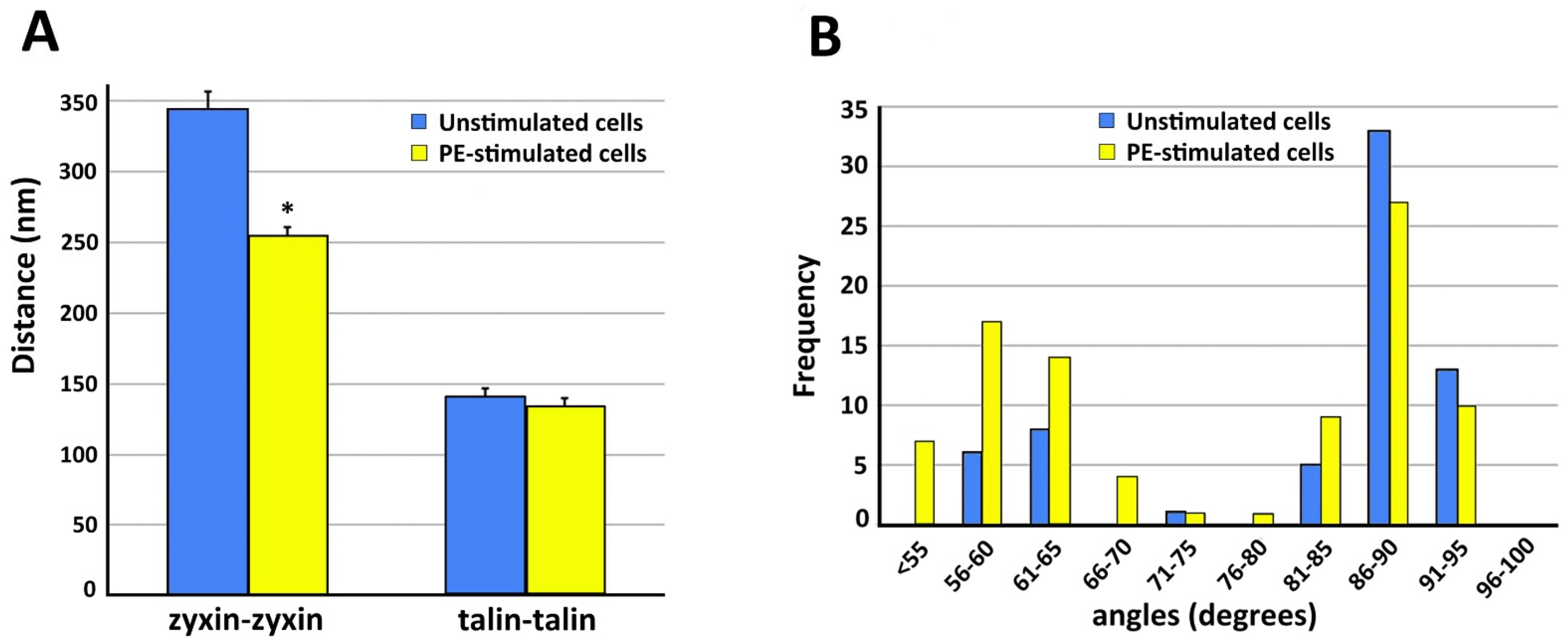
Figure 4. As is shown in
Figure 4A, in the presence of PE the zyxin–zyxin spacing decreases in a statistically significant manner but the talin–talin spacing is not significantly changed. Functionally, this suggests that the lack of change in talin spacing would maintain a constant size of the focal adhesion, as has been reported for contractile VSMCs
[37][31].
Figure 3. Talin versus zyxin labeling in response to agonist activation. (A) Double labeling of talin and zyxin (left, −10° tilted image; right, +10° tilted image) in unstimulated VSMC. Arrows = 10 nm beads, anti-talin labeling. Arrowheads = 6 nm beads of anti-zyxin labeling. Bars = 100 nm. Depth estimation is 160–180 nm. (B) Double labeling of talin and zyxin (left, −10° tilted image; right, +10° tilted image) in phenylephrine-stimulated VSMC. Arrows = 10 nm beads of anti-talin labeling. Arrowheads = 6 nm beads of anti-zyxin labeling. Bars = 100 nm. Depth estimation is 200–220 nm. Clusters of talin and zyxin are shown in the black circles. In both panels, the inset shows a larger view of actin cytoskeleton in the VSMC where talin (arrows) and zyxin (arrowheads) colocalized close to actin filaments.
Figure 4. Quantitation of structural features of VSMC cortical actin cytoskeleton. In all panels, analysis is from five different cells in each category. Data = mean ± standard error with statistical significance, p < 0.05 (*). (A) Spacing between zyxin beads is significantly decreased with PE stimulation. Talin spacing is not significantly different. (B) Frequency distribution of angles measured from actin branches in VSMC.