Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Aleksandra Djikic Rom.
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary hepatic malignancy, with mass-forming growth pattern being the most common. The typical imaging appearance of mass-forming ICC (mICC) consists of irregular ring enhancement in the arterial phase followed by the progressive central enhancement on portal venous and delayed phases. However, atypical imaging presentation in the form of hypervascular mICC might also be seen, which can be attributed to distinct pathological characteristics. Ancillary imaging features such as lobular shape, capsular retraction, segmental biliary dilatation, and vascular encasement favor the diagnosis of mICC.
- mass-forming cholangiocarcinoma
- mimickers
- magnetic resonance imaging
1. Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary malignant liver tumor after hepatocellular carcinoma (HCC) [1]. These tumors arise from the intrahepatic biliary duct epithelium, proximal to the second-order bile ducts [2]. Although the majority of cases occur sporadically, there are certain medical conditions that are considered to be risk factors for ICC development, in particular primary sclerosing cholangitis (PSC), choledochal cyst, intrahepatic lithiasis, Caroli disease, clonorchiasis, and viral hepatitis (especially type C) [3,4][3][4].
According to the growth pattern, ICC can be classified into mass-forming, periductal-infiltrating, or intraductal growth types [5]. Among the three different growth patterns, mass-forming cholangiocarcinoma (mICC) is the most common, accounting for 80% of all cases [5]. The second most common type is mixed type consisting of mass-forming and periductal infiltrating growth pattern [5]. Even though mixed type was initially introduced as a distinctive type, it is now grouped together with mass-forming type according to its imaging presentation [5,6][5][6]. However, recent studies have shown that gross morphological classification is insufficient in explaining the atypical presentations of ICC [7]. In order to provide better understanding of varying imaging appearances of mICC and its correlation with clinicopathological features, Kim et al. have proposed new dichotomous