Ethanol can be produced from sugary, starchy, and lignocellulosic feedstocks. Each feedstock requires different procedures for its conversion to fermentable sugar. Lignocellulosic biomass requires extra pretreatment compared to sugar and starch feedstocks to disrupt the structure and improve enzymatic hydrolysis efficiency. However, the greatest concern regarding the pretreatment process is inhibitor formation, which might retard enzymatic hydrolysis and fermentation. In addition to the inhibitors from pretreatment, chemicals used during the pretreatment and fermentation of byproducts may remain in the final product if they are not removed by ethanol distillation and dehydration. Maintaining the quality of ethanol during storage is another concerning issue. Initial impurities of ethanol being stored and its nature, including hygroscopic, high oxygen and carbon dioxide solubility, influence chemical reactions during the storage period and change ethanol’s characteristics (e.g., water content, ethanol content, acidity, pH, and electrical conductivity).
- bioethanol
- ethanol specification
- quality control
1. Introduction
| Specification | Unit | European Union |
USA | Brazil | Thailand | ||||
|---|---|---|---|---|---|---|---|---|---|
| prEN 15376 | ASTM D-4806-16a |
ANP Resolution nº 19 | TIS 2324 | TIS 640-1 | TIS 640-2 | ||||
| Ethanol type | - | - | Anhydrous | Denatured anhydrous |
Anhydrous | Denatured anhydrous |
Anhydrous | Anhydrous | |
| Ethanol | % by volume | Min. | - | - | 98 | - | - | - | |
| Ethanol and higher saturated alcohols | % by volume, (% by mass) |
Min. | (98.7) | 92.1 | (99.3) | 99 | 99.5 | 99.5 | |
| Higher saturated mono-alcohols-C3-C5 | % by volume, (% by mass) |
Max. | (2) | - | 3 | 2 | - | - | |
| Methanol | % by volume, (% by mass) |
Max. | (1) | 0.5 | 0.5 | 0.5 | 0.02 | 0.05 | |
| Water content | % by volume, (% by mass) |
Max. | (0.3) | 1 | (0.7) | 0.3 | - | - | |
| Density at 20 °C | kg/m | 3 | Max. | - | - | 791.5 | - | 790–793 | - |
| Total acidity (as acetic acid) | mg/L, (% by mass) | Max. | (0.007) | 56 (0.007) | 30 | 30 | 30 | (0.005) | |
| Electrical conductivity | µS/m | Max. | - | - | 300 | 500 | - | - | |
| pHe | - | - | 6.5~9.0 | - | 6.5~9.0 | - | - | ||
| Copper | mg/kg, (mg/L) | Max. | 0.1 | 0.1 | 0.07 | 0.07 | - | - | |
| Inorganic chloride | mg/kg, (mg/L) | Max. | 1.5 | 6.7 (5) | 1 | (20) | - | - | |
| Solvent-washed gum | mg/100 mL | Max. | - | 5 | - | 5 | - | - | |
| Sulfur | mg/kg, (ppm) | Max. | 10 | (30) | Report | - | - | - | |
| Total sulfate | mg/kg | Max. | 3 | 4 | 4 | - | - | - | |
| Phosphorus content | mg/L | Max. | 0.15 | - | - | - | - | - | |
| Non-volatile material | mg/100 mL, (% by mass) |
Max. | 10 | - | 5 | - | 2.5 | (0.005) | |
| Denaturant content | vol. % | Max. | - | 1.96~2.5 | - | - | - | - | |
| Iron | mg/kg | Max. | - | - | 5 | - | - | - | |
| Benzene | mL/kL | Max. | - | - | - | - | 2 | - | |
| Acetaldehyde and acetal (as acetaldehyde) |
% by volume, (% by mass) |
Max. | - | - | - | - | 0.001 | (0.10) | |
| Any other volatile impurity (as 4-methylpentan-2-ol) | mL/kL | Max. | - | - | - | - | 300 | - | |
