Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Nora Tang and Version 1 by Akbar Niaz.
Reinforced concrete (RC) has been commonly used as a construction material for decades due to its high compressive strength and moderate tensile strength. However, these two properties of RC are frequently hampered by degradation. The main degradation processes in RC structures are carbonation and the corrosion of rebars. The scientific community is divided regarding the process by which carbonation causes structural damage. Some researchers suggest that carbonation weakens a structure and makes it prone to rebar corrosion, while others suggest that carbonation does not damage structures enough to cause rebar corrosion.
- concrete carbonation
- concrete protection
- inhibitor addition
- corrosion testing
1. Factors Contribute to Carbonation
The main factors that cause carbonation are dry-wet cycles, relative humidity, temperature, and CO2 concentration, all of which are environmental factors. Moreover, certain factors that lead to carbonation are related to concrete’s microstructural properties and materials, such as concrete’s porous structure and the number of chemical compounds that can react to form carbonation [7][1].
Low-permeability concrete can restrict CO2 penetration if it has a low water-to-cement ratio, high cement content, and high compressive strength [2]. This is because an increase in the water-to-cement ratio can be related to increased porosity and CO2 transport. Furthermore, if the concrete is completely dry or wet, carbonation does not occur unless the relative humidity is between 50% and 70%. If the relative humidity is below 50%, the moisture level is not adequate for reactions to take place; if the relative humidity is above 70%, the high level of moisture in pores restricts CO2 penetration [10][3]. The optimum relative humidity for the carbonation process is 65% [11][4].
Moreover, a previous study revealed that CO2 penetration increases as temperature increases, as higher temperatures make concrete more porous [12,13][5][6]. In 2005, the atmospheric CO2 concentration was about 380 parts per million (PPM), up from 280 PPM in 1975; this increase occurred because of temperature changes. Therefore, climate change has accelerated the initiation of corrosion in RC by enabling harmful substances from the environment, such as CO2 and chloride, to penetrate RC. For example, if the temperature increases by 2 °C because of global warming, the steel corrosion rate may increase by 15% [1,10][3][7]. In addition, the mean daily temperature affects the carbonation depth. For example, if a concrete structure is located in an area with a mean daily temperature of 27 °C, it will undergo more carbonation than the same structure located in an area with a mean daily temperature of 9 °C [11][4]. Furthermore, carbonation often occurs in areas of building facades that are exposed to rainfall, shaded from sunlight, or located in indoors [12,14][5][8].
CO2 diffusion and carbonation reactions occur only in mortar, which consists of water, cement, and sand. This means that using less mortar or a coarser aggregate will decrease the carbonation depth. Carbonation depth is further decreased if small coarse aggregates are used, because they have a larger surface area thus providing a longer and more arduous path for CO2 diffusion Figure 21 [14][8]. Using small coarse aggregates with fine aggregates is preferable when designing durable concrete with ordinary Portland cement. While coarse aggregate grading does not affect the depth of carbonation, increasing the size of large coarse aggregates will increase the size and porosity of the interfacial transition zone, which may increase the carbonation depth. The interfacial transition zone is a thin shell between aggregate particles and hydrated cement paste, which significantly affects the permeability and durability of concrete [15][9].
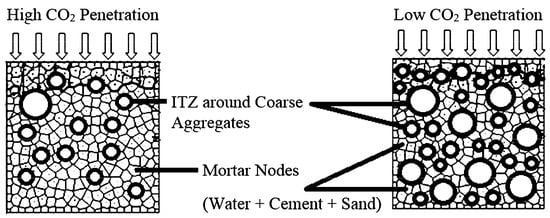
Figure 21. The effects of mortar and coarse aggregate contents on a concrete mixture’s carbonation resistance [16].
The effects of mortar and coarse aggregate contents on a concrete mixture’s carbonation resistance [10].
A previous study showed that CO2 gas penetrates the concrete surface through the sand-cement connection gap [14][8]. This means that increasing the sand volume in concrete also increases CO2 penetration. However, another study revealed that increasing the sand volume of concrete reduces the water-to-cement ratio and makes the path of CO2 penetration more complex, thus reducing the depth of carbonation and air permeability [15][9].
