Ethanol can be produced from sugary, starchy, and lignocellulosic feedstocks. Each feedstock requires different procedures for its conversion to fermentable sugar. Lignocellulosic biomass requires extra pretreatment compared to sugar and starch feedstocks to disrupt the structure and improve enzymatic hydrolysis efficiency. However, the greatest concern regarding the pretreatment process is inhibitor formation, which might retard enzymatic hydrolysis and fermentation. In addition to the inhibitors from pretreatment, chemicals used during the pretreatment and fermentation of byproducts may remain in the final product if they are not removed by ethanol distillation and dehydration. Maintaining the quality of ethanol during storage is another concerning issue. Initial impurities of ethanol being stored and its nature, including hygroscopic, high oxygen and carbon dioxide solubility, influence chemical reactions during the storage period and change ethanol’s characteristics (e.g., water content, ethanol content, acidity, pH, and electrical conductivity).
- bioethanol
- ethanol specification
- quality control
1. Introduction
| Specification | Unit | European Union |
USA | Brazil | Thailand | ||||
|---|---|---|---|---|---|---|---|---|---|
| prEN 15376 | ASTM D-4806-16a |
ANP Resolution nº 19 | TIS 2324 | TIS 640-1 | TIS 640-2 | ||||
| Ethanol type | - | - | Anhydrous | Denatured anhydrous |
Anhydrous | Denatured anhydrous |
Anhydrous | Anhydrous | |
| Ethanol | % by volume | Min. | - | - | 98 | - | - | - | |
| Ethanol and higher saturated alcohols | % by volume, (% by mass) |
Min. | (98.7) | 92.1 | (99.3) | 99 | 99.5 | 99.5 | |
| Higher saturated mono-alcohols-C3-C5 | % by volume, (% by mass) |
Max. | (2) | - | 3 | 2 | - | - | |
| Methanol | % by volume, (% by mass) |
Max. | (1) | 0.5 | 0.5 | 0.5 | 0.02 | 0.05 | |
| Water content | % by volume, (% by mass) |
Max. | (0.3) | 1 | (0.7) | 0.3 | - | - | |
| Density at 20 °C | kg/m | 3 | Max. | - | - | 791.5 | - | 790–793 | - |
| Total acidity (as acetic acid) | mg/L, (% by mass) | Max. | (0.007) | 56 (0.007) | 30 | 30 | 30 | (0.005) | |
| Electrical conductivity | µS/m | Max. | - | - | 300 | 500 | - | - | |
| pHe | - | - | 6.5~9.0 | - | 6.5~9.0 | - | - | ||
| Copper | mg/kg, (mg/L) | Max. | 0.1 | 0.1 | 0.07 | 0.07 | - | - | |
| Inorganic chloride | mg/kg, (mg/L) | Max. | 1.5 | 6.7 (5) | 1 | (20) | - | - | |
