Neurodegenerative diseases (NDs) constitute a global challenge to human health and important social and economic burdens worldwide, mainly due to their growing prevalence in an aging population and to their associated disabilities. Clinically, NDs are characterized by the progressive damage and death of nerve cells from a particular region of the brain, resulting in disease-specific clinical symptoms. They are devastating disorders that lead to severe impairments of patients’ abilities such as cognitive decline, dementia or motor deficits, and ultimately to death
[1]. Despite their differences at the clinical level, NDs share fundamental pathological mechanisms such as abnormal protein deposition, intracellular Ca
2+ overload, mitochondrial dysfunction, redox homeostasis imbalance and neuroinflammation, among others
[2][3][2,3]. Although important progress is being made in deciphering the mechanisms underlying NDs, the availability of effective therapies is still scarce. Indeed, current therapies generally lack disease-modifying activity and act to alleviate symptoms, but they cannot delay or halt the progress of the disease. Therefore, exploring new therapeutic options is urgently required to identify new treatments for NDs either using synthetic or natural products or even existing drugs. Obviously, the existence of shared pathological mechanisms leading to neurodegeneration in different NDs reinforces the importance of those pathways as common targets for intervention strategies
[4]. Therefore, therapeutic approaches directed to reduce oxidative stress (OS) levels, protein aggregation or neuroinflammation could be beneficial for diverse NDs
[1].
Carnosine is a natural endogenous molecule discovered more than 100 years ago as an abundant non-protein, nitrogen-containing compound of meat
[5]. However, it has been extensively studied during the last years due to its promising beneficial effects for human health
[6][7][6,7]. Carnosine (β-alanyl-L-histidine) is a histidine-containing dipeptide (HCD) that, together with its analogs homocarnosine, anserine and ophidine/balenine, is widely distributed in mammalian tissues
[8][9][8,9]. This dipeptide is mainly located in skeletal and cardiac muscles but is also present in the brain. Interestingly, all these tissues exhibit a very active oxidative metabolism. However, while most studies have been directed to determine the role of carnosine in muscles, its physiological role in the brain is still unclear (reviewed in
[7][9][10][7,9,10]). Carnosine is present throughout the brain but is particularly enriched in the olfactory bulb and cortex. β-alanine (β-ala) and L-histidine (L-his) are the two amino acids forming carnosine, which can be easily obtained from the circulatory system for its synthesis and have different origins. While β-ala is synthesized in the liver or can be obtained from the diet in humans, L-His must be ingested since it cannot be synthesized de novo
[9]. In addition, they can be obtained from proteolysis of endogenous proteins