imaging classification introducing “parenchymal” and “ductal” types of mICC [8]. This is in accordance with the new histological classification that divides ICC into small duct and large duct types [9]. With regard to new imaging classification, parenchymal type originates from small bile ducts or canals of Hering and presents as a mass-forming lesion without gross involvement or bile duct dilatation [8]. On the other hand, ductal type develops from mature cholangiocytes of the large bile ducts and presents usually as mixed mass-forming and periductal infiltrating lesion causing biliary dilatation [8,10][8][10]. In addition, it has been shown that the ductal type tends to show hypovascularity while the parenchymal type frequently displays hypervascularity on imaging studies [11,12][11][12]. Furthermore, Hayashi et al. have shown that large bile duct type was more commonly associated with poor differentiation due to the rich fibrous stroma in contrast to the small duct type, which had better postsurgical outcomes [12,13][12][13]. Therefore, recognition of this different imaging appearances of mICC provides additional clinical information regarding the prognosis and clinical outcome, which may influence treatment decisions in certain cases [8].
2. Imaging Findings of Mass-Forming ICC
The imaging appearance of mICC depends on its pathohistological composition and is determined by the amount of fibrous stroma, viable tumor cells, intralesional mucin, and necrosis [14].2.1. Typical Imaging Features of mICC
Mass-forming ICC typically presents as a large, lobulated, irregularly shaped lesion with well-defined borders [14]. On MRI, the tumor is usually hypointense on T1-weighted images, while the appearance on T2-weighted images varies from hypointensity in highly fibrotic lesions to hyperintensity in necrotic or mucin-rich tumors [15]. Although central T2-weighted hypointensity is considered a characteristic MRI feature of mICC, it can also be seen in colorectal metastasis due to intralesional coagulative necrosis [14]. Nevertheless, in most of the cases mICC presents as a heterogeneous lesion on T2-weighted images containing both areas of hyperintensity and areas of hypointensity [14,15][14][15]. The characteristic