You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Md. Habibur Rahman and Version 2 by Catherine Yang.
Several plants and essential oils, as well as isolated bioactive compounds, such as phenolic acids, flavonoids, terpenes, lignans, coumarins, alkaloids, or proteins, showed a potential role as anti-viral agents. Nanoparticles have also been utilized in noninvasive imaging techniques to diagnose and monitor illnesses, as well as treatment responses. Nanomaterials can prevent viral binding to the host cell-surface receptor, which is crucial in the context of CoVs.
- bioavailability
- coronavirus
- nano-formulations
1. General Structure of Viruses
Viruses are intracellular parasites, as depicted in Figure 1, composed of DNA or RNA inside a protein coat that lacks cell walls and cell membranes and does not carry out metabolic processes, so they must attach and enter the host’s cells to use their energy for protein, DNA and RNA synthesis for their survival. It is difficult to kill and manage viral infections, as they live inside the host’s cells and an anti-viral drug that kills viruses may also damage and kill the host’s cells; therefore, for pharmaceutical scientists, it is quite challenging to develop an effective anti-viral drug and vaccine. Therapy for viral diseases using anti-viral drugs is further complicated by the fact that clinical symptoms appear late and until then, most viral particles have been replicated [1][20]. The key points that need to be considered for the successful development of anti-viral therapy include the development of a molecule that can enter the infected cells to interfere with the nucleic acid synthesis, or to prevent the binding and entry of the virus into the host cells, Most herbal bioactive compounds are known to work efficiently using this strategy, in addition to the synthesis of molecules that strengthen the body’s immune system to combat infection. [2][21].
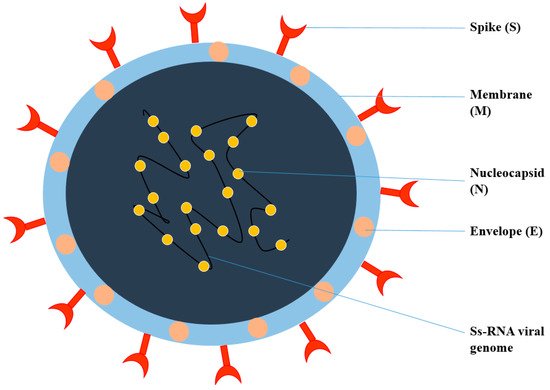
Figure 1.
Structure of Coronavirus.
Coronavirus is an enveloped, single-stranded RNA virus, known to cause different disorders, such as hepatic, enteric, neurological, and respiratory diseases [3][22]. Coronaviruses are classified into four genera according to the taxonomic analysis, which includes α, β, γ, and δ coronavirus. The α and β coronavirus assemblies trigger respiratory track information. SARS-CoV-2 falls under β and shares approximately 79.6% of SARS-CoV genome identity [4][23]. SARS-CoV-2 consists of structural proteins (S, E, M, and N) and non-structural proteins (nsp1−16). The S protein of the virus is a trimeric S glycoprotein that facilitates adhesion to the host cell’s receptor. In most coronaviruses, the S protein is fragmented into two polypeptides called S1 and S2 via the host cell’s furin-like protease. The S1 is a component of the S protein’s large RBD, while the S2 is the stalk of the spike protein structure [4][23]. The non-structural protein Nsp1 is involved in RNA processing and replication. Nsp2 influences the host cell’s survival signaling system. Nsp3 is thought to be involved in the separation of the translated protein. Nsp4 is a transmembrane domain 2 (TM2) protein that alters ER membranes. In replication, Nsp5 participates in the polyprotein process. Nsp6 is a transmembrane domain. The existence of nsp7 and nsp8 boosted the combination of nsp12 with template-primer RNA, considerably. Nsp9 is a protein that binds to ssRNA. Nsp10 is required for viral mRNA cap methylation. The COVID-19 genome codes four-wide structural and five accessory proteins, including ORF3a, ORF6, ORF-7, OR8, and ORF9, with a size of approximately 29 kb [5][24]. Coronaviruses replicate their genomes and transcribe their genes via an RNA-dependent RNA polymerase (RdRp) complex. The RdRp complex of SARS-CoV-2 consists of subunits nsp12, nsp7, and nsp8, which enhance their processes and template binding [5][24].