Using graded fine aggregates in concrete retards CO2 penetration more than using only sand as a fine aggregate. In other words, graded aggregates help to delay CO2 penetration. In addition, applying thicker concrete covers or plastering covers delays CO2 penetration in RC structures, thereby extending the life of the passive film around steel bars [17][11].
2. Carbonation Depth with Time
Carbonation depth is one of the most important parameters when considering concrete damage [6,9,13][6][12][13]. As stated above, carbonation lowers the pH of concrete; therefore, measuring the low pH region of concrete can indicate carbonation depth. A commonly used method for measuring carbonation depth is to apply phenolphthalein (a pH indicator) spray on freshly fractured sections. The spray turns the section color from gray to dark pink if the pH has not been altered (i.e., the pH is around 13). Meanwhile, the color of the concrete does not change if the pH of the region is 8–9, thus indicating the carbonation depth [18,19,20][14][15][16].
Multiple factors contribute to carbonation depth, including the concentration of CO2 in the environment, relative humidity, and the type of cement. Table 1 highlights some of the major factors that control carbonation depth in field and laboratory environments. It can be seen that carbonation in concrete structures does not occur in isolation; induced or produced stresses, progressive changes within the concrete and the environment, inhomogeneity, and additives also contribute significantly to carbonation depth. CO2 enters concrete within a few hours of casting, and the depth of its penetration increases gradually [21][17].
Models used to measure carbonation depth can be modified according to the factors mentioned above; the simplest is that the carbonation depth (in mm) increases with the square root of the time (years). The rate of carbonation slows due to existing factors and factors that arise over time. CO2 easily diffuses through pores, but reactions with Ca(OH)2 are slow since they are gas-solid phase reactions. Continuous carbonation and hydration of concrete causes the sealing of micro-pores, which, in turn, hinders diffusion. As a result, the rate of CO2 diffusion decreases, thus slowing the carbonation rate [22,23,24][18][19][20].
Another method used to measure carbonation depth is microstructural analysis. CaCO3 is a chemical product formed during carbonation, and locating its presence in the depth profile via microstructural analysis can indicate the depth of the carbonation.
The contribution of carbonation to corrosion is similar to that of chloride corrosion, which decreases passivity and damages the passive layer. If the passive layer is not damaged, electron and ion transport across the passive layer remain limited. Once the passive layer is damaged, transport across the interface begins, and corrosion is initiated. Corrosion spreads as further physicochemical processes take place on the rebar surface.
Table 1.
Previous research on factors that control carbonation depth.
| Carbonation Depth | Environment | Major Findings | Research Focus |
|---|---|---|---|
| Depth increases with stress Carbonation depth increases |
Lab exposure Natural exposure |
Low load decreases carbonation, while high load increases carbonation. Unstressed structure life decreased by 1/3 under combined stress. |
Flexural stress effect on carbonation [25][21] Stress effect on carbonation [26][22] |
| Carbonation time increases | Field exposure | More corrosion is needed to generate cracks on the surface. | Cover depth effect on carbonation-induced corrosion [27][23] |
| Carbonation depth varies | Recycled mixed concrete | Recycled coarse aggregate controls carbonation more effectively than nono-SiO2. | Inhomogeneities in carbonation depth recycled aggregate [28][24] |
| Carbonation depth decreases under sCO2 | Lab exposure | Among porosity, aggregate size, and their distribution, porosity in the interfacial transition zone makes the greatest contribution to carbonation | Interfacial transition zone changes under supercritical carbon dioxide [29][25] |
| Carbonation depth increases | Accelerated lab environment | Carbonation depth increases exponential function for temperature, power function for CO2, and polynomial function for relative humidity. | Carbonation depth as a function of temperature, humidity, and CO2 [24][20] |
| Carbonation depth decreases for BF slag | Natural carbonation | Among Portland cement, fly ash, and blast furnace slag, the latter yields the lowest uptake. | Changes in CO2 uptake due to different mineral additions [30][26] |
| Carbonation depth depends on additives | Natural and accelerated environment | It is hard to establish a correlation between lab-simulated and natural data. Only a simplistic model can be useful. | Carbonation modeling under normal and accelerated conditions [31][27] |
| Carbonation changes with temperature, humidity, and CO2 | Natural environment | Climatic changes (e.g., changes in CO2 concentration, humidity, and temperature) contribute to carbonation and induce damage. | Carbonation depth changes under climate changes [32][28] Climate change impact on faster CO2 ingress [33][29] |
| Uniform carbonation | Lab accelerated environment | Ambient pressure carbonation corrosion delays corrosion initiation. | Ambient pressure carbonation curing [34][30] |
| Carbonation increases | Accelerated carbonation | Carbonation decreases the strength of concrete and increases corrosion damage. | Accelerated carbonation testing in a sewage environment [35][31] |
| Corrosion increases with carbonation | Accelerated environment | Chloride penetration significantly increases with carbonation and crack size. | Combined impact of carbonation and crack width on chloride-assisted corrosion [36][32] |
| Carbonation depth increases with sulfates | Lab simulated environment | Sodium sulfate, which sometimes exists in slag materials, increases carbonation. | Sulfate-assisted carbonation [37][33] |
References
- Neves, R.; Branco, F.; de Brito, J. Field assessment of the relationship between natural and accelerated concrete carbonation resistance. Cem. Concr. Compos. 2013, 41, 9–15.