enhancement pattern using conventional gadolinium-based extracellular agents consists of an irregular ring enhancement on the arterial phase followed by progressive central enhancement in the portal venous and delayed phases (Figure 1) [14,15][14][15]. This postcontrast behavior could be explained by a rim of viable cells at the periphery of the tumor and rich edematous internal fibrous stroma [15].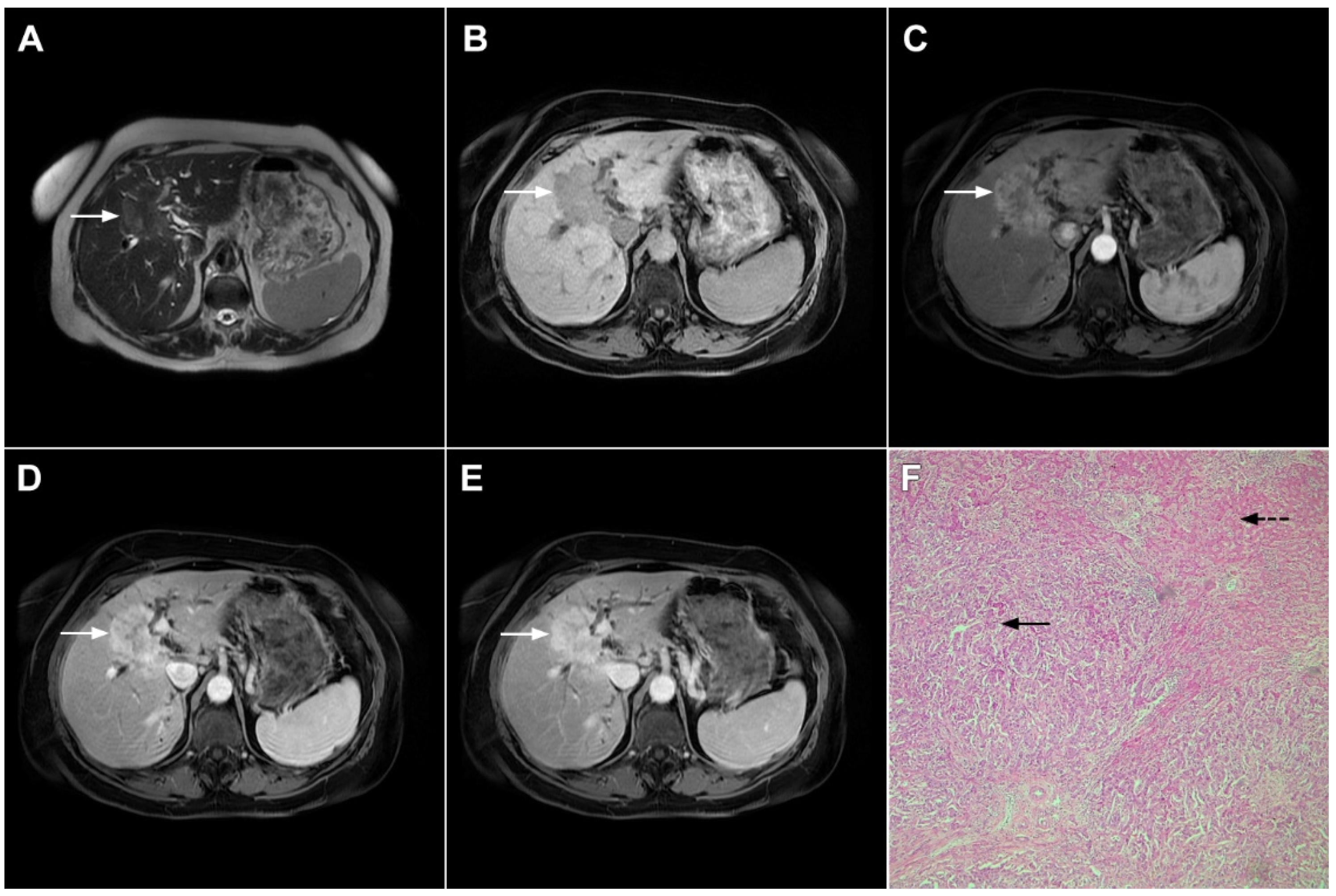
Figure 1. Typical intrahepatic mass-forming cholangiocarcinoma in 68-year-old woman. On axial T2-weighted image a lobular heterogeneously hyperintense tumor (arrow) is seen, located centrally in the liver segment IVB (A). The lesion (arrow) is hypointense in a plain T1-weighted image (B) with irregular ring enhancements in the arterial phase (C) and progressive enhancement in the portalvenous (D) and delayed phase (E). Note the perilesional biliary dilatation. Hematoxylin and eosin (H&E) staining (F) showed cholangiocarcinoma (arrow) and normal liver parenchyma next to the tumor (dashed arrow); original magnification ×40.
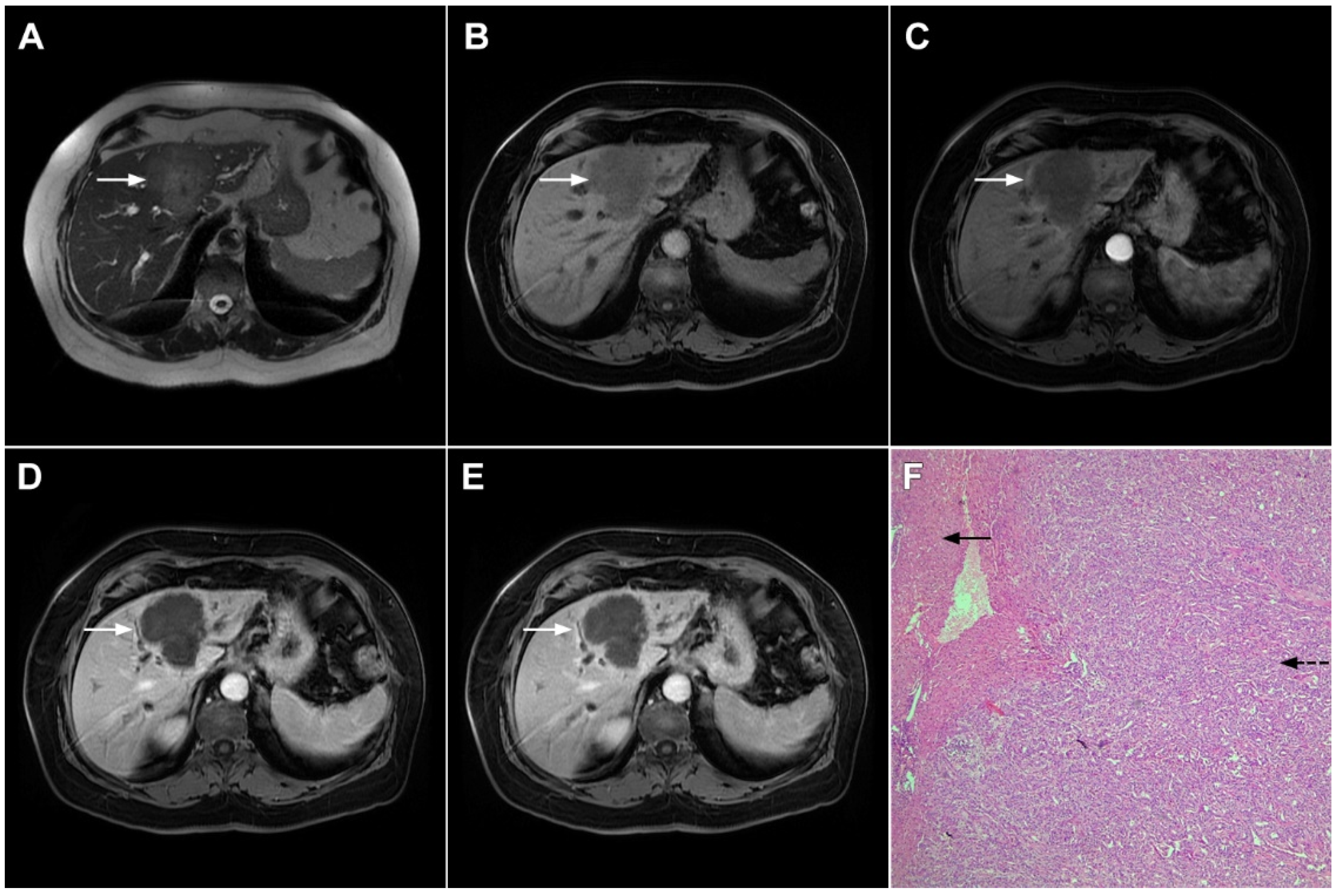
Figure 2. Mass-forming intrahepatic cholangiocarcinoma in 72-year-old man. Irregular heterogeneously hyperintense lesion (arrow) on T2-weighted image (A) located in liver segments IVB and III with peripheral biliary dilatation is shown. On a plain T1-weighted image (B) the lesion (arrow) is hypointense with only discrete ring enhancement in the arterial phase (C) but without detectable enhancements in the portal venous (D) and delayed phases (E). Hematoxylin and eosin (H&E) staining (F) showed poorly differentiated cholangiocarcinoma (dashed arrow); normal liver parenchyma is also shown (arrow); original magnification ×40.
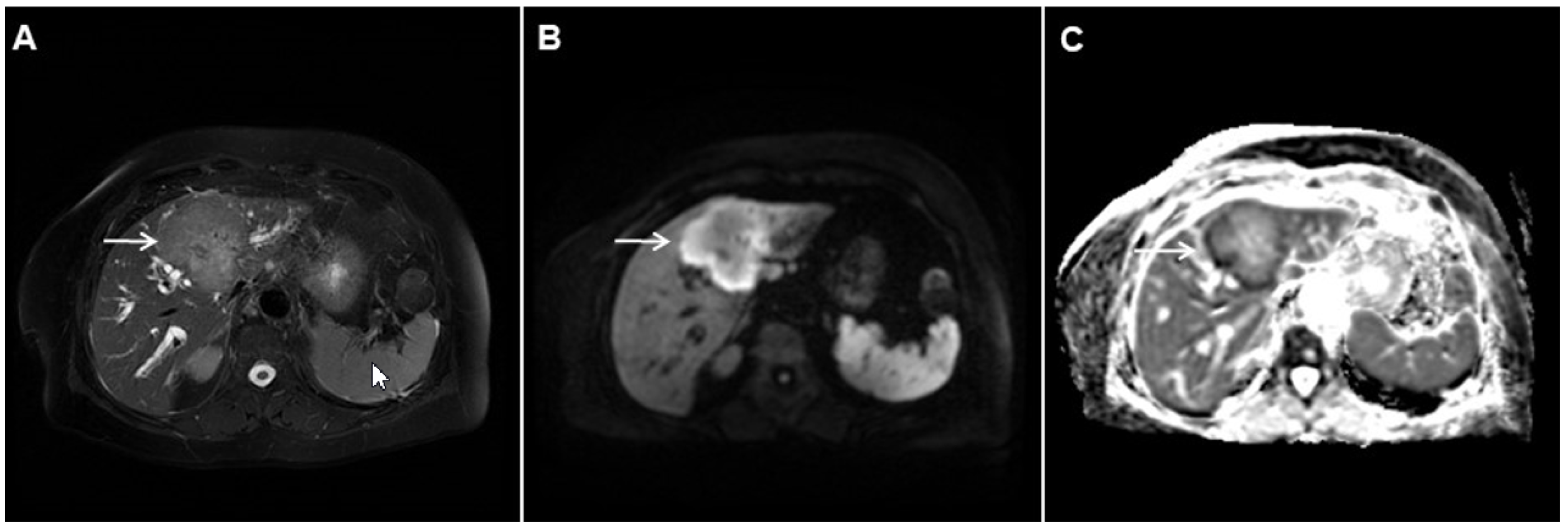
Figure 3. Mass-forming intrahepatic cholangiocarcinoma in the left liver lobe of a 76-year-old man. Axial T2-weighted FS image shows lobulated hetrogeneously hyperintense hepatic tumor (arrow) with perilesional biliary dilatation (A). Axial diffusion-weighted image (b = 800 s/mm2) shows target-like appearance (arrow) of the lesion that consists of a central darker area and a peripheral hyperintense area (B). Corresponding ADC map is shown on (C).