2. Pathophysiology of COVID-19
The three major COVID-19 routes are as follows: (1) transmission by aerosol, (2) transmission by droplets, and (3) transmission by contact [6][25].
The incidence of COVID-19 infection in individuals is highly impacted by disorders, such as diabetes, hypertension, and lung diseases. This may be attributable to the increased ACE2 receptor expression in several organs, including the kidney, lungs, heart, and host epithelial cells. As COVID-19 enters the human body, it interacts with the ACE2 receptor and releases the RNA inside epithelial cells (EC). It then replicates and is released for further infection and spread via a nasal passage to the alveolar area of the lung [7][26]. The alveoli are generally the arbitration for gaseous exchange but, because of COVID-19 infection, there is increased permeability, pulmonary edema, disseminated intravascular coagulation (ICD) activation, pulmonary ischemia, hypoxia, respiratory failure, and severe lung damage [8][27]. It is then transported across the body, including GIT, brain, kidney, liver, and heart, through the blood from the respiratory tract, leading to various neurological disorders, comas, cerebral blood clots, ischemic strokes, and eventually death [9][28]. COVID-19 infects endothelial cells through ACE-2 binding, causing localized inflammation, endothelial activation, damaging tissue, and impaired release of cytokine. This severe aggregation of cytokine storm through vascular growth factor secretion, monocyte protein-1, interleukin-8, and decreased E-cadherin expression in epithelial cells contribute to vascular permeability and leakages, which are part of the acute respiratory distress syndrome pathophysiology (ARDS). Most Coronavirus-infected patients die from ARDS, in which pulmonary epithelial cells help to begin and transmit ARDS with a shift in the veracity of a vessel’s barrier, encourage the condition of pro coagulation, induce vascular inflammation and reconcile inflammatory cell infiltration [10][29]. The epithelial cells are the key cause of pathogenesis of ARDS and multi-organ failure in COVID-19 patients, according to the hypothesis. Extreme COVID-19 infection stimulates coagulation mechanisms, which can potentially induce angiogenesis and possible epithelial cell hyperplasia, with the formation of DICs and blockages of small capillaries by the inflammatory cell, as well as significant thrombosis in larger vessels [11][12][30,31]. According to Teuwen et al., there are multiple mechanisms proposed for increased vascular permeability and vascular leakage in severely infected patients. The virus may have a direct effect on epithelial cells with widespread epithelial dysfunction, lysis, and death. It binds to ACE-2, decreasing ACE2 activity, indirectly turns on a kallikren–bradykinin route with enhanced vascular permeability to reach the host cells, recruited in pulmonary epithelial cells, stimulated neutrophils to generate cytotoxic mediators, such as reactive oxygen species, immune cells, inflammatory cytokines, and vasoactive molecules contribute to improved contractility and a weakening of inter-endothelial connections in the epithelial cells. The cytokines IL-1β and tumor necrosis factor cause glucuronidases that both degrade glycocalyx and activate hyaluronic acid synthase 2, which results in increased hyaluronic acid deposition within the extracellular matrix and encourages fluid retention [13][32].
3. Natural Anti-Viral Plants and Phytochemicals
Phytoconstituents are naturally isolated bioactive molecules from plant parts, such as vegetables, fruits, flowers, leaves, and roots, that together with nutrients and fibers act as a defense system against disease or, more specifically, help to fight against the disease. Polyphenols, alkaloids, flavonoids, saponins, quinones, terpenes, proanthocyanidins, lignans, tannins, polysaccharides, steroids, organosulfur compounds, and coumarins are protruding bioactive phytochemicals, which have been used in experiments to combat viral infections and have attracted the attention of formulators because of their considerable advantages over synthetic molecules including low toxicity, side effects and low cost and have less potential to develop anti-viral resistance [14][33]. Phytochemicals are classified into primary and secondary constituents according to their metabolic activities. Primary constituents comprise common sugars, amino acids, proteins, and chlorophyll, while secondary constituents include alkaloids, terpenoids, phenolic compounds, and many more, such as flavonoids, tannins, and so on [15][34]. Plants have been established naturally over the years in various climatic environments on the planet and are enriched with a wide pharmacokinetic complexity of secondary metabolites. Some of the plants containing natural bioactive constituents are discussed below and play specific roles in combating diverse viral infections and diseases.