| Solvent-washed gum | mg/100 mL | Max. | - | 5 | - | 5 | - | - | |
| Sulfur | mg/kg, (ppm) | Max. | 10 | (30) | Report | - | - | - | |
| Total sulfate | mg/kg | Max. | 3 | 4 | 4 | - | - | - | |
| Phosphorus content | mg/L | Max. | 0.15 | - | - | - | - | - | |
| Non-volatile material | mg/100 mL, (% by mass) |
Max. | 10 | - | 5 | - | 2.5 | (0.005) | |
| Denaturant content | vol. % | Max. | - | 1.96~2.5 | - | - | - | - | |
| Iron | mg/kg | Max. | - | - | 5 | - | - | - | |
| Benzene | mL/kL | Max. | - | - | - | - | 2 | - | |
| Acetaldehyde and acetal (as acetaldehyde) |
% by volume, (% by mass) |
Max. | - | - | - | - | 0.001 | (0.10) | |
| Any other volatile impurity (as 4-methylpentan-2-ol) | mL/kL | Max. | - | - | - | - | 300 | - | |
| Absorbance - Lower than 240 nm - 250 to 260 nm - 270 to 340 nm |
Max. | - | - | - | - | 0.4 0.3 0.1 |
- | ||
| Sodium | % by mass | Max. | - | - | 0.0002 | - | - | - | |
| Permanganate time | Minute | Min. | - | - | - | - | - | 15 | |
| Aspect | - | Clear and colorless | Clear and colorless | Clear and no impurities | Clear, colorless and no visible suspended solids | Clear and colorless | Corresponding to ISO 2211 | ||
2. Ethanol Production from Different Types of Feedstock
Ethanol can be produced from different feedstocks. There are two main types of ethanol production feedstock in first-generation technology: sugar-containing feedstock and starch-containing feedstock. An increase in fuel demand and concern regarding the potential negative risks of using food feedstock led to the utilization of lignocellulosic feedstock for fuel ethanol production in second-generation technology. Ethanol production processes from any feedstocks can be divided into three main steps: (1) converting feedstock into fermentable sugar; (2) the fermentation process to convert fermentable sugar to ethanol; and (3) the ethanol recovery and storage process. Although the production feedstocks are different, the fermentation and downstream processes are significantly similar. Hence, when considering different feedstocks, the difference in contamination is mainly affected by the feedstock stage involving the conversion to fermentable sugar [24].3. Impact of Different Feedstocks on Impurities in Fuel Ethanol
