Skin hyperpigmentation resulting from excessive tyrosinase expression has long been a problem for beauty lovers, which has not yet been completely solved. Although researchers are working on finding effective tyrosinase inhibitors, most of them are restricted, due to cell mutation and cytotoxicity. Therefore, functional foods are developing rapidly for their good biocompatibility. Food-derived peptides have been proven to display excellent anti-tyrosinase activity, and the mechanisms involved mainly include inhibition of oxidation, occupation of tyrosinase’s bioactive site and regulation of related gene expression. For anti-oxidation, peptides can interrupt the oxidative reactions catalyzed by tyrosinase or activate an enzyme system, including super-oxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) to scavenge free radicals that stimulate tyrosinase. In addition, researchers predict that peptides probably occupy the site of the substrate by chelating with copper ions or combining with surrounding amino acid residues, ultimately inhibiting the catalytic activity of tyrosinase.
- bioactive peptides
- tyrosinase activity
- hyperpigmentation
- mechanism
- molecular docking
1. Introduction
Tyrosinase (TYR) is a metalloenzyme with a highly conserved copper binding region and exists in fruits, fungi, vegetables, mammals, cuticle sclerosis and wound healing in insects [1–4]. Two copper ions are essential for the catalytic activity of tyrosinase regardless of source [3]. In mammals, melanin regulated by tyrosinase is responsible for pigmentation of skin, hair and eyes [5]. When skin is exposed to UV radiation or oxidative stress, the melanocytes produce melanin, which accumulates in melanosomes, and is then transported to keratinocytes surrounding the melanocytes through dendrites to form supranuclear melanin caps to protect skin from photoaging [6]. Therefore, melanin is generally considered the perfect protection against UV damage. However, melanin is also the main reason for skin disorders, such as age spots, freckles and malignant melanoma [7].
Tyrosinase activity determines the synthesis content of melanin. Hence, inhibiting tyrosinase is one of the most effective ways to solve excessive pigment deposition [8]. At present, a large number of natural ingredients have been found to inhibit tyrosinase, among which phenols (flavonoids are the main ingredient), organic acids, glycosides, terpenes, aldehydes, esters, coumarins and their derivatives have better effects [9,10]. Kojic acid, hydroquinone and arbutin are mostly used in the treatment of melanin dermatosis. Although they have a strong inhibitory effect on tyrosinase activity, they are limited because of poor penetration and potential mutagenicity [11]. Finding inhibitors with high activity and low side effects has practical value in the prevention, or early treatment, of pigmented skin diseases [12].
As a new type of therapeutic drug, bioactive peptides have been of immense interest in recent years. Food-derived tyrosinase inhibitory peptides (TIPs) are favored, due to their high biological safety and easy absorption [13,14]. In addition to TIPs, amino acids released by gastrointestinal digestion can be absolutely absorbed without consumption. Active TIPs have been obtained from a wide range of animal and plant sources, and further animal and clinical trials are ongoing [15–17].
The present overview focuses on the sources, preparation, and inhibitory mechanisms on tyrosinase of TIPs and the emerging bioinformatic technologies used in studying TIPs, aiming to provide a theoretical basis and scientific guidance for dietary nutrition and cosmetics.
2. The Origin of Anti-Tyrosinase Peptides from Food Proteins
TIPs are short sequences including 3-20 units of amino acids obtained by enzymatic or chemical hydrolysis. Numerous studies reported that the anti-tyrosinase effect of TIPs is equivalent to, or even better than, that of natural or chemical synthetic inhibitors [18–20]. TIPs come from terrestrial and aquatic sources, and they are abundantly found in mammals’ milk [21] and agricultural products [22], as well as in aquatic products. In order to reduce costs, food industry by-products, such as peels [23], seeds [15], feathers [24], fish scales [25] and fish skin [26], are also utilized to produce potential TIPs.