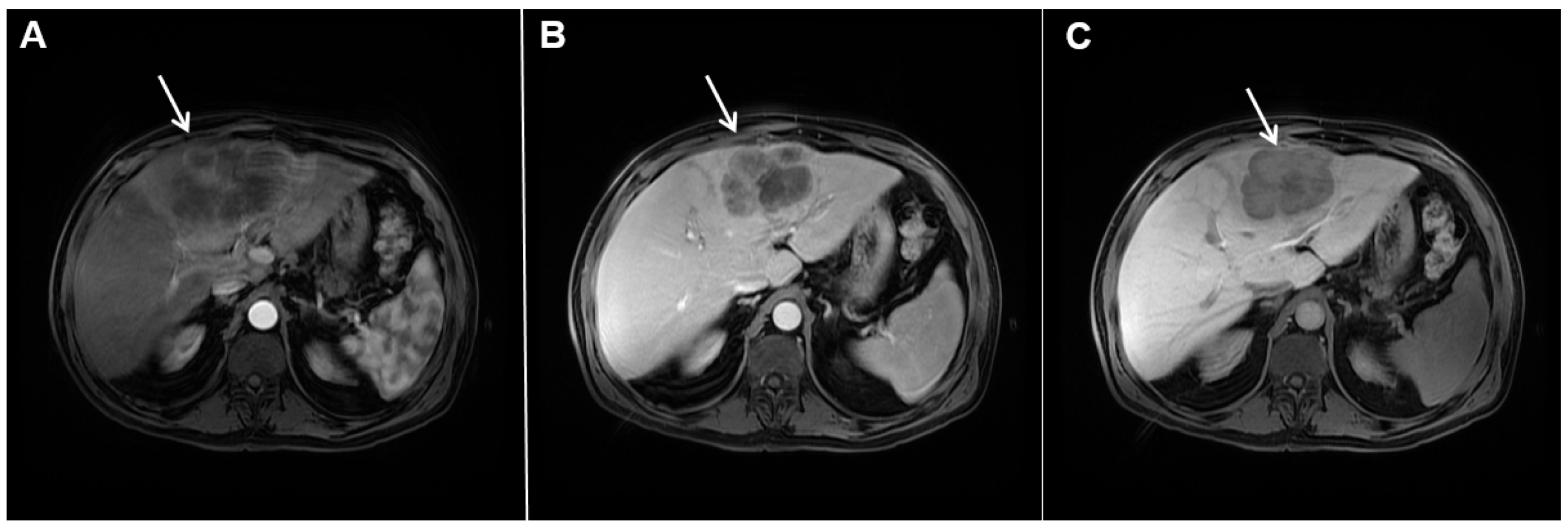
Figure 4. Mass-forming intrahepatic cholangiocarcinoma in a 68-year-old woman. Axial T1-weighted image after gadoxetic acid administration obtained in arterial phase (A) shows peripherally enhancing lesion (arrow). Portal venous phase in the same patient (B) shows progressive centripetal enhancement of the lesion (arrow) with cloud-like appearance in the hepatobiliary phase (C) consisting of an area of central enhancement and a thin, peripheral, hypointense rim.
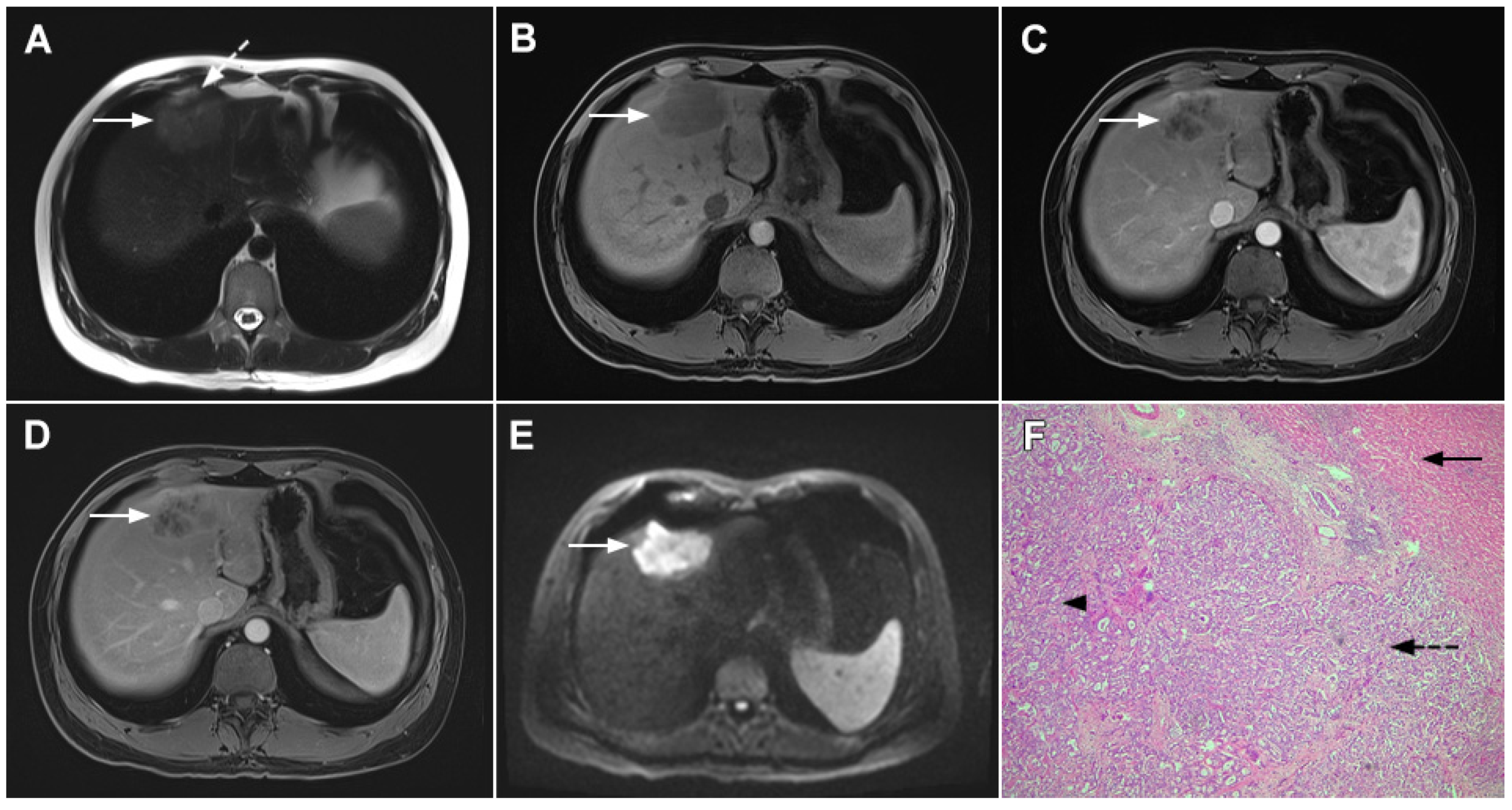
Figure 5. Parenchymal mass-forming cholangiocarcinoma in a 36-year-old man. The lobular slightly hyperintense lesion (arrow) is seen in the liver segment IVA in a T2-weighted image (A) with subtle capsular retraction (dashed arrow). On a plain T1-weighted image (B), the tumor (arrow) is hypointense with irregular discrete peripheral and central enhancements in the arterial phase (C), mild progressive enhancement in the portal venous phase (D), and high signal intensity in DWI (E). Hematoxylin and eosin (H&E) staining (F) showed cholangiocarcinoma (arrowhead) with poorly differentiated components (dashed arrow). Normal liver parenchyma is also shown (arrow); original magnification ×40.
2.2. Atypical Imaging Features of mICC
Besides the typical imaging presentation of mICC, atypical appearance can also be observed in imaging studies [24]. Hypervascular mICCs are usually small lesions and this vascular behavior might be explained by less intralesional fibrosis and abundant vascular stroma [25]. The incidence of hypervascular mICC ranges from 12.5% up to 47% in