As mentioned previously, the ethanol production process from each type of feedstock includes three major steps: conversion of feedstock, fermentation, and ethanol recovery. This section describes the conversion of each separate feedstock. The key to this process is to release sugar molecules from the feedstock structure. The difficulties in releasing sugar molecules depend on feedstock type, which involve different required steps to convert feedstock, and consequently result in various contamination profiles in the ethanol product.3.1. Conversion of Sugar-Containing Feedstock
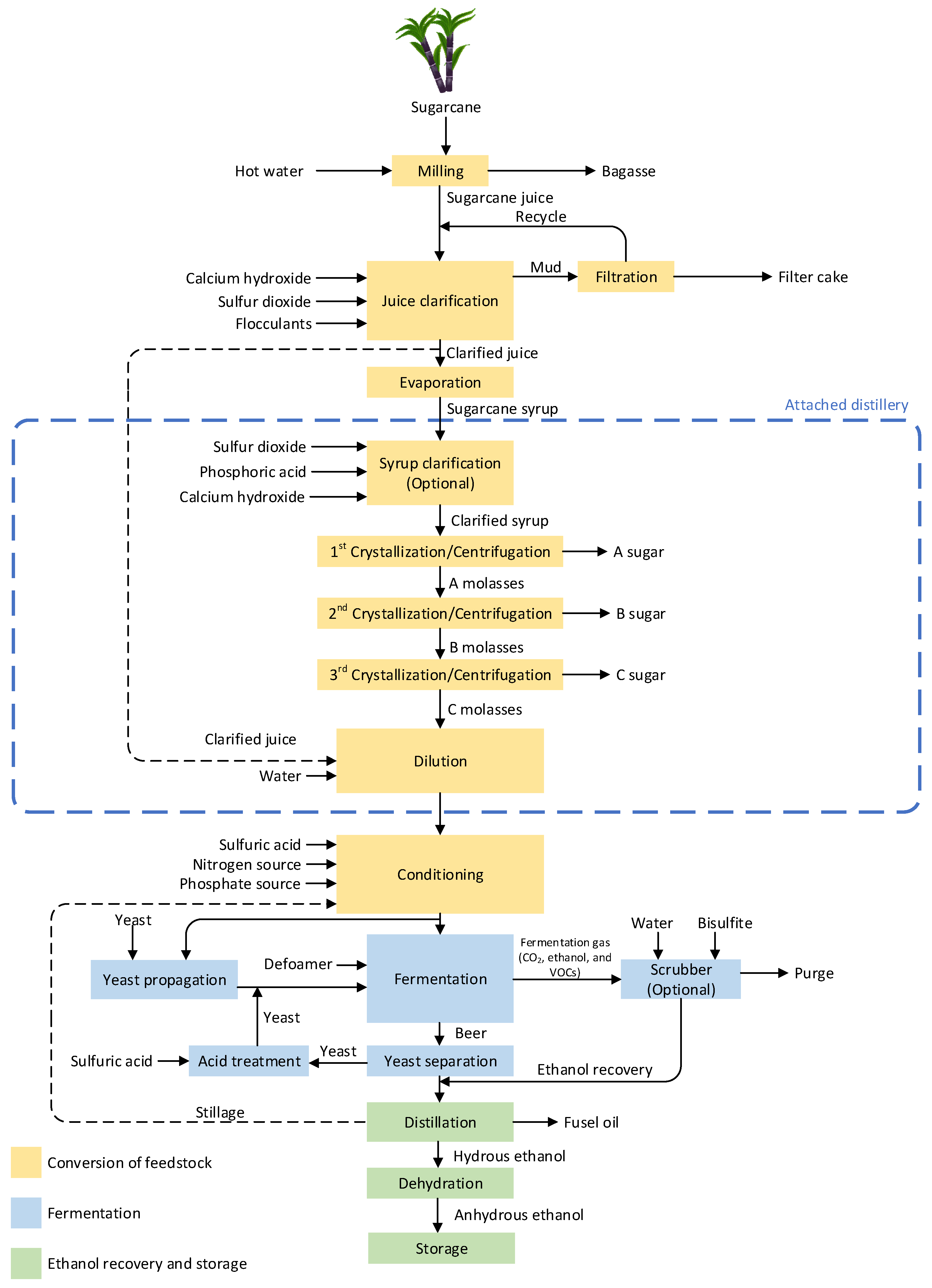
In many countries, such as Thailand, Brazil, India, and Colombia, sugarcane is cultivated for sugar production [25,26][25][26]. The valuable byproduct from sugar production is molasses, which is used in ethanol production. Besides, sugarcane juice is also utilized to produce ethanol in some countries such as Thailand [25,27,28][25][27][28]. Therefore, the sugar production process needs to be considered, as it determines the quality and impurities of the feedstock during ethanol production. Attached and autonomous distilleries are two types of sugarcane-derived ethanol production plants, classified by ethanol feedstocks. The overall production process and chemical additions in each step for these two categorized sugarcane-derived ethanol production plants are shown in Figure 1. In the case of autonomous distilleries, the process section in the dashed–blue box can be excluded.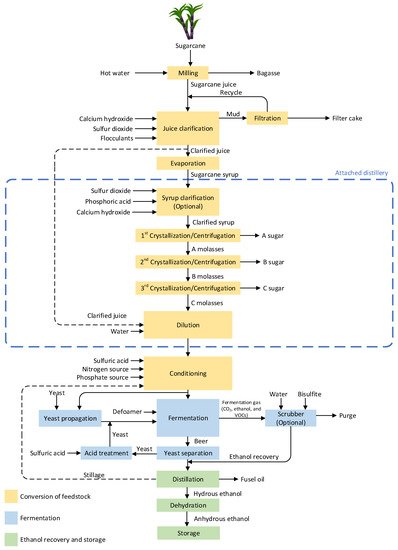
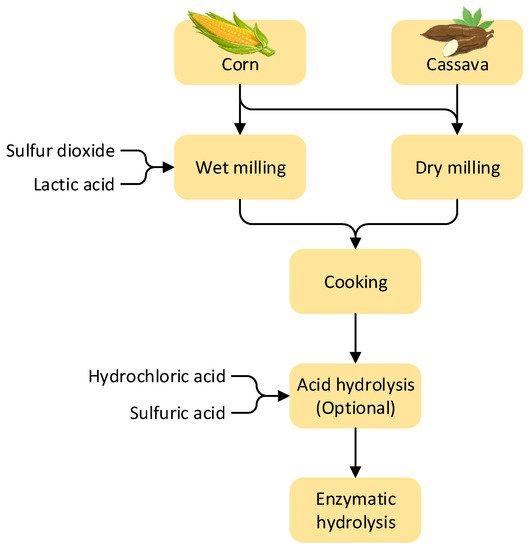
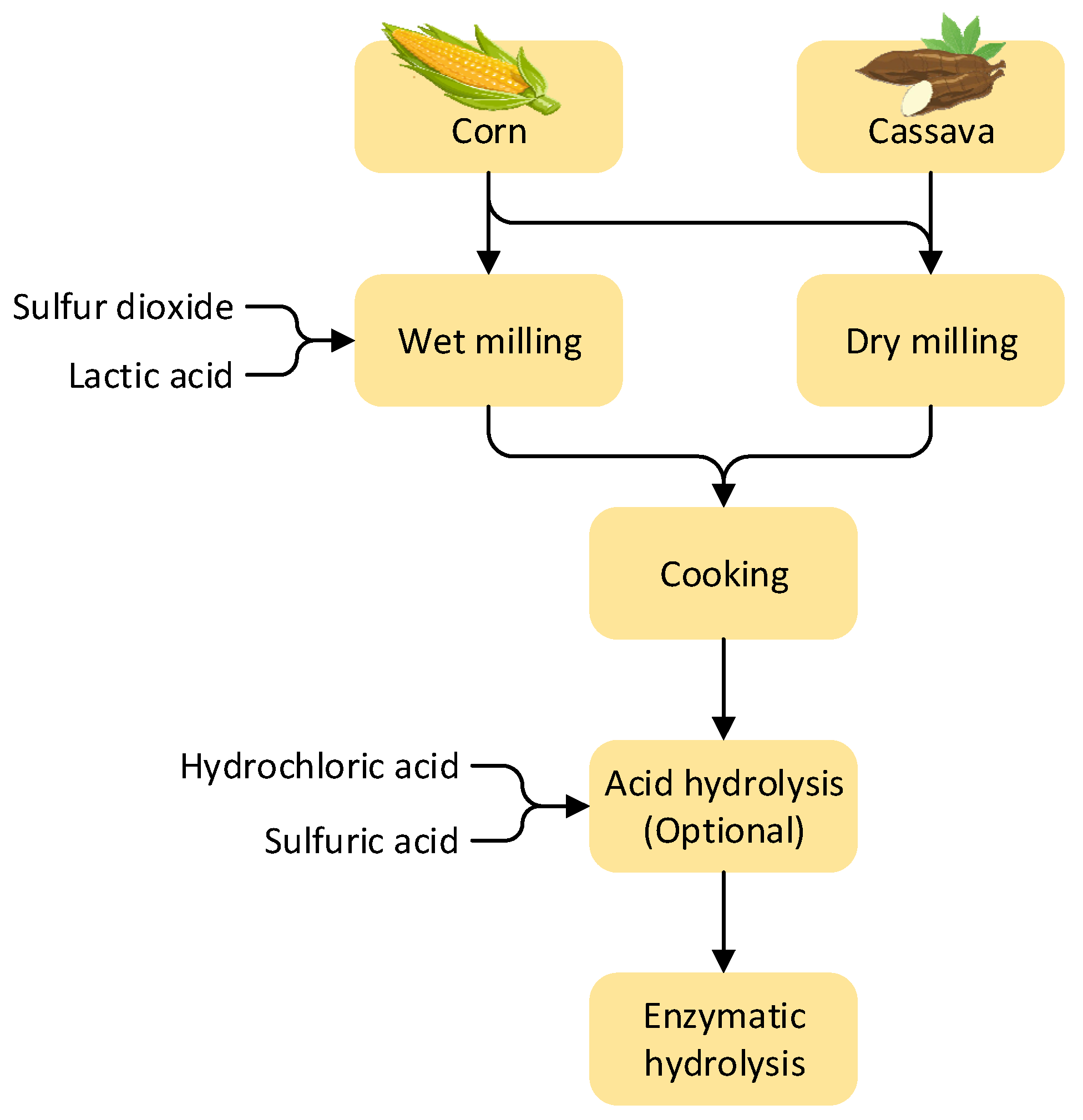
3.2. Conversion of Starch-Containing Feedstocks
4. Ethanol Recovery
4.1. Distillation Process
In sugar and starch fermentation, other alcohols, aldehydes, ketones, fatty acids, and esters are produced as volatile byproducts, whereas cyclic and heterocyclic compounds are volatile byproducts in lignocellulosic ethanol fermentation [91][29]. After the fermentation process is finished, the centrifuged broth is obtained by separating the yeast from the fermented beer. The centrifuged broth containing ethanol at about 5–15 wt.% is passed to the distillation column to remove the water. The distillation column consists of two columns. The first one is called the distillation column, or the beer column. In this column, approximately 50 wt.% ethanol can be achieved. The second column is the rectifying column. Hydrous ethanol (about 93 wt.