2.1. Anti-Tyrosinase Peptides of Terrestrial Origin
TIPs play an important physiological role in organisms whose molecular weights are usually less than 6000 Da. The advantages of food protein hydrolysates include improved solubility, thermal stability and strong anti-precipitation ability [24]. Plant protein is a good material to obtain TIPs. The oldest study on natural TIPs isolated from Agaricus hortensis was reported by Madhosingh and Sundberg [27]. TIPs derived from land plants are usually found in crops, such as potato [22], rice [28], quince [15], and so on. These materials have also been utilized to cure other skin problems like inflammation and photoaging [29]. Peptide P4 (YRSRKSSWP) was known as one of the most effective anti-tyrosinase inhibitors (IC50 = 123 μmol/mL), and Ochiai et al. [30] found peptide TH10 (MRSRGRSSWP), similar to the P4 sequence from rice, had higher inhibitory activity with 102 μmol/mL of its IC50. It was also found that TIPs can be obtained from the hydrolysates of rice by-products, such as rice brans [28] and rice paste [31]. Additionally, mammalian proteins are primary materials to produce TIPs, due to their large quantity. In Table 1, TIPs of terrestrial origin can be seen. IRW and GYSLGNWVCAAK from egg white with anti-tyrosinase activity were identified [5,32]. The most studied peptides like MHIR, MYSLAMAA were derived from milk proteins, such as αS-casein, κ-casein, and β-lactoglobulin [21]. Addar et al. [17] hydrolyzed αS-casein isolated from camel milk, and found that the fraction with low molecular weight (<0 kDa) not only exhibited the highest anti-oxidative activity, but also strongly inhibited 51.21% of tyrosinase activity at a concentration of 0.2 mg/mL. At the same time, researchers also looked at the usefulness of other animal proteins with potential activities. Pongkai et al. [24] reported that protein hydrolysates from chicken feather meal, containing cysteine disulfide bonds, exhibited strong tyrosinase inhibition activity for both monophenolase (IC50 = 5.780 µg/mL) and diphenolase activities (IC50 = 0.040 µg/mL).
Although terrestrial plant protein is a common source of TIPs, studies have found that some natural plant ingredients exhibit higher anti-tyrosinase activity with lower concentration. Furthermore, terrestrial animal proteins are no longer popular in health care products and cosmetics due to religious beliefs, the risk of zoonotic disease transmission and other factors. The market needs new TIPs with better activity and safety.
2.2. Anti-Tyrosinase Peptides of Aquatic Origin
Oceans cover more than 70% of the earth’s surface, and aquatic species account for about half of the total global biodiversity. With the rise of blue resources, researchers have begun to explore more nutritional values and bioactive activities of aquatic organisms. Collagen is a kind of aquatic protein, which plays a vital role in several organs of the body, particularly in the bone, skin and cartilage. Aquatic collagen peptides were verified to show significant effects in human skin, such as anti-oxidation, anti-photoaging, and moisturizing, etc. [33,34]. In recent years, they have been found to reduce melanogenesis through inhibiting tyrosinase activity [35]. Generally, collagen hydrolysates with low molecular weight display better dispersion and higher hydrophobicity, thus exhibiting better bioactivity [36]. It was proved that the presence of hydrophobic amino acids at the beginning and end of the peptide chain formed extra interactions with copper active sites of tyrosinase [37]. Wang et al. [35] reported a low-molecular-weight (700–1700 Da) gelatin hydrolysate isolated from the sea cucumber wall with 55.7% of hydrophobic amino acids. The isolated peptides exhibited excellent inhibitory characteristics against tyrosinase activity and melanin synthesis in B16 cells. In contrast, Park et al. [26] gave the opposite result. They found Thunnus obesus collagen hydrolysate fractions with large molecular (>10,000 Da) weight exhibited higher anti-tyrosinase activity than those with small molecular weights (500–10,000 Da). To a great extent, the bioactivity of TIPs depends on the composition, quantity and position of characteristic amino acids [38–40].
It was estimated that fish waste, such as fish scales, skin and bones, accounted for approximately 60-75% of total fish weight and crude protein levels of aquatic waste were 8-35% [32]. The use of these discarded parts may be conducive to a circular economy. Therefore, waste hydrolysates have gained much attention as potential materials for TIPs. Fish by-products like grass carp scales [13], milk fish scales [25], thunnus obesus skin [26] and tuna backbone [32] have been hydrolyzed to obtain active TIPs. A modified peptide CNGVQPK derived from crocodile blood was verified to reduce melanin content in B16F1 cells through significantly inhibiting tyrosinase activity, and showed no damage to cell proliferation in human skin keratinocytes [41].
Compared with terrestrial protein sources, TIPs of aquatic origin normally have shorter peptide chains, which is beneficial for skin penetration and intestinal digestion and absorption. On account of their special ecological environment, the denatured temperature of TIPs from aquatic origin is low, which does not harm cell proliferation. Furthermore, TIPs of aquatic origin have higher solubility so as to simplify the technological process. To sum up, it is speculated that aquatic biological proteins could replace terrestrial biological proteins as the main sources for preparing active peptides, especially TIPs, in future years.
Table 1. The sources of food-derived tyrosinase inhibitory peptides (TIPs) and their activity.