[11]. Both can be delivered to the brain via the circulatory system and cross the blood–brain barrier (BBB) through amino acid transporters for carnosine synthesis. For example, β-ala transport in the brain seems to occur via the β-amino acid transporter
[12]. Carnosine synthesis takes place specifically in glial cells, mostly in oligodendrocytes; however, neurons and astrocytes are the main utilizers of this dipeptide in the brain
[10]. In addition, it is assumed that the majority of brain carnosine comes from de novo synthesis, despite the fact that it can also cross BBB and can penetrate this tissue when it comes from the diet
[7][10][7,10].
Over the years, several properties have been assigned to carnosine. However, although it is a multifunctional dipeptide, it is mainly described to play an important role as a molecule with antioxidant, anti-aggregant and anti-inflammatory activities
[9][13][9,13]. Here,
reswe
archers discuss the most interesting mechanisms of action of carnosine, especially those associated with the nervous system that are relevant in the context of NDs (
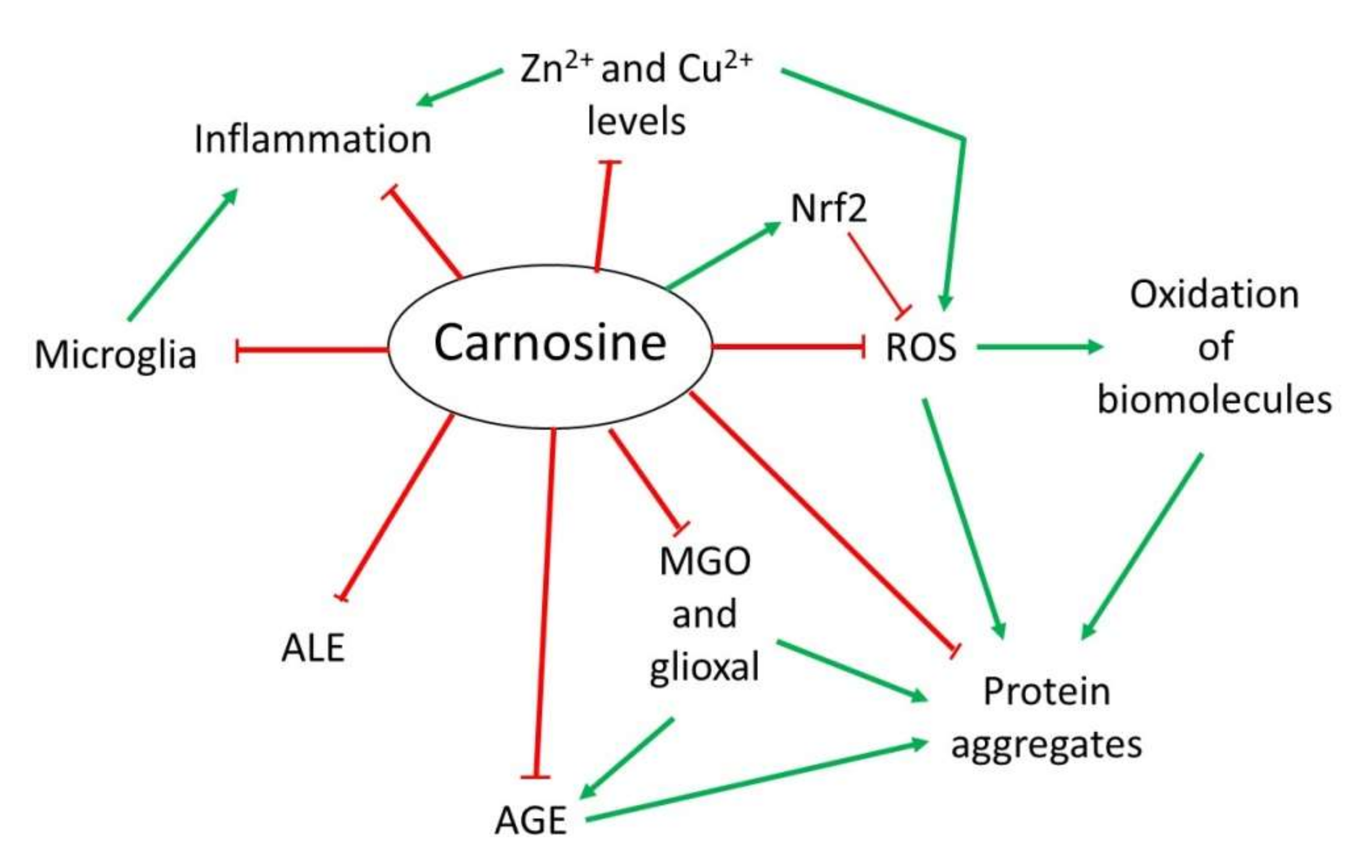
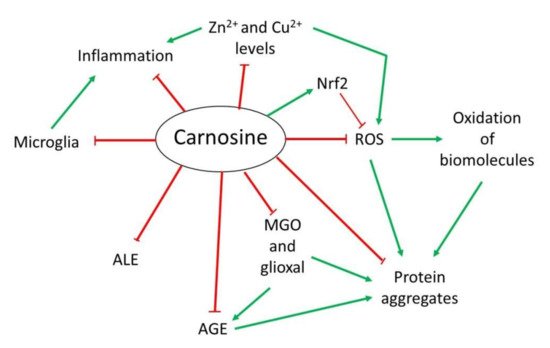
Figure 12).
Figure 12. Overview of the mechanism of action of carnosine as antioxidant, anti-aggregant, anti-glycation and anti-inflammatory molecule.
Overview of the mechanism of action of carnosine as antioxidant, anti-aggregant, anti-glycation and anti-inflammatory molecule.
2.1. Antioxidant Activity
OS is defined as an imbalance between cellular production of reactive oxygen species (ROS) and the mechanisms that remove these species
[14][33]. Principal detoxification pathways include antioxidant tripeptide glutathione (GSH) and the activity of the superoxide dismutase 1 (SOD1) enzyme, among others
[14][15][33,34]. However, an important antioxidant role has been also assigned to carnosine in the last years
[9][13][9,13]. Due to its composition, this dipeptide presents in its structure an imidazole ring that belongs to the this residue, responsible for its activity as a ROS scavenger and its protective capacity against hypochlorous acid (HOCl)
[9][13][16][9,13,20]. Indeed, it has been demonstrated that carnosine is oxidized to 2-oxo-carnosine in SH-SY5Y human neuroblastoma cells stably expressing CARNS1 after H
2O
2 exposure through the oxidation of the imidazole ring. A similar modification was detected for its analogs homocarnosine and anserine
[17][35]. Furthermore, 2-oxo-carnosine seems to have a stronger antioxidant effect than GSH, one of the main cellular detoxifying mechanisms
[15][17][34,35]. Carnosine can also protect cells against HOCl, another toxic species produced from H
2O
2 and Cl
− in mammalian cells, mainly in leukocytes. In excess, HOCl is toxic to cells since it can modify proteins. In fact, it has been linked to several disorders such as Alzheimer’s disease (AD) and cancer associated with inflammation
[18][36]. The imidazole ring of carnosine rapidly reacts with HOCl producing an imidazole chloramine, thus limiting its oxidative activity in a highly efficient manner
[13][16][19][13,20,37].