% ethanol) can be achieved in this column [30,35][30][31]. Distillation can remove some impurity from ethanol with increasing ethanol concentration. Furthermore, chemical molecules with low boiling points, or those similar to ethanol, show up in distillate because distillation is ineffective in removing them [17]. For example, volatile impurities (acetaldehyde, acetone, ester, methanol) still show up in distillate. These contaminants result in lower engine efficiency when ethanol is used as fuel [7,8,15,91,242][7][8][15][29][32].4.2. Stillage Recycles
The remaining bottom liquid product after distillation of the ethanol from the beer column is called whole stillage. The whole stillage can contain ethanol up to 0.02 wt.%. Not only ethanol, but also solid particles, such as yeast cells, dissolved matter, and minerals, can be found [26,243][26][33]. After removing solid particles through a solid–liquid separation unit (e.g., centrifuge or decanter), the obtained liquid product called thin stillage can be recycled back to different process steps, e.g., fermentation or saccharification, to minimize effluent treatment cost. However, thin stillage recycling can possibly cause some drawbacks, such as the accumulation of lactic acid, minerals, and unutilized substrates [26,243,244][26][33][34]. The difference in the type of feedstock affects the impurities in the stillage. When stillage is recycled, it causes different contaminations. In the case of cane molasses feedstocks, whole stillage (without yeast cell separation) can be recycled in the fermentation step [26]. In the case of starch-containing feedstock, 25–75% of the thin stillage can be recycled in the fermentation or saccharification processes [26]. Other feedstocks, such as corn, wheat, and triticale, can be recycled at 75%, 60%, and 60% of thin stillage, respectively [243,245][33][35]. In Thailand, produced stillage during ethanol production from molasses or cassava is often treated and converted into methane gas. Stillage can also be distributed to farmers because stillage provides minerals for plants [246,247][36][37].4.3. The Fate of Electrolytes during Distillation