previous reports [18,25][18][25]. Since hypervascular mICCs are frequently seen in cirrhotic livers, the differential diagnosis with HCC may be very difficult. In this regard, the absence of washout and the presence of progressive enhancement together with the lack of fibrous pseudocapsule favor the diagnosis of mICC over HCC [25]. However, if hypervascular mICC shows washout in the portal venous phase, preoperative differential diagnosis with HCC is hardly possible (Figure 6). In such cases, additional findings, such as cloud appearance in the hepatobiliary phase, multiplicity around the main tumor, or intrahepatic metastasis, capsule retraction and tumor markers may be helpful for differentiating between these tumors [18,19][18][19]. Hypervascular mICC differs from typical hypovascular mICC not only in terms of vascularity but also in patient outcome, as it has been shown that the former has much better prognosis [12]. Therefore, the assessment of tumor vascularity on preoperative imaging could represent an important marker for predicting malignant characteristics in mICC [26].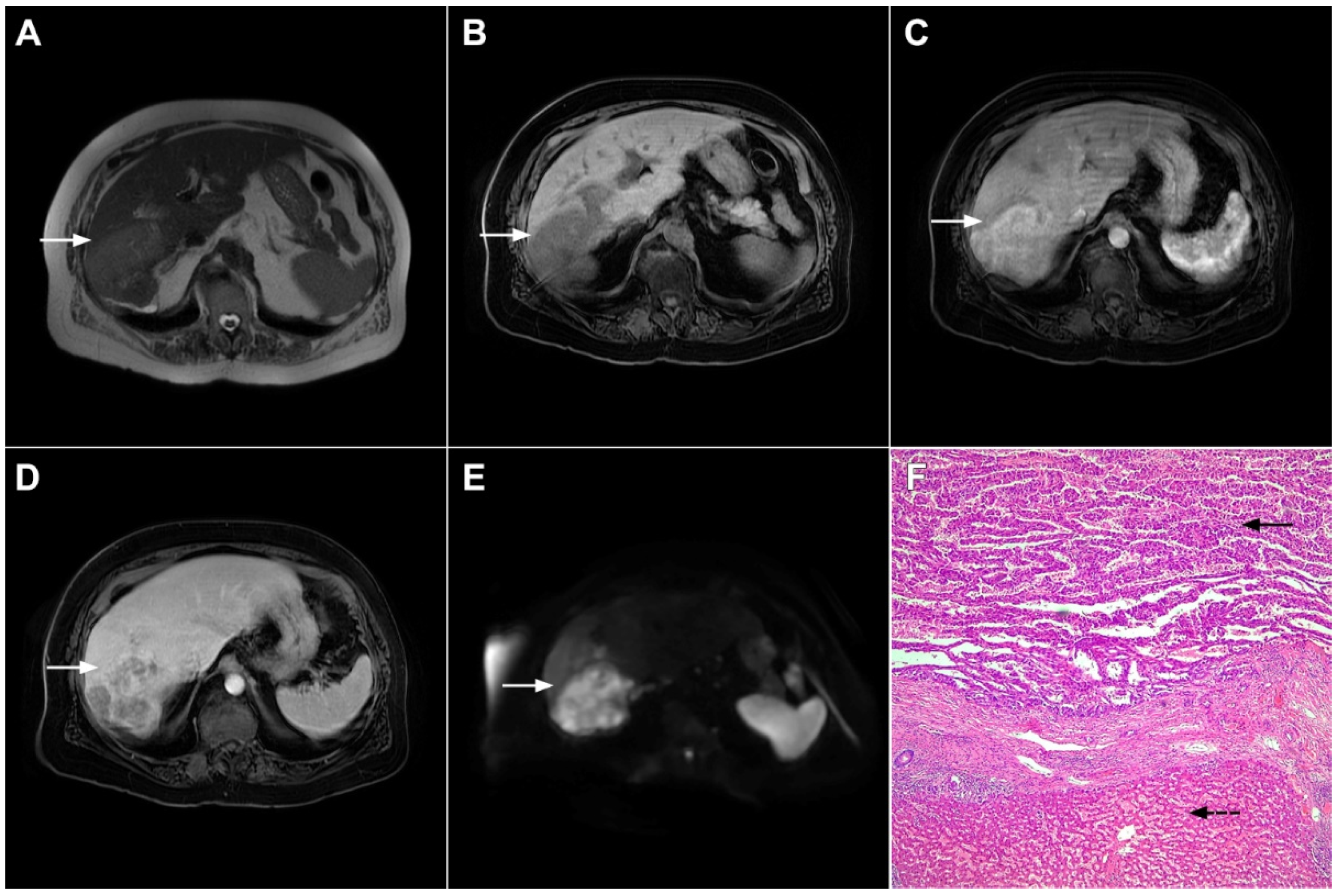
Figure 6. Hypervascular mass-forming cholangiocarcinoma in a 63-year-old woman. The axial T2-weighted image (A) shows a moderately hyperintense tumor (arrow) located in liver segments VI and VII with a subtle medial capsular retraction. The lesion (arrow) is hypointense on the plain T1-weighted image (B), hypervascular in the arterial phase (C) with washout on the portal venous phase (D). The tumor (arrow) is hyperintense on DWI (E). Hematoxylin and eosin (H&E) staining (F) showed well-differentiated cholangiocarcinoma (arrow) surrounded by normal liver parenchyma (dashed arrow); original magnification ×40.