- Marques, P.F.; Chastre, C.; Nunes, Â. Carbonation service life modelling of RC structures for concrete with Portland and blended cements. Cem. Concr. Compos. 2013, 37, 171–184.
- Stewart, M.G.; Wang, X.; Nguyen, M.N. Climate change adaptation for corrosion control of concrete infrastructure. Struct. Saf. 2012, 35, 29–39.
- Roy, S.K.; Northwood, D.O.; Poh, K.B. Effect of plastering on the carbonation of a 19-year-old reinforced concrete building. Constr. Build. Mater. 1996, 10, 267–272.
- Huang, N.; Chang, J.; Liang, M. Effect of plastering on the carbonation of a 35-year-old reinforced concrete building. Constr. Build. Mater. 2012, 29, 206–214.
- Talakokula, V.; Bhalla, S.; Ball, R.J.; Bowen, C.R.; Pesce, G.L.; Kurchania, R.; Bhattacharjee, B.; Gupta, A.; Paine, K. Diagnosis of carbonation induced corrosion initiation and progression in reinforced concrete structures using piezo-impedance transducers. Sens. Actuators A Phys. 2016, 242, 79–91.
- Stewart, M.G.; Wang, X.; Nguyen, M.N. Climate change impact and risks of concrete infrastructure deterioration. Eng. Struct. 2011, 33, 1326–1337.
- Huang, Q.; Jiang, Z.; Zhang, W.; Gu, X.; Dou, X. Numerical analysis of the effect of coarse aggregate distribution on concrete carbonation. Constr. Build. Mater. 2012, 37, 27–35.
- Basheer, L.; Basheer, P.A.M.; Long, A.E. Influence of coarse aggregate on the permeation, durability and the microstructure characteristics of ordinary Portland cement concrete. Constr. Build. Mater. 2005, 19, 682–690.
- Jiang, Z.-L.; Gu, X.-L.; Huang, Q.-H.; Zhang, W.-P. Statistical analysis of concrete carbonation depths considering different coarse aggregate shapes. Constr. Build. Mater. 2019, 229, 116856.
- Zhou, Y.; Gencturk, B.; Willam, K.; Attar, A. Carbonation-Induced and Chloride-Induced Corrosion in Reinforced Concrete Structures. J. Mater. Civ. Eng. 2014, 27, 04014245.
- Marques, P.F.; Costa, A. Service life of RC structures: Carbonation induced corrosion. Prescriptive vs. performance-based methodologies. Constr. Build. Mater. 2010, 24, 258–265.
- Marie-Victoire, E.; Cailleux, E.; Texier, A. Carbonation and historical buildings made of concrete. J. Phys. IV 2006, 136, 305–318.
- Elsalamawy, M.; Mohamed, A.; Kamal, E. The role of relative humidity and cement type on carbonation resistance of concrete. Alex. Eng. J. 2019, 58, 1257–1264.
- Ferreira, M.; Jalali, S. Software for probability-based durability analysis of concrete structures. Concr. Repair Rehabil. Retrofit. 2005, 01, 117.