3.1. Scutellaria baicalensis
Scutellaria baicalensis has been used for over 200 years in TCM as a remedy for viral infection and inflammation. The roots of this plant have been used for viral infections, anti-inflammation, and anti-cancer activities. This plant also possesses diuretic, chalagogic and cathartic actions. It contains a variety of sterols, flavonoids, phenylethanoids, essential oils, etc. Its dried roots contain over 30 kinds of flavonoids, including baicalein, baicalin, wagonin, oroxylin A, etc. Baicalin is the most important component and has an anti-SARS coronavirus effect, anti-HIV, free radicle scavenging [16][17][18][35,36,37], etc. Another constituent named oroxylin A has anti-respiratory syncytial viral activity [19][38]. Current research confirmed that baicalin has in-vitro and in-vivo activity against dengue virus, influenza viruses, and enter virus-71 [20][39]. The compounds particularly hit cell attachment and intracellular replication of H3N2 and H1N1. In the murine RSV infection model, baicalin significantly reduced macrophages and T-lymphocyte infiltration to the lungs.
3.2. Tanacetum vulgare
Tanacetum vulgare L. also acknowledged as tansy is an herbaceous plant found in the temperate region of North Africa and Europe. It has been practiced as a traditional medicine that exhibits various properties, such as carminative, anti-diabetic, diuretic, anti-hypertensive, emmenagogue, and so on [21][40]. The extracts and active compounds isolated from this plant have shown several therapeutic applications, such as anti-bacterial, anti-fungal, anti-oxidant, immunomodulatory, anti-viral, etc. The chemical constituent of this plant includes flavonoids, vacuolar flavonoids (such as apigenin) glycosides, sterols (such as cholesterol and campesterol), triterpenes (taraxasterol and amyrin), and caffeic acid [22][41]. In 2009, Álvarez demonstrated the anti-viral activity of tansy against the HSV-1 virus from ethyl acetate extract from its aerial parts corresponding to parthenolide isolation [23][42]. Furthermore, the study revealed the anti-viral properties of methanol extract of the tansy aerial parts and was active against both HSV-1 and HSV-2 viruses [24][43]. Methanol extract from the blossom of the tansy plant showed anti-tumor properties against potato virus Y (PVY) and cucumber mosaic virus (CMV) [25][44].
3.3. Ruta Angustifolia
Ruta angustifolia Pers has been used as a traditional remedy for treating inflammation, curing mal conditions during pregnancy, respiratory problems, musculoskeletal systems, and so on [26][45]. In Indonesia, it was used prominently for treating liver disease and jaundice. Its chemical constituent consists of angustifolin and aromatic derivatives, including moskachan A, B, C, and D. Other constituents include ostruthin, xanthotoxin, dictamnine, xanthyletin, limonoid, psoralen, etc. [27][46]. The extract from the leaves of this plant exhibited potent anti-HCV properties. The constituent chalepin and pseudo IX from its leaves inhibited post-entry of HSV, RNA replication and synthesis of viral proteins.
3.4. Liriope platyphylla
Liriope platyphylla is a perennial plant present across the areas of China, Korea, and Japan and was demonstrated to exhibit biological activities against chronic diseases, such as cough, sputum, and neurodegenerative disorders, obesity, and diabetes [28][47]. Its chemical constituent spicatoside A interferes with lipopolysaccharide-induced (LPS) activation of extracellular signal-regulated kinases, c-Jun N-terminal kinase (JNK), and NF-kB [29][48]. It was found that L. platyphylla roots contain a principal active moiety that inhibits HBV viral promoter activity via interfering with the NF-kB signaling pathway [30][49].