During ethanol distillation, sulfite as sulfur dioxide can be distilled into the final ethanol product. The presence of sulfite in distilled ethanol appears to be a common experience in the distilled spirits industry [7,248][7][38]. Zhang et al. [249][39] reported that the distillate of chardonnay contained 12% ethanol and 176 mg/L sulfite as SO2. After two stages of distillation, the concentration of ethanol and sulfite as SO2 were increased to 69 vol% and 654 ppm, respectively. This phenomenon can be explained with the vapor–liquid equilibria for dilute aqueous solutions of SO2 as volatile weak electrolyte [250][40].4.4. Dehydration Process
The distillation process produces 95 vol% ethanol, approximately, because of the azeotropic mixture of ethanol and water (95.6 wt.% at 78.15 degrees Celsius). Before mixing ethanol with gasoline, it is necessary to increase the ethanol concentration to 99.3 wt.%, to make anhydrous ethanol. Anhydrous ethanol can be obtained by several dehydration methods such as molecular sieves, azeotropic distillation, and pervaporation. The molecular sieve is most commonly used because it has lower investment costs than pervaporation and requires less steam than azeotropic distillation [30,35][30][31]. The most common dehydration methods in Brazil are heterogeneous azeotropic distillation, extractive distillation, and molecular sieve adsorption [35][31]. The heterogeneous azeotropic distillation method requires an entrainer to increase separation. Many entrainers, such as benzene, toluene, and cyclohexane can be used to separate ethanol from water [35,251][31][41]. However, using an entrainer can cause product contamination [252,253][42][43]. Extractive distillation, as an alternative method, requires the addition of a third component to change the relative volatility of ethanol and water. The third component acts as a separating agent, such as ethylene glycol, glycerol, 1,3 diamino pentane, diethylenetriamine, or hexachlorobutadiene. The separating agent and water mixture is obtained at the bottom of the column, which is fed to the second column to recover the separating agent. Anhydrous ethanol is obtained at the top of the extractive column. Compared to azeotropic distillation, this method provides less energy consumption and less ethanol contamination [35][31]. In the case of molecular sieve adsorption, there is no requirement to add solvent. Ethanol vapor is fed to zeolite beds. When hydrated ethanol contacts zeolite, water molecules are