] |
VY | |||
/ |
Hydrogen bond: His85, His263, Gly281, His296 π–π stacking: His263 π–Alkyl: His85 Alkyl: Val283 π–Sigma: Val283 π–Amide stacking: His85 |
[60] |
|
|
GYSLGNWVCAAK |
IC50: 3.04 mmol/L |
Hydrogen bond: Tyr65, His259, His263, Arg268, Gly281, Glu322 Hydrophobic Interaction: Ala80, Cys83, Arg321, His85, Val283, Asn81, Glu189, His244, Val248, Asn260, Phe264, Ala323 Covalent bond: CuB |
[5] |
|
ECGYF |
IC50: 0.46 mmol/L |
Hydrogen bond: Met280, Tyr65, Asn260, His263 Hydrophobic interaction: Phe264, Pro284 |
[39] |
|
FPY |
IC50: 3.22 mmol/L |
Hydrogen bond: Asn260, Pro81 π–π stacking: Ser282, His263 π–Alkyl: Ala286, Val283 π–Sigma: Val283 π–Cation: His259 |
[42] |
|
Origin |
Source |
Peptides Sequences or Hydrolysates |
Molecular Weight(Da) |
Acitivity Evaluation |
References |
|
Terrestrial origin |
Potato |
Solunum tuberosum peels hydrolysates |
485,980 |
990.44 μg KE/μg peptides |
[22] |
|
Vicia faba pods |
Broad bean pods hydrolysates |
26,102 |
135.80 μg KE/μg peptides |
[23] |
|
|
Chinese quince seeds |
RHAKF |
658 |
IC50: 0.93 mg/mL |
[15] |
|
|
Defatted walnut meal |
FPY |
425 |
IC50: 1.11 mmol/L |
[42] |
|
|
Liquid rice starch |
LQPSHY |
744 |
IC50: 0.16 mmol/L |
[28] |
|
|
Rice starch |
Strain hydrolysates |
<1000 |
107.70 mgKAeq/g |
[31] |
|
|
Chicken feather meal |
Proteolysates |
<3000 |
IC50: 0.04 µg/mL |
[24] |
|
|
Egg white |
IRW |
340 |
IC50: 2.90 mmol/L |
[32] |
|
|
Egg white |
GYSLGNWVCAAK |
1268 |
IC50: 3.04 mmol/L |
[5] |
|
|
Milk |
MHIR |
555.30 |
IC50: 0.08 mmol/L |
[21] |
|
|
Camel milk |
αS-casein hydrolysates |
>10,000 |
0.2 mg/mL(peptides): 39.26% |
[17] |
|
|
Ganoderma lucidum |
VLT |
639 |
5.0 mg/mL(peptides): 16.00% |
[43] |
|
|
Porcine skin |
Proteolysates |
<3000 |
5.0 mg/mL(peptides): 69.80% |
[44] |
|
|
Chia seeds |
Proteolysates |
<3000 |
IC50: 0.66 mg/mL |
[45] |
|
|
Sorghum grain kafirins |
Proteolysates |
<1000 |
Peptides solution: 14.20% |
[46] |
|
|
Aquatic origin |
Rhopilema hispidum |
Collagen hydrolysates |
<10000 |
Collagen solution: 64.00% |
[47] |
|
Sea cucumber |
Body wall gelatin |
700–1700 |
0.1 mg/mL(peptides): 30.80% |
[35] |
|
|
Grass carp fish |
FTGML |
567 |
IC50: 1.89 mmol/L |
[13] |
|
|
Mackerel meat |
VWWW |
680 |
IC50: 1.25 mmol/L |
[32] |
|
|
Tuna (backbone protein) |
VKAGFAWTANQQLS |
1519 |
IC50: 0.60 mmol/L |
[32] |
|
|
Milk fish scale |
MSCP |
/ |
IC50: 0.75 mg/mL |
[25] |
|
|
Bigeye tuna and thunnus obesus skin |
Proteolysates |
50,000–100,000; <1000 |
5.0 mg/mL: 63.10% (50,000–100,000); 56.10% (<1000) |
[26] |
|
|
Zebrafish |
Phosvitin-derived peptide Pt5 |
/ |
0.1 mg/mL (peptides): 16.00% |
[48] |
3. The Preparation of Anti-Tyrosinase Peptides from Food Protein
The methods for TIPs preparation include enzymatic hydrolysis, chemical hydrolysis, microbial fermentation and chemical synthesis, among which the enzymatic hydrolysis and solid phase synthesis are the two more common technologies.