However, in addition to its direct antioxidant mechanism, recent studies have demonstrated that carnosine may indirectly exert its antioxidant function through different molecular pathways. Carnosine can modulate the activity of Nrf2 (nuclear factor erytroid 2-related factor 2), the main regulator of the cellular antioxidant response
[13][20][21][22][13,38,39,40]. In OS conditions, carnosine is able to increase Nrf2 expression and translocation to the nucleus, where it regulates the transcription of hundreds of genes that contain an antioxidant response element (ARE) in their promoter region, such as thioredoxin 1, SOD1 or catalase
[13][23][13,41]. Currently, it is unclear how carnosine produces Nrf2 pathway activation
[13]. Nevertheless, a recent study performed in podocytes from a mouse model of diabetes mellitus demonstrated that carnosine also activates the PI3K (phosphatidylinositol 3-kinase)/AKT (protein kinase B) pathway, which can result in Nrf2 activation
[20][38].
2.2. Anti-Glycating and Anti-Aggregant Activity
OS significantly contributes to the impairment of neuronal cells function through modifications of biomolecules, such as oxidation of lipids, DNA or proteins. Among them, proteins are the main targets of ROS due to the presence of residues that can easily react with these species, producing the addition of carbonyl groups
[24][42]. In addition to proteins, the carbonylation of sugars and lipids leads to the formation of advanced glycation end-products (AGEs) and advanced lipoxidation end-products (ALEs), respectively, which are a cluster of heterogeneous molecules generated in a non-enzymatic reaction that play an important role in several NDs
[13][16][25][13,20,43]. Carnosine can inhibit AGEs and ALEs formation by detoxifying reactive carbonyl species through its imidazole ring, in a similar manner than ROS
[13][25][13,43]. In this context, carnosine has been proved useful in preventing the reactivity of methylglyoxal (MGO) and glyoxal, which are carbonyl compounds that can produce protein glycation and aggregation
[13][26][13,44]. In particular, carnosine was shown to reduce OS and carbonylation levels in rats treated with MGO
[27][45]. As for antioxidant activity, the increase in Nrf2 pathway activity by carnosine should also be considered in the carbonyl quenching mechanism. Nrf2 activates the expression of several antioxidant enzymes and oxidoreductases, which convert carbonyl compounds in less reactive products such as alcohols or carboxyl derivatives
[13]. Interestingly, the activation of Nrf2 also explains the ability of carnosine to decrease AGEs formation from MGO and glyoxal
[25][43].
In addition to the effect of MGO regarding AGEs and ALEs, this compound can also produce protein aggregation and deposition leading to neuron death in some NDs
[16][20]. There is evidence that carnosine prevents oxidation and glycation, both of which contribute to the crosslinking of proteins. Excitingly, a direct anti-crosslinking activity has been attributed to carnosine that also depends on the imidazole ring
[16][26][28][20,44,46]. In addition, carnosine has been shown to exhibit protease activity in cell extracts from nervous and cardiac cell from rats
[16][20]. In sum, this dipeptide could present therapeutic potential for NDs in which protein aggregation plays an important physiopathological role.
2.3. Anti-Inflammatory and Metal Ion Chelator Activity
Some NDs are characterized by neuroinflammation produced by microglial cells and astrocytes that, along with the formation of protein aggregates, ultimately leads to cell death
[29][30][47,48]. In this respect, a possible anti-inflammatory activity of carnosine through the regulation of pro-inflammatory cytokine release was described in human epithelial CaCo-2 cells
[31][49]. Although it is still unknown how carnosine produced this effect, it could be due to its ability to form complexes with metal ions, particularly with zinc (Zn
2+)
[16][20]. Zn is an essential mineral that is required as a cofactor of several enzymes, in cell proliferation and in wound healing
[32][50]. Carnosine can form a complex with Zn
2+ called polaprezinc, a molecule that exhibits several beneficial properties, such as anti-inflammatory effect in the colon through inhibition of NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) signaling, reduction in interleukin-8 release, and induction in Hsp72
[32][33][34][50,51,52]. Currently, polaprezinc is widely used for Zn supplementation therapy and to protect the mucosa against ulceration. Therefore, due to the link between Zn and NDs and considering the neuroprotective effects of carnosine, this complex has been proposed to be used in NDs to reduce inflammation through its chelator activity
[33][51]. Regarding the brain, it has also been reported that carnosine may regulate Zn and copper (Cu) homeostasis in the synaptic cleft. The dipositive ions of these metals are necessary to modulate synaptic transmissions; for example, Zn
2+ and Cu
2+ can inhibit NMDA (N-methyl-D-aspartate)-type glutamate receptors in the post-synaptic membrane. It has been shown that carnosine directly binds to Zn
2+ and Cu
2+, thus regulating the synaptic impulse
[16][33][20,51].