3.5. Citrus reticulate
Citrus reticulate is an evergreen tree with aromatic flowers and glossy leaves that belongs to the family Rutaceae. It is referred to as tangerine, mandarin, or Kamla lebu in Bengal. The existence of different bioactive compounds confirms the uses of this plant for various ailments by traditional practitioners [31][50]. In 2009, Kirbaslar et al. demonstrated the various chemical components in this plant, which include limonene, myrcene, γ-terpinene, sabinene, and so on [32][51]. Guo et al. in 2016 and Choi et al. in 2011 demonstrated that, in China, it has been used for treating gastrointestinal and respiratory disorders [33][34][52,53]. Tangerine has also been documented to express an inhibitory effect against the respiratory syncytial virus (RSV) [35][54] and rotavirus replication [36][55]. Nobiletin, a key bioactive constituent isolated from its pericarps, affects the intracellular replication of RSV. The efficacy of tangerine was also reported against VHF-causing arenavirus entry.
3.6. Tinospora cardifolia
Tinospora cardifolia, also known as giloya, is an Indian native medicinal plant that belongs to the family Menispermaceace. It is extensively used in Ayurveda because of its therapeutic potential (anti-inflammatory, anti-allergic, anti-diabetic, and immunomodulatory activity). The aqueous extract of Tinospora cardifolia boosts immunity in children and is adjuvant to vaccination. In a patient with HIV infection, treatment with Tinosopra cardifolia extract is found to reduce the TLC, neutrophile, and eosinophil count, suggesting anti- HIV activity [37][56]. S. Krupanidhi et al. carried out screening of the phytochemicals present in Tinospora cardifolia for an inhibitory effect against SARS-Cov-2. Based on the study, the phytochemicals berberine, tinosponone, xanosporic acid, and tembetarine were identified as the possible lead compounds that can be considered to have activity against SARS-Cov-2. The research proved tinosponone as a potent, selective, and non-toxic inhibitor of 3 CL protease of SARS-Cov-2 [38][57].
3.7. Andrographis paniculata
Andrographis paniculata, generally known by the name of kalmegh, is one of the most commonly used medications in Ayurveda, belonging to the family Acanthaceae. The principal constituents present in this plant are flavonoids, diterpenoids, and polyphenols. The major bioactive compound responsible for its therapeutic activity is andrographolide [39][58]. Both 25µg/mL of ethanolic extract of Andrographis paniculata and 5 µg/mL of Andrographolide demonstrated anti-viral activity against a variety of viruses, including influenza A virus, hepatitis B and C, herpes simplex virus, Epstein–Barr virus, and human immunodeficiency virus. They inhibit viral entry inside the viral cell and prevent the replication of genetic material by DNA and RNA polymerase, protein synthesis, and functional mature proteins [40][59]. Du et al. used alcohol as a co-surfactant, tween 80 as a surfactant, isopropyl alcohol as an oil phase, and water to prepare an andrographolide-loaded microemulsion [41][60]. Sermkaew et al. used caproyl, cremphor, and labrasol [42][61], while Syukri et al. used caproyl, tween 20, and polyethylene glycol 400 for the formulation of microemulsions. It was concluded in all the studies that the solubility and bioavailability of andrographolide were increased significantly [43][62].
3.8. Saururus chinensis
Saururus chinensis, belonging to the family Saururaceae, is a perennial herbaceous plant found in Korea and China. Traditionally, it has been used as an anti-pyretic, anti-inflammatory, and diuretic agent. A phytochemical investigation led to the discovery of various compounds, including flavonoids, aristolactamus, furanoditerpens, anthraquinones, etc. [44][63]. Lignans are the main bioactive constituent of plants that exhibit various activities, such as HIV-1 protease, NF-kB, and HIF1 inhibitory effects. Manassantin B, a bioactive compound extracted from the roots of Saururus, reported inhibitory activity against Epstein–Barr virus (EBV) lytic replication [45][64]. Some of the plants with anti-viral activities, along with their phytochemicals, have been summarized in Table 1.