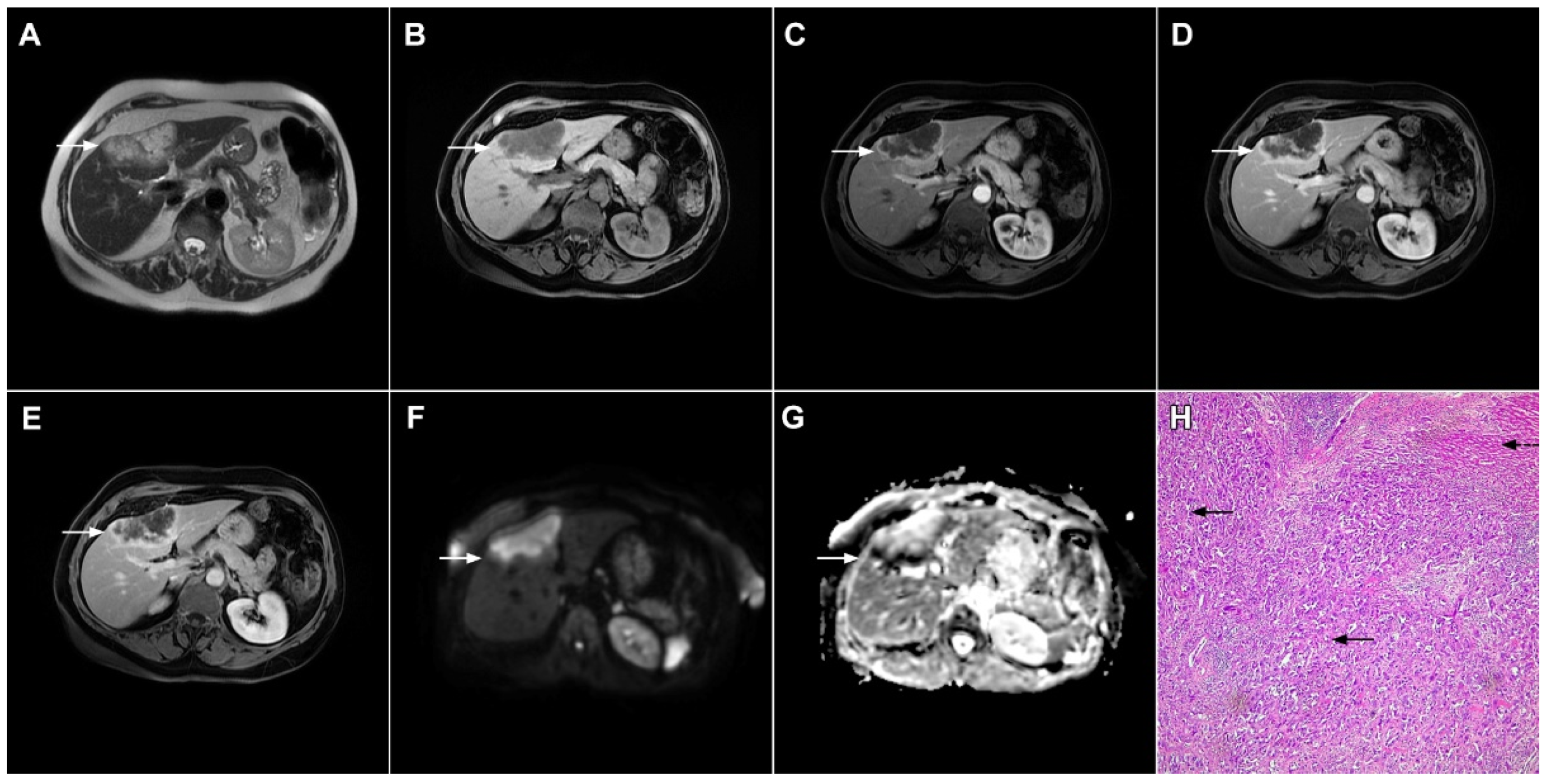
Figure 7. Mucin-rich mass-forming cholangiocarcinoma in a 78-year-old woman. The axial T2-weighted image (A) shows the lobulated hyperintense lesion (arrow) located in the subcapsular region of liver segment IVB, which is associated with capsular retraction. On the plain T1-weighted image (B) the lesion (arrow) is hypointense. In the arterial phase (C), ring enhancement can be seen with slight “ragged” central enhancement in the portal venous (D) and delayed phase (E). On DWI, diffusion restriction is noted on the periphery of the lesion (arrow) while no restriction is seen in the central part of the tumor (F). Corresponding ADC map showing targetoid appearance of the lesion is shown on (G). Hematoxylin and eosin (H&E) staining (H) showed cholangiocarcinoma (arrows) adjacent to normal liver parenchyma (dashed arrow); original magnification ×40.
References
- Choi, B.I.; Lee, J.M.; Han, J.K. Imaging of intrahepatic and hilar cholangiocarcinoma. Abdom. Imaging 2004, 29, 548–557.
- Lazaridis, K.N.; Gores, G.J. Cholangiocarcinoma. Gastroenterology 2005, 128, 1655–1667.
- Shaib, Y.H.; El-Serag, H.B.; Nooka, A.K.; Thomas, M.; Brown, T.D.; Patt, Y.Z.; Hassan, M.M. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A hospital-based case-control study. Am. J. Gastroenterol. 2007, 102, 1016–1021.
- El-Diwany, R.; Pawlik, T.M.; Ejaz, A. Intrahepatic Cholangiocarcinoma. Surg. Oncol. Clin. N. Am. 2019, 28, 587–599.