absorbed. When compared to azeotropic distillation and extractive distillation, molecular sieve adsorption offers lower energy consumption and no chemical contamination [35][31]. Pervaporation, a membrane dehydration method, is a relatively new alternative to the dehydration process. While adsorbents need regeneration, membrane separation offers continuous operation and energy saving. Industrial applications of zeolite membranes have been reported [254][44].References
- Gallo, J.M.; Bueno, J.; Schuchardt, U. Catalytic transformations of ethanol for biorefineries. J. Braz. Chem. Soc. 2014, 25, 2229–2243.
- Galante-Fox, J.; Von Bacho, P.; Notaro, C.; Zizelman, J. E-85 fuel corrosivity: Effects on port fuel injector durability performance. SAE Trans. 2007, 116, 989–994.
- Sriroth, K.; Wanlapatit, S.; Piyachomkwan, K. Cassava bioethanol. In Bioethanol; IntechOpen: London, UK, 2012.
- SEWPAC. Regulation Impact Statement Fuel Quality Standard: Ethanol (E85) Automotive Fuel; Department of Sustainability, Environment, Water, Population and Communities: Canberra, Australia, 2012. Available online: https://parlinfo.aph.gov.au/parlInfo/download/publications/tabledpapers/HSTP012629_2010-13/upload_pdf/12629_2010-13.pdf;fileType=application%2Fpdf#search=%22publications/tabledpapers/HSTP012629_2010-13%22 (accessed on 7 January 2021).
- Cortez, L.A.B. Sugarcane Bioethanol: R&D for Productivity and Sustainability; Blucher: São Paulo, Brazil, 2010.
- TTF. White Paper on Internationally Compatible Biofuel Standards. Tripartite Task Force. 2007. Available online: https://www.nist.gov/document/biofuelsreportpdf (accessed on 15 December 2020).
- McCormick, R.L.; Alleman, T.; Yanowitz, J. Sulfate Salts in Gasoline and Ethanol Fuels—Historical Perspective and Analysis of Available Data; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2017.
- Stepien, Z.; Krasodomski, W. Investigation into Engine Deposit-Forming Tendency due to Sulfate Salt Contamination of Gasoline–Ethanol Blends. Energy Fuels 2019, 33, 4244–4253.
- ASTM D5798-10a; Standard Specification for Fuel Ethanol (Ed70-Ed85) for Automotive Spark-Ignition Engines. ASTM International: West Conshohocken, PA, USA, 2011.
- Habe, H.; Shinbo, T.; Yamoto, T.; Sato, S.; Shimada, H.; Sakaki, K. Chemical analysis of impurities in diverse bioethanol samples. J. Jpn. Pet. Inst. 2013, 56, 414–422.