3.1. Enzymatic Hydrolysis
Enzymatic hydrolysis has become one of the most common methods for preparing TIPs in recent years, due to mild reaction conditions and ease of process control. A variety of commercial enzymes are currently used for TIPs production, including flavourzyme, alkaline protease, neutral protease, trypsin, chymotrypsin, papain, etc. On the one hand, different enzymes have different hydrolytic effects on the same material due to the binding specificity between enzyme and substrate. On the other hand, the enzymolytic effect of the same enzyme on different raw materials is different. El-sayed used the immobilized lettuce protease to hydrolyze potato peels [22] and broad bean pods [23] respectively, and found that the tyrosinase inhibitory activity of broad bean pods hydrolysate was better than that of potato peels.
It is of importance to correctly select raw materials and proteases before enzymatic hydrolysis. In the previous discussion, lactoproteins, such as milk [17,21] and eggs [32,37], are good materials to prepare TIPs. In addition, researchers found that trypsin and chymotrypsin had specific cleavage characteristics of amino acids that contribute to tyrosinase inhibition, such as Arg, Lys, Phe, Leu, Tyr, etc. For example, trypsin as an endopeptidase cleaves Arg and Lys at the C-terminal of peptide chains, and chymotrypsin specifically cleaves Phe, Leu, Tyr, Met and Try at the C-terminal of peptide chains. Addar et al. [17] demonstrated that chymotrypsin could produce hydrophobic aromatic amino acids from αS-casein. Yap et al. [14] found that the egg albumin hydrolysate with the highest monophenolase inhibition was produced by the complex of 55% trypsin + 45% chymotrypsin. Protein materials with tyrosinase inhibitory activity usually had high contents of hydrophobic amino acids (Trp, Phe, Gly, Val, Leu, Ile, Ala, Pro and Met) and aromatic amino acids (Tyr, Trp and Phe). Hydrophobic amino acids react with other residues, free radicals or metal ions as hydrogen donor while aromatic amino acids have conjugated planar rings, which can not only absorb the ultraviolet rays, but also form π-π interactions with Cu2+ of tyrosinase. The conjugation with Cu2+ can interrupt the oxidative action of tyrosinase, thereby inhibiting the synthesis of melanin. In short, the common method to obtain TIPs at present includes enzyme species screening and enzymolytic process optimization, accompanied by effective purification.
3.2. Solid Phase Synthesis
Chemical synthesis of TIPs includes solid phase synthesis and liquid phase synthesis, among which the former has been developed since the 1960s. Solid phase synthesis to synthesize TIPs uses the continuous reaction of amino acids on insoluble porous carriers. It is mainly divided into 9-fluorene methoxy-carbonyl (Fmoc) synthesis and tert-butyl-carbon (Boc) synthesis according to the different protective groups added at the N-terminal and side chains of the peptide sequence [38]. Compared with Boc synthesis, The protective groups by Fmoc synthesis have the advantage of being stable in an acidic medium and being removed easily in basic solution, making Fmoc synthesis more popular in TIPs production. Ookubo et al. [49] synthesized an octapeptide LILVLLAI by Fmoc synthesis and found it could enter B164A5 cells through the skin delivery system and significantly inhibit melanin production. Kim et al. [50] established a kojic acid-tripeptide library (KO-X1X2X3) by Fmoc synthesis and verified that the tyrosinase inhibitory activity of convergence was greatly enhanced. Compared with liquid phase synthesis, solid-phase synthesis omits the purification step and overcomes the difficulty in dissolving long-chain TIPs in solution. However, it is still unsatisfactory to achieve in large-scale production, due to its complex operation and high cost.
Enzymatic hydrolysis is limited for its low yield of target peptides as a result of protease choice blindness, and solid phase synthesis is limited for its high cost. Virtual enzymatic hydrolysis on a mass data base has developed well in recent years for it can predict the sequence fragments and their corresponding bioactivities hydrolyzed by one or more specific protease(s). Virtual enzymatic hydrolysis would help experimental work go further by improving the probability of each peptide site being cleaved and difference in cleaved sites.