- Lim, J.H. Cholangiocarcinoma: Morphologic classification according to growth pattern and imaging findings. AJR Am. J. Roentgenol. 2003, 181, 819–827.
- Yamasaki, S. Intrahepatic cholangiocarcinoma: Macroscopic type and stage classification. J. Hepatobiliary Pancreat. Surg. 2003, 10, 288–291.
- Jeong, H.T.; Kim, M.J.; Chung, Y.E.; Choi, J.Y.; Park, Y.N.; Kim, K.W. Gadoxetate disodium-enhanced MRI of mass-forming intrahepatic cholangiocarcinomas: Imaging-histologic correlation. AJR Am. J. Roentgenol. 2013, 201, W603–W611.
- Kim, M.J.; Rhee, H.; Woo, H.Y. A dichotomous imaging classification for cholangiocarcinomas based on new histologic concepts. Eur. J. Radiol. 2019, 113, 182–187.
- Nakanuma, Y.; Sato, Y.; Harada, K.; Sasaki, M.; Xu, J.; Ikeda, H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J. Hepatol. 2010, 2, 419–427.
- Cardinale, V.; Carpino, G.; Reid, L.; Gaudio, E.; Alvaro, D. Multiple cells of origin in cholangiocarcinoma underlie biological, epidemiological and clinical heterogeneity. World J. Gastrointest. Oncol. 2012, 4, 94–102.
- Fujita, N.; Asayama, Y.; Nishie, A.; Ishigami, K.; Ushijima, Y.; Takayama, Y.; Okamoto, D.; Moirta, K.; Shirabe, K.; Aishima, S.; et al. Mass-forming intrahepatic cholangiocarcinoma: Enhancement patterns in the arterial phase of dynamic hepatic CT-Correlation with clinicopathological findings. Eur. Radiol. 2017, 27, 498–506.
- Nam, J.G.; Lee, J.M.; Joo, I.; Ahn, S.J.; Park, J.Y.; Lee, K.B.; Han, J.K. Intrahepatic Mass-Forming Cholangiocarcinoma: Relationship Between Computed Tomography Characteristics and Histological Subtypes. J. Comput. Assist. Tomogr. 2018, 42, 340–349.