- Christensen, E.; Fioroni, G.M.; Kim, S.; Fouts, L.; Gjersing, E.; Paton, R.S.; McCormick, R.L. Experimental and theoretical study of oxidative stability of alkylated furans used as gasoline blend components. Fuel 2018, 212, 576–585.
- Haaz, E.; Fozer, D.; Toth, A.J. Development of Anhydrous Ethanol Purification: Reduction of Acetal Content and Vapor–Liquid Equilibrium Study of the Ethanol–Acetal Binary System. ACS Omega 2021, 6, 1289–1298.
- Naegeli, D.W.; Lacey, P.I.; Alger, M.J.; Endicott, D.L. Surface corrosion in ethanol fuel pumps. SAE Trans. 1997, 106, 564–571.
- Hoekman, S.K.; Broch, A. Investigation into Filter Plugging Due to Sulfate Salt Contamination of Ethanol, Gasoline, and Gasoline-Ethanol Blends; Coordinating Research Council, Inc.: Alpharetta, GA, USA, 2018.
- Styarini, D.; Aristiawan, Y.; Aulia, F.; Abimanyu, H.; Sudiyani, Y. Determination of organic impurities in lignocellulosic bioethanol product by GC-FID. Energy Procedia 2013, 32, 153–159.
- Sánchez, R.; Sánchez, C.; Lienemann, C.-P.; Todolí, J.-L. Metal and metalloid determination in biodiesel and bioethanol. J. Anal. At. Spectrom. 2015, 30, 64–101.
- Sánchez, C.; Santos, S.; Sánchez, R.; Lienemann, C.-P.; Todolí, J.-L. Profiling of Organic Compounds in Bioethanol Samples of Different Nature and the Related Fractions. ACS Omega 2020, 5, 20912–20921.
- RFA. Fuel Ethanol: Industry Guidelines, Specifications, and Procedures; Renewable Fuels Association: Washington, DC, USA, 2018.
- API 1626: 2010; Storing and Handling Ethanol and Gasoline-Ethanol Blends at Distribution Terminals and Service Stations. American Petroleum Institute: Washington, DC, USA, 1985.
- Costenoble, O. Worldwide Fuels Standards. In Overview of Specifications and Regulations on (Bio) Fuels; NEN–Netherlands Standardization Institute: Delft, The Netherlands, 2017.
- TIS 640-1; Ethanol for Pharmaceutical Use. Thai Industrial Standards Institute (TISI): Bangkok, Thailand, 2010.
- TIS 640-2; Ethanol for Industrial Use. Thai Industrial Standards Institute (TISI): Bangkok, Thailand, 2010.
- TIS 2324; Denatured Ethanol for Gasohol Production. Thai Industrial Standards Institute (TISI): Bangkok, Thailand, 2006.
- Vohra, M.; Manwar, J.; Manmode, R.; Padgilwar, S.; Patil, S. Bioethanol production: Feedstock and current technologies. J. Environ. Chem. Eng. 2014, 2, 573–584.
- Nguyen, T.L.T.; Gheewala, S.H. Life cycle assessment of fuel ethanol from cane molasses in Thailand. Int. J. Life Cycle Assess. 2008, 13, 301.
- Cardona, C.A.; Sanchez, O.J.; Gutierrez, L.F. Process Synthesis for Fuel Ethanol Production; CRC Press: Boca Raton, FL, USA, 2009.
- Modesto, M.; Nebra, S.A.; Zemp, R.J. Improving the Ethanol Production From Sugar Cane Biomass. In Proceedings of the ASME 8th Biennial Conference on Engineering Systems Design and Analysis, Torino, Italy, 4–7 July 2006; pp. 203–210.
- Dias, M.O.; Junqueira, T.L.; Cavalett, O.; Cunha, M.P.; Jesus, C.D.; Rossell, C.E.; Maciel Filho, R.; Bonomi, A. Integrated versus stand-alone second generation ethanol production from sugarcane bagasse and trash. Bioresour. Technol. 2012, 103, 152–161.