4. The Possible Hypopigmentation Mechanisms of Anti-Tyrosinase Peptides from Food Proteins
4.1. Mechanism of Anti-Tyrosinase Peptides by Anti-Oxidation
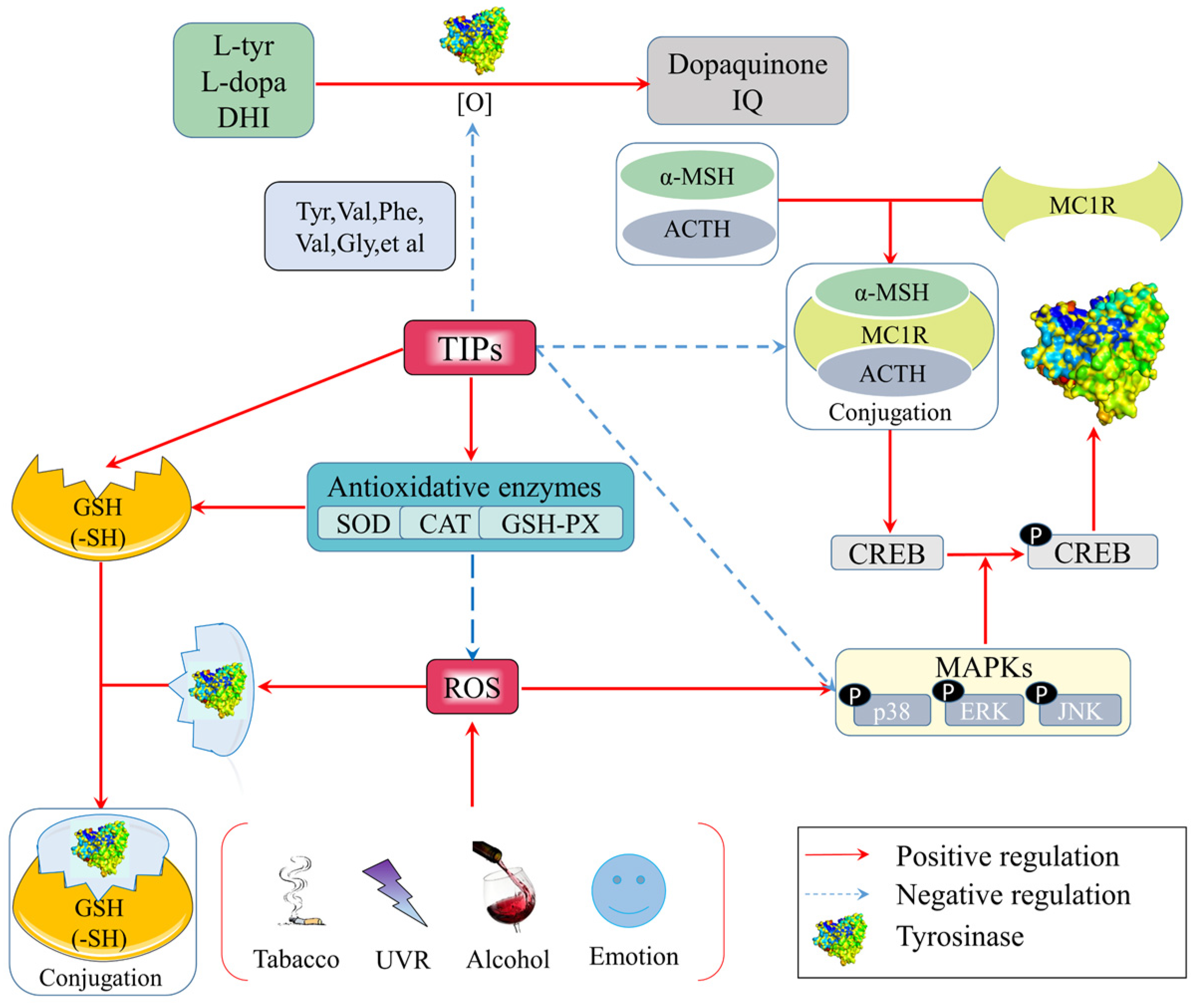
Figure 1. Anti-tyrosinase mechanisms of food-derived tyrosinase inhibitory peptides (TIPs) by anti-oxidation.
4.2. Mechanism of Anti-Tyrosinase Peptides by Occupying the Bioactive Site of Tyrosinase
4.2.1. TIPs Inhibit Tyrosinase Activity by Chelating with Binuclear Copper II Ions Catalytic Core
4.2.2. TIPs Inhibit Tyrosinase Activity by Binding to Amino Acid Residues of Tyrosinase Hydrophobic Cavity
Table 2. Interactive forces and amino acid residues between TIPs and tyrosinase.