| Absorbance - Lower than 240 nm - 250 to 260 nm - 270 to 340 nm |
Max. | - | - | - | - | 0.4 0.3 0.1 |
- | ||
| Sodium | % by mass | Max. | - | - | 0.0002 | - | - | - | |
| Permanganate time | Minute | Min. | - | - | - | - | - | 15 | |
| Aspect | - | Clear and colorless | Clear and colorless | Clear and no impurities | Clear, colorless and no visible suspended solids | Clear and colorless | Corresponding to ISO 2211 | ||
2. Ethanol Production from Different Types of Feedstock
Ethanol can be produced from different feedstocks. There are two main types of ethanol production feedstock in first-generation technology: sugar-containing feedstock and starch-containing feedstock. An increase in fuel demand and concern regarding the potential negative risks of using food feedstock led to the utilization of lignocellulosic feedstock for fuel ethanol production in second-generation technology. Ethanol production processes from any feedstocks can be divided into three main steps: (1) converting feedstock into fermentable sugar; (2) the fermentation process to convert fermentable sugar to ethanol; and (3) the ethanol recovery and storage process. Although the production feedstocks are different, the fermentation and downstream processes are significantly similar. Hence, when considering different feedstocks, the difference in contamination is mainly affected by the feedstock stage involving the conversion to fermentable sugar [24].3. Impact of Different Feedstocks on Impurities in Fuel Ethanol
As mentioned previously, the ethanol production process from each type of feedstock includes three major steps: conversion of feedstock, fermentation, and ethanol recovery. This section describes the conversion of each separate feedstock. The key to this process is to release sugar molecules from the feedstock structure. The difficulties in releasing sugar molecules depend on feedstock type, which involve different required steps to convert feedstock, and consequently result in various contamination profiles in the ethanol product.3.1. Conversion of Sugar-Containing Feedstock
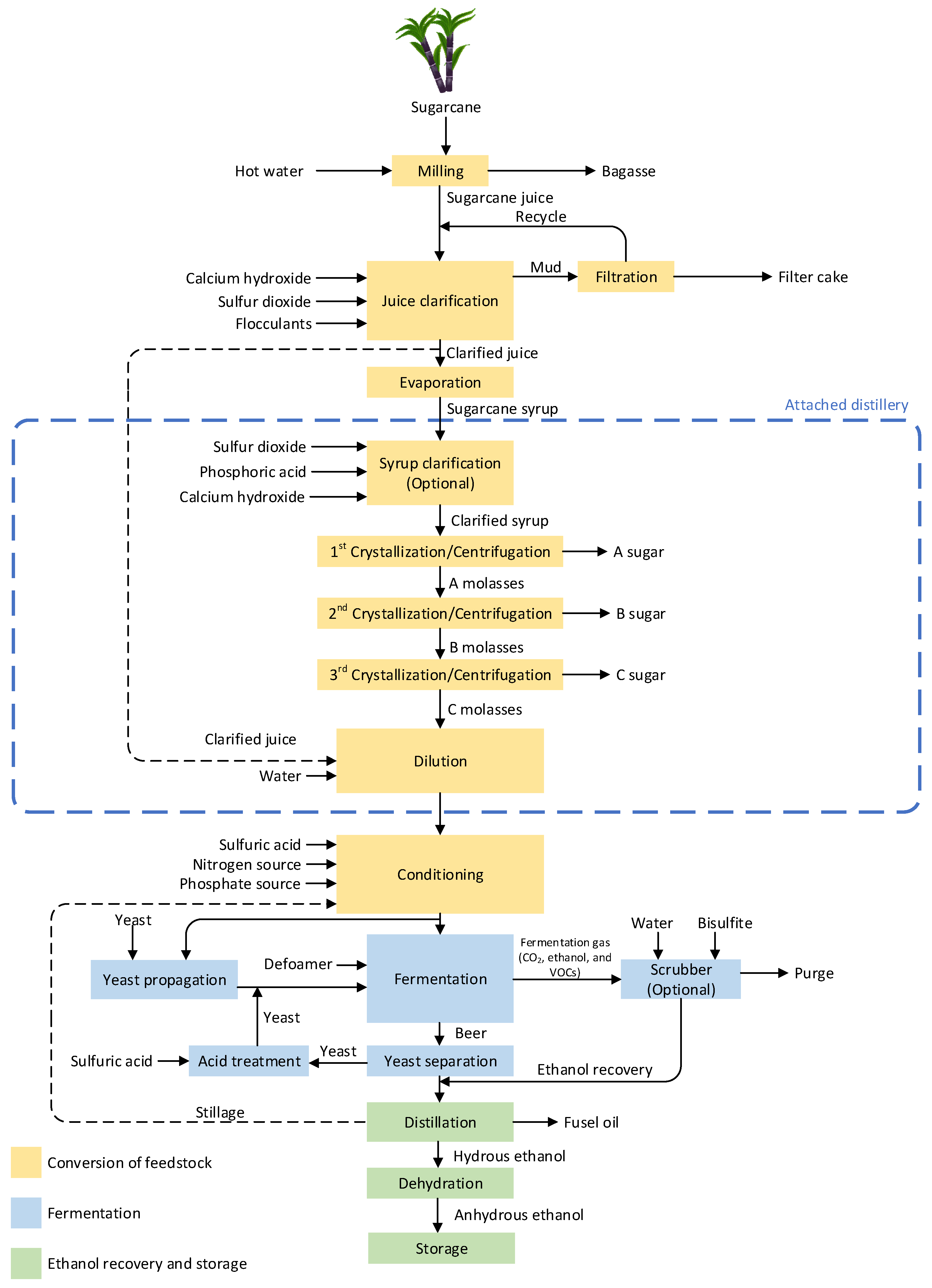
In many countries, such as Thailand, Brazil, India, and Colombia, sugarcane is cultivated for sugar production [25][26][25,26]. The valuable byproduct from sugar production is molasses, which is used in ethanol production. Besides, sugarcane juice is also utilized to produce ethanol in some countries such as Thailand [25][27][28][25,27,28]. Therefore, the sugar production process needs to be considered, as it determines the quality and impurities of the feedstock during ethanol production. Attached and autonomous distilleries are two types of sugarcane-derived ethanol production plants, classified by ethanol feedstocks. The overall production process and chemical additions in each step for these two categorized sugarcane-derived ethanol production plants are shown in Figure 1. In the case of autonomous distilleries, the process section in the dashed–blue box can be excluded.
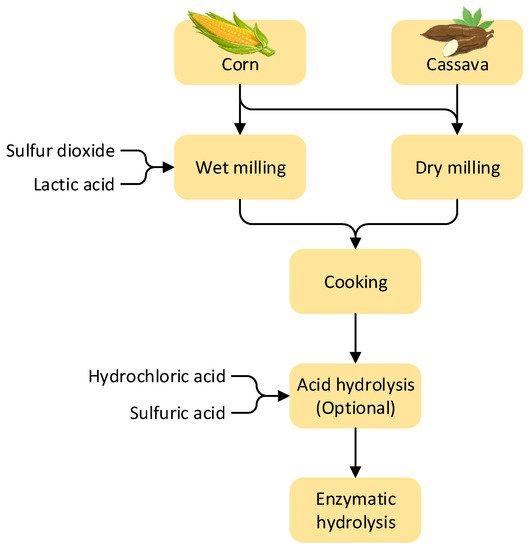
3.2. Conversion of Starch-Containing Feedstocks