- Onuki, S.; Koziel, J.A.; Jenks, W.S.; Cai, L.; Grewell, D.; van Leeuwen, J. Taking ethanol quality beyond fuel grade: A review. J. Inst. Brew. 2016, 122, 588–598.
- de Castro, R.E.N.; de Brito Alves, R.M.; do Nascimento, C.A.O.; Giudici, R. Assessment of Sugarcane-Based Ethanol Production. In Fuel Ethanol Production from Sugarcane; IntechOpen: London, UK, 2018.
- de Souza Dias, M.O.; Maciel Filho, R.; Mantelatto, P.E.; Cavalett, O.; Rossell, C.E.V.; Bonomi, A.; Leal, M.R.L.V. Sugarcane processing for ethanol and sugar in Brazil. Environ. Dev. 2015, 15, 35–51.
- Onuki, S.; Koziel, J.A.; van Leeuwen, J.H.; Jenks, W.S.; Grewell, D.; Cai, L. Ethanol production, purification, and analysis techniques: A review. In Proceedings of the 2008 Providence, Providence, RI, USA, 29 June–2 July 2008; p. 1.
- Friedl, A. Bioethanol from Sugar and Starch. In Energy from Organic Materials (Biomass): A Volume in the Encyclopedia of Sustainability Science and Technology, 2nd ed.; Kaltschmitt, M., Ed.; Springer: New York, NY, USA, 2019; pp. 905–924.
- Stenberg, K.; Tengborg, C.; Galbe, M.; Zacchi, G.; Palmqvist, E.; Hahn-Hägerdal, B. Recycling of process streams in ethanol production from softwoods based on enzymatic hydrolysis. Appl. Biochem. Biotechnol. 1998, 70, 697.
- Stout, B.A. Handbook of Energy for World Agriculture; Elsevier: Amsterdam, The Netherlands, 2012.
- Nguyen, T.L.T.; Gheewala, S.H. Fuel ethanol from cane molasses in Thailand: Environmental and cost performance. Energy Policy 2008, 36, 1589–1599.
- Mangmeechai, A.; Pavasant, P. Water footprints of Cassava-and Molasses-based ethanol production in Thailand. Nat. Resour. Res. 2013, 22, 273–282.
- Schill, S.R. Sulfur Compounded in Ethanol Regulations. Ethanol Prod. Mag. 2016. Available online: http://www.ethanolproducer.com/articles/13513/sulfur-compounded-in-ethanol-regulations (accessed on 12 October 2020).
- Zhang, Q.; Du, J.; Jin, Y.; Zhao, Z.; Li, Y. SO2 reduction in distilled grape spirits by three methods. J. Inst. Brew. 2013, 119, 314–320.
- Edwards, T.J.; Newman, J.; Prausnitz, J.M. Thermodynamics of aqueous solutions containing volatile weak electrolytes. AIChE J. 1975, 21, 248–259.
- Huang, H.-J.; Ramaswamy, S.; Tschirner, U.W.; Ramarao, B. Separation and Purification processes for lignocellulose-to-bioalcohol production. In Bioalcohol Production; Elsevier: Amsterdam, The Netherlands, 2010; pp. 246–277.
- Ramos, W.B.; Figueiredo, M.F.; Brito, R.P. Optimization of extractive distillation process with a single column for anhydrous ethanol production. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2014; Volume 33, pp. 1411–1416.
- Ortuño-Boter, D.; Plesu, V.; Ruiz, A.E.B.; Ruiz, J.B.; Iancu, P.; Llorens, J. Enhanced Distillation Based on Feed Impurities. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2016; Volume 38, pp. 1923–1928.
- Morigami, Y.; Kondo, M.; Abe, J.; Kita, H.; Okamoto, K. The first large-scale pervaporation plant using tubular-type module with zeolite NaA membrane. Sep. Purif. Technol. 2001, 25, 251–260.