|
Peptide Sequences |
Tyrosinase Inhibition Activity |
Interaction Forces and Residues |
References |
|
IQSPHFF |
IC50: 1.70 mmol/L |
Hydrogen bond: Lys229, Gly250, Ser276 π–π stacking: His266 |
[56] |
|
FTGML |
IC50: 1.89 mmol/L |
Hydrogen bond: Lys147, Trp53 π–π stacking: Trp53 π–Alkyl: Ile39, Phe41 Attractive Charge: Asp51 |
[13] |
|
NGVQPKY |
/ |
Hydrogen bond: Asn260, His94, His296 π–π stacking: His263 π–Alkyl: Val283 |
[41] |
|
CNGVQPK |
/ |
Hydrogen bond: Pro277, Leu275, Gly281, Gly257 π–Alkyl: Asn260, Glu256, Met257 |
[41] |
|
IR |
/ |
Hydrogen bond: His85, His94, Glu256, His259, Asn260, His263, Gly281, His296 π–Alkyl: His244, His263 Alkyl: Val283, Ala286 |
[60] |
|
LK |
/ |
Hydrogen bond: His61, His85, Glu256, His259, His263, Met280, His296 π–Alkyl: His244, His263 π–Amide stacking: His244, His263 Alkyl: Val283, Ala286 |
[60 |
4.2.3. Emerging Bioinformatic Technologies Used in Exploring Novel TIPs
4.3. Mechanism of TIPs by Regulating Related Gene Expression
4.3.1. CAMP/PKA/CREB Signaling Pathway
4.3.2. PI3K/AKT/GSK3β Signaling Pathway
4.3.3. MEK/ERK/MITF Signaling Pathway
4.3.4. P38 MAPK/CREB/MITF Signaling Pathway
5. Current Discussion
The collection of studies presented highlight the normal sources and mechanisms in melanocytes of TIPs and emerging technologic tools used in TIPs study, aiming to improve the pigmentation problem that the cosmetics industry still cannot completely solve.
The initial sources of TIPs were plants, and later animals became the main focus. With the popularity of blue economy, aquatic TIPs have become a new research hotspot. Compared with terrestrial protein sources, TIPs of aquatic origin have better physical and biological properties, which deserve further study. Whether it is terrestrial or aquatic protein, TIPs with small molecular weight (<3000 Da) seem more active. In addition, food industry by-products are also implicated in obtaining TIPs, but their anti-tyrosinase activity do not seem to be as high as that of edible proteins. It makes more commercial sense to prepare TIPs with higher activity from edible proteins than from by-products. Until now, the production of TIPs has almost always occurred through enzymatic or chemical hydrolysis, which have disadvantages of high cost and difficulty in separating products from the reaction system. Novel extraction technologies, such as microbial fermentation, pulse electric field, supercritical fluids and virtual enzyme hydrolysis, combined with conventional methods are able to keep costs lower and increase yields. Virtual enzymatic hydrolysis can predict the peptides likely to be produced after the protein has been hydrolyzed by the specific proteases in practice, and it will be widely applied in the future, employing a mathematical base as the primary means of production of TIPs.
In silico analysis plays a very important role in simulating the conjugation of enzymes and ligands, because there is no technology that can directly observe the dynamic binding between them. Many computer tools are capable of quickly screening TIPs and predicting their activities according to the established database. However, there is neither a database of anti-tyrosinase peptides nor molecular dynamics experience for TIPs. The Quantitative structure-activity relationship model (QSAR) is an effective tool for predicting biological activity of compounds. Its principle is to describe the quantitative relationships between structures with similar characteristics and their biological activity, which is lacking in spatial structure investigation between receptor and ligand; molecular docking can better solve this problem. However, current studies are only using molecular docking to verify the interaction sites between receptor proteins and active peptides. Therefore, the effective integration of these two technologies can improve the accuracy of screening efficiency of active peptides. Meanwhile, the time-consuming and laborious purification process of traditional experimental methods can be avoided by making full use of structural bioinformatics technology, thus laying a theoretical foundation for efficient screening of bioactive peptides based on the structure-activity relationship.
It is well known that most tyrosinase inhibitors work through powerful anti-oxidation. However, the specific mechanism of oxidation, especially regarding photoaging and tyrosinase activity, is not fully understood. The skin is exposed to light all the time, triggering a complex set of reactions that goes beyond just sun tanning. In addition to freckle-removing and whitening activities, TIPs are required to have more functionalities, such as moisturizing, elasticity, anti-aging and anti-inflammation, which need further investigation. The pure peptide may exhibit a higher toxicity and weaker bioactivity than the crude extracts in practical application, suggesting a non-negligible synergetic effect of multiple compounds. What’s more, chemical inhibitors usually exhibit stronger tyrosinase inhibition rather than bioactive peptides, although they pose a security risk. Therefore, the synergetic effect of various tyrosinase inhibitors and agents with different bioactivities deserves study. More importantly, future studies should consider mechanisms as to how TIPs regulate tyrosinase activity, activated by other factors, such as nicotine, alcohol, emotion, diet and endocrine function.
TIPs can inhibit tyrosinase activity by chelating with tyrosinase’s copper Ⅱ ions’ centers. Copper is an essential trace mineral in the human body, and it is associated with some pathogenesis, due to its pro-oxidation effect. Therefore, the copper chelating ability of TIPs should be emphasized and utilized. Peptides with copper chelating ability can be isolated by copper-immobilized affinity chromatography and prepared by tyrosinase inhibition activity as an evaluation index. There have been no reports about this method. It can be underscored that more research should focus on how copper ions in tyrosinase catalyze L-tyr or L-dopa and how TIPs inhibit the catalytic reaction of copper ions.