Table 1. Plants with anti-viral activities, along with their phytochemicals.
| Plant | Part | Phytochemical | Class | Active against Virus | Reference |
|---|
Various other formulations contain anti-viral phytoconstituents.
| Active Phytoconstituent | Formulation | Applications | References | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ziziphus jujuba | Roots | Jubanines | Alkaloids | PEDV | [46][65] | ||||
| Oxymatrine | Phytosome | Enhanced bioavailability | [65][121] | ||||||
| Rheum palmatum | Roots | Sennoside A | Glycoside | HIV-1 | |||||
| Artemisia arborescens | Liposomal | Raised anti-viral activity and improved stability | [66][122] | [ | 47][66] | ||||
| Swietenia macrophylla | Stem | Limonoids | Lignin | HSV | [48][67] | ||||
| Hypocrellins | Nanoparticulate | Enhanced hydrophilicity and stability | [67][123] | Embelia ribes | Seeds | Quercetin | Flavonoid | HSV | [49 |
| Matrine | Emulsion | ] | [ | 68 | ] | ||||
| Enhanced sustained released activity | [ | 68 | ][124] | Humulus lupulus | Whole plant | Xanthohumol | Chalcone | BVDV | [50][69] |
| Glycyrrhiza inflate | Roots | Chalcones | Ketone | Influenza A | [51][70] |
4. Anti-Viral Bioactive-Based Nanocarrier Systems
Natural medicines can give beneficial and promising therapeutic results when incorporated into pharmaceutical nanotechnology. Boosted clinical and therapeutic responses are possible with nanoformulations used as carriers for delivering poorly soluble phytoconstituents and plant extracts [52][108]. Various delivery systems, such as self-nano emulsifying drug delivery systems (SNEDDS), hydrogels, phytosomes, microspheres, transferases, etc., have been used for the delivery of phytoconstituents with anti-viral potential. These nanoformulations displayed numerous effects, such as improved oral solubility, systemic bioavailability, delayed metabolism, and enhanced therapeutic activity. For example, chitosan nanoparticles containing catechin and EGCG resulted in an enhanced rate of intestinal absorption [53][109]. According to studies, myricetin, a natural flavonoid, was loaded into a polymeric nano-particle carrier, which significantly increased its solubility profile [54][110]. The encapsulation of flavonoids into red blood cells can boost anti-viral activity and bioavailability. The flavonoids showed positive impacts in reducing oxidative damage to the erythrocyte membrane [55][111]. Several researchers reported that RBCs play a vital role in the distribution and bioavailability of circulating quercetin [56][112]. Surprisingly, flavonoids in chitosan particles retain their anti-oxidant activity and can be used to combat free radicals in the bloodstream [57][113]. The most evenly distributed type of polylactic acid-4 nanoparticle was effectively used to encapsulate quercetin, which permitted the delayed release of quercetin [58][114]. Kim et al. [59][115] boosted the oral bioavailability of apigenin by incorporating apigenin into the water-in-oil emulsion system. Due to the poor solubility and permeability of baicalein, Zhang et al. [60][116] used a micellar composition including the carriers Pluronic P123 copolymer and sodium taurocholate, which significantly increased oral absorption of baicalein. Oleanolic acid was incorporated into SMEEDS, which enhanced its systemic bioavailability [61][117]. Andrographolide has poor absorption properties and limited oral bioavailability. To circumvent these constraints, PLGA was used to formulate andrographolide-loaded microspheres [62][118]. According to studies, methanolic extracts of strawberries (Fragaria ananassa Duch.) and ginger (Zingiber officinale) were utilized to synthesize silver nanoparticles (AgNPs) to investigate their SARS-CoV-2 inhibitory capability [63][119]. Several studies are being carried out on the development of nanotechnology for Indonesian Jamun to fight against SARS-CoV-2 [64][120]. Various other formulations containing anti-viral phytoconstituents are summarized in Table 2.