- Leemann, A.; Moro, F. Carbonation of concrete: The role of CO2 concentration, relative humidity and CO2 buffer capacity. Mater. Struct. 2016, 50, 30.
- Jaffer, S.J.; Hansson, C.M. Chloride-induced corrosion products of steel in cracked-concrete subjected to different loading conditions. Cem. Concr. Res. 2009, 39, 116–125.
- Czarnecki, L.; Woyciechowski, P. Prediction of the reinforced concrete structure durability under the risk of carbonation and chloride aggression. Bull. Pol. Acad. Sci. Tech. Sci. 2013, 61, 173–181.
- Zhang, J.-s.; Cheng, M.; Zhu, J.-h. Carbonation Depth Model and Prediction of Hybrid Fiber Fly Ash Concrete. Adv. Civ. Eng. 2020, 2020, 9863963.
- Liu, P.; Yu, Z.; Chen, Y. Carbonation depth model and carbonated acceleration rate of concrete under different environment. Cem. Concr. Compos. 2020, 114, 103736.
- Wang, J.; Su, H.; Du, J. Influence of coupled effects between flexural tensile stress and carbonation time on the carbonation depth of concrete. Constr. Build. Mater. 2018, 190, 439–451.
- Shi, X.; Yao, Y.; Wang, L.; Zhang, C.; Ahmad, I. A modified numerical model for predicting carbonation depth of concrete with stress damage. Constr. Build. Mater. 2021, 304, 124389.
- Otieno, M.; Ikotun, J.; Ballim, Y. Experimental investigations on the influence of cover depth and concrete quality on time to cover cracking due to carbonation-induced corrosion of steel in RC structures in an urban, inland environment. Constr. Build. Mater. 2018, 198, 172–181.
- Mi, R.; Pan, G. Inhomogeneities of carbonation depth distributions in recycled aggregate concretes: A visualisation and quantification study. Constr. Build. Mater. 2022, 330, 127300.
- Bao, H.; Xu, G.; Yu, M.; Wang, Q.; Li, R.; Saafi, M.; Ye, J. Evolution of ITZ and its effect on the carbonation depth of concrete under supercritical CO2 condition. Cem. Concr. Compos. 2021, 126, 104336.
- Younsi, A.; Turcry, P.; Aït-Mokhtar, A. Quantification of CO2 uptake of concretes with mineral additions after 10-year natural carbonation. J. Clean. Prod. 2022, 349, 131362.
- Rathnarajan, S.; Dhanya, B.S.; Pillai, R.G.; Gettu, R.; Santhanam, M. Carbonation model for concretes with fly ash, slag, and limestone calcined clay-using accelerated and five-year natural exposure data. Cem. Concr. Compos. 2021, 126, 104329.
- Chen, G.; Lv, Y.; Zhang, Y.; Yang, M. Carbonation depth predictions in concrete structures under changing climate condition in China. Eng. Fail. Anal. 2020, 119, 104990.
- Al-Ameeri, A.S.; Rafiq, M.I.; Tsioulou, O.; Rybdylova, O. Impact of climate change on the carbonation in concrete due to carbon dioxide ingress: Experimental investigation and modelling. J. Build. Eng. 2021, 44, 102594.
- Xian, X.; Zhang, D.; Lin, H.; Shao, Y. Ambient pressure carbonation curing of reinforced concrete for CO2 utilization and corrosion resistance. J. CO2 Util. 2021, 56, 101861.
- Kong, L.; Han, M.; Yang, X. Evaluation on relationship between accelerated carbonation and deterioration of concrete subjected to a high-concentrated sewage environment. Constr. Build. Mater. 2019, 237, 117650.
- Al-Ameeri, A.S.; Rafiq, M.I.; Tsioulou, O. Combined impact of carbonation and crack width on the Chloride Penetration and Corrosion Resistance of Concrete Structures. Cem. Concr. Compos. 2020, 115, 103819.
- Liu, Z.; Hu, W.; Hou, L.; Deng, D. Effect of carbonation on physical sulfate attack on concrete by Na2SO4. Constr. Build. Mater. 2018, 193, 211–220.
More