The Reconstructed human epidermal model (RHE) was developed as an alternative to animal tests to predict acute skin and eye irritation, and now is further used to verify whitening activity. On this basis, the model may be used in studying TIPs’ inhibitory pathway. In addition, the zebrafish model can also serve as an excellent model for studying the melanogenesis signaling pathway, as it has up to 75% homology with human gene sequences. Finally, cytokine inhibitors make a reverse argumentation that is more convincing. Although the signaling pathways regulating tyrosinase expression have been roughly clarified, the specific targets of TIPs in these pathways are still obscure. Most important of all, more studies should be conducted instead of just observing the expression changes of several upstream factors. Future research will need to combine genomics with proteomics and clinical trials to provide better evidence for TIP function.
References
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Recent development of signaling pathways inhibitors of melanogenesis. Cell Signal. 2017, 40, 99–115.
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549.
- Castro-Jacome, T.P.; Alcantara-Quintana, L.E.; Montalvo-Gonzalez, E.; Chacon-Lopez, A.; Kalixto-Sanchez, M.A.; Rivera, M.D.; Lopez-Garcia, U.M.; Tovar-Perez, E.G. Skin-protective properties of peptide extracts produced from white sorghum grain kafirins. Ind. Crop. Prod. 2021, 167, 113551.
- Cunha, S.A.; Pintado, M.E. Bioactive peptides derived from marine sources: Biological and functional properties. Trends. Food Sci. Tech. 2022, 119, 348–370.
- Li, C.Y.; Fu, Y.; Dai, H.J.; Wang, Q.; Gao, R.C.; Zhang, Y.H. Recent progress in preventive effect of collagen peptides on photoaging skin and action mechanism. FSHW 2022, 11, 218–229.
- Wang, J.F.; Wang, Y.M.; Tang, Q.J.; Wang, Y.; Chang, Y.J.; Zhao, Q.; Xue, C.H. Anti-oxidation activities of low-molecular-weight gelatin hydrolysate isolated from the sea cucumber Stichopus japonicus. J. Ocean Univ. China. 2010, 9, 94–98.
- Lakhan, M.K.; Lynch, M. Skin pigmentation. Pigment. Probl. 2021, 49, 447–452.
- Addar, L.; Bensouici, C.; Zennia, S.S.A.; Haroun, S.B.; Mati, A. Antioxidant, tyrosinase and urease inhibitory activities of camel αS-casein and its hydrolysate fractions. Small Ruminant. Res. 2019, 173, 30–35.
- Park, S.H.; Jo, Y.J. Static hydrothermal processing and fractionation for production of a collagen peptide with anti-oxidative and anti-aging properties. Process. Biochem. 2019, 83, 176–182.
- Thaha, A.; Wang, B.S.; Chang, Y.W.; Hsia, S.M.; Huang, T.C.; Shiau, C.Y.; Hwang, D.F.; Chen, T.Y. Food-derived bioactive peptides with anti-oxidative capacity, xanthine oxidase and tyrosinase inhibitory activity. Processes 2021, 9, 747.
- Shen, Z.W.; Wang, Y.J.; Guo, Z.; Tan, T.Y.; Zhang, Y. Novel tyrosinase inhibitory peptide with free radical scavenging ability. J. Enzyme Inhib. Med. Chem. 2019, 34, 1633–1640.
- Joompang, A.; Jangpromma, N.; Choowongkomon, K.; Payoungkiattikun, W.; Tankrathok, A.; Viyoch, J.; Luangpraditkun, K.; Klaynongsruang, S. Evaluation of tyrosinase inhibitory activity and mechanism of Leucrocin I and its modified peptides. J. Biosci. Bioeng. 2020, 130, 239–246.
- Nie, H.L.; Liu, L.; Yang, H.Q.; Guo, H.Z.; Liu, X.; Tan, Y.H.; Wang, W.; Quan, J.; Zhu, L.M. A novel heptapeptide with tyrosinase inhibitory activity identified from a phage display library. Appl. Biochem. Biotechnol. 2020, 181, 219–232.
- Peng, Z.Y.; Wang, G.C.; Zeng, Q.H.; Li, Y.F.; Wu, Y.; Liu, H.Q.; Wang, J.J.; Zhao, Y. Synthesis, antioxidant and anti-tyrosinase activity of 1,2,4-triazole hydrazones as antibrowning agents. Food Chem. 2021, 341, 128265.