- Hayashi, A.; Misumi, K.; Shibahara, J.; Arita, J.; Sakamoto, Y.; Hasegawa, K.; Kokudo, N.; Fukayama, M. Distinct Clinicopathologic and Genetic Features of 2 Histologic Subtypes of Intrahepatic Cholangiocarcinoma. Am. J. Surg. Pathol. 2016, 40, 1021–1030.
- Jhaveri, K.S.; Hosseini-Nik, H. MRI of cholangiocarcinoma. J. Magn. Reson. Imaging 2015, 42, 1165–1179.
- Maetani, Y.; Itoh, K.; Watanabe, C.; Shibata, T.; Ametani, F.; Yamabe, H.; Konishi, J. MR imaging of intrahepatic cholangiocarcinoma with pathologic correlation. AJR Am. J. Roentgenol. 2001, 176, 1499–1507.
- Park, H.J.; Kim, Y.K.; Park, M.J.; Lee, W.J. Small intrahepatic mass-forming cholangiocarcinoma: Target sign on diffusion-weighted imaging for differentiation from hepatocellular carcinoma. Abdom. Imaging 2013, 38, 793–801.
- Kovač, J.D.; Galun, D.; Đurić-Stefanović, A.; Lilić, G.; Vasin, D.; Lazić, L.; Mašulović, D.; Šaranović, Đ. Intrahepatic mass-forming cholangiocarcinoma and solitary hypovascular liver metastases: Is the differential diagnosis using diffusion-weighted MRI possible? Acta Radiol. 2017, 58, 1417–1426.
- Kim, S.H.; Lee, C.H.; Kim, B.H.; Kim, W.B.; Yeom, S.K.; Kim, K.A.; Park, C.M. Typical and atypical imaging findings of intrahepatic cholangiocarcinoma using gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging. J. Comput. Assist. Tomogr. 2012, 36, 704–709.
- Kang, Y.; Lee, J.M.; Kim, S.H.; Han, J.K.; Choi, B.I. Intrahepatic mass-forming cholangiocarcinoma: Enhancement patterns on gadoxetic acid-enhanced MR images. Radiology 2012, 264, 751–760.
- Koh, J.; Chung, Y.E.; Nahm, J.H.; Kim, H.Y.; Kim, K.S.; Park, Y.N.; Kim, M.J.; Choi, J.Y. Intrahepatic mass-forming cholangiocarcinoma: Prognostic value of preoperative gadoxetic acid-enhanced MRI. Eur. Radiol. 2016, 26, 407–416.
- Kim, S.; An, C.; Han, K.; Kim, M.J. Gadoxetic acid enhanced magnetic resonance imaging for prediction of the postoperative prognosis of intrahepatic mass-forming cholangiocarcinoma. Abdom. Radiol. 2019, 44, 110–121.
- Soyer, P.; Bluemke, D.A.; Vissuzaine, C.; Beers, B.V.; Barge, J.; Levesque, M. CT of hepatic tumors: Prevalence and specificity of retraction of the adjacent liver capsule. AJR Am. J. Roentgenol. 1994, 162, 1119–1122.
- Han, J.K.; Choi, B.I.; Kim, A.Y.; An, S.K.; Lee, J.W.; Kim, T.K.; Kim, S.W. Cholangiocarcinoma: Pictorial essay of CT and cholangiographic findings. Radiographics 2002, 22, 173–187.
- Lee, W.J.; Lim, H.K.; Jang, K.M.; Kim, S.H.; Lee, S.J.; Lim, J.H.; Choo, I.W. Radiologic spectrum of cholangiocarcinoma: Emphasis on unusual manifestations and differential diagnoses. Radiographics 2001, 21, S97–S116.
- Kim, S.A.; Lee, J.M.; Lee, K.B.; Kim, S.H.; Yoon, S.H.; Han, J.K.; Choi, B.I. Intrahepatic mass-forming cholangiocarcinomas: Enhancement patterns at multiphasic CT, with special emphasis on arterial enhancement pattern--correlation with clinicopathologic findings. Radiology 2011, 260, 148–157.
- Ariizumi, S.; Kotera, Y.; Takahashi, Y.; Katagiri, S.; Chen, I.P.; Ota, T.; Yamamoto, M. Mass-forming intrahepatic cholangiocarcinoma with marked enhancement on arterial-phase computed tomography reflects favorable surgical outcomes. J. Surg. Oncol. 2011, 104, 130–139.
- Hayashi, M.; Matsui, O.; Ueda, K.; Kadoya, M.; Yoshikawa, J.; Gabata, T.; Takashima, T.; Izumi, R.; Nakanuma, Y. Imaging findings of mucinous type of cholangiocellular carcinoma. J. Comput. Assist. Tomogr. 1996, 20, 386–389.
- Vijgen, S.; Terris, B.; Rubbia-Brandt, L. Pathology of intrahepatic cholangiocarcinoma. Hepatobiliary Surg. Nutr. 2017, 6, 22–34.
- Chung, Y.E.; Kim, M.J.; Park, Y.N.; Choi, J.Y.; Pyo, J.Y.; Kim, Y.C.; Cho, H.J.; Kim, K.A.; Choi, S.Y. Varying appearances of cholangiocarcinoma: Radiologic-pathologic correlation. Radiographics 2009, 29, 683–700.
More