- Hu, Z.Z.; Sha, X.M.; Zhang, L.; Huang, S.; Tu, Z.C. Effect of grass carp scale collagen peptide FTGML on cAMP-PI3K/Akt and MAPK signaling pathways in B16F10 melanoma cells and correlation between anti-melanin and antioxidant properties. Foods 2022, 11, 391.
- Jara, J.R.; Aroca, P.; Solano, F.; Martinez, J.H.; Lozano, J.A. The role of sulfhydryl compounds in mammalian melanogenesis effect of cysteine and glutathione upon tyrosinase and the intermediates of the pathway. Biochim. Biophys. Acta. 1988, 967, 296–303.
- Kong, S.; Choi, H.R.; Kim, Y.J.; Lee, Y.S.; Park, K.C.; Kwak, S.Y. Milk protein-derived antioxidant tetrapeptides as potential hypopigmenting agents. Antioxidants 2020, 9, 1106.
- Baskaran, R.; Chauhan, S.S.; Parthasarathi, R.; Mogili, N.S. In silico investigation and assessment of plausible novel tyrosinase inhibitory peptides from sesame seeds. LWT Food Sci. Technol. 2021, 147, 111619.
- Bagherzadeh, K.; Talari, F.S.; Sharifi, A.; Ganjali, M.R.; Saboury, A.A.; Amanlou, M. A new insight into mushroom tyrosinase inhibitors: Docking, pharmacophore-based virtual screening, and molecular modeling studies. J. Biomol. Struct. Dyn. 2015, 33, 487–501.
- Sepehri, N.; Khoshneviszadeh, M.; Farid, S.M.; Moayedi, S.S.; Asgari, M.S.; Moazzam, A.; Hosseini, S.; Adibi, H.; Larijani, B.; Pirhadi, S.; et al. Design, synthesis, biological evaluation, and molecular docking study of thioxo-2,3-dihydroquinazolinone derivative as tyrosinase inhibitors. J. Mol. Struct. 2022, 125, 132283.
- Feng, Y.X.; Wang, Z.C.; Chen, J.X.; Li, H.R.; Wang, Y.B.; Ren, D.F.; Lu, J. Separation, identification, and molecular docking of defatted walnut (Juglans regia L.) meal. Food Chem. 2021, 353, 129471.
- Yap, P.G.; Gan, C.Y. Multifunctional tyrosinase inhibitor peptides with copper chelating, UV-absorption and antioxidant activities: Kinetic and docking studies. Foods 2021, 10, 675.
- Wang, H.M.; Chen, C.Y.; Chen, C.Y.; Oh, M.L.; Chou, Y.T.; Chang, H.C.; Lee, C.H.; Wang, C.Z.; Chu, I.M. (−)-N-Formylanonaine from michelia alba as a human tyrosinase inhibitor and antioxidant. Bioogra. Med. Chem. 2010, 18, 5241–5247.
- Ghani, U. Carbazole and hydrazone derivatives as new competitive inhibitors of tyrosinase: Experimental clues to binuclear copper active site binding. Bioorg. Chem. 2019, 83, 235–241.
- Deng, Y.J.; Huang, L.X.; Zhang, C.H.; Xie, P.J.; Cheng, J.; Wang, X.; Liu, L.J. Skin-care functions of peptides prepared from Chinese quince seed protein: Sequences analysis, tyrosinase inhibition and molecular docking study. Ind. Crop. Prod. 2020, 148, 112331.
- Vo, T.D.L.; Pham, K.T. Copper-chelating peptide from salmon by-product proteolysate. Int. J. Food Eng. 2020, 16, 20190280.
- Deri, B.; Kanteev, M.; Goldfeder, M.; Lecina, D.; Guallar, V.; Adir, N.; Fishman, A. The unravelling of the complex pattern of tyrosinase inhibition. Sci. Rep. 2016, 6, 34993.
- Yu, Q.; Fan, L.P. Understanding the combined effect and inhibition mechanism of 4-hydroxycinnamic acid and ferulic acid as tyrosinase inhibitors. Food Chem. 2021, 352, 129369.
- Hu, Z.Z.; Ma, T.X.; Sha, X.M.; Zhang, L.; Tu, Z.C. Improving tyrosinase inhibitory activity of grass carp fish scale gelatin hydrolysate by gastrointestinal digestion: Purification, identification and action mechanism. LWT Food Sci. Technol. 2020, 159, 113205.
- Goldfeder, M.; Kanteev, M.; Isaschar-Ovdat, S.; Adir, N.; Fishman, A. Determination of tyrosinase substrate-binding modes reveals mechanistic differences between type-3 copper proteins. Nat. Commun. 2014, 5, 4505.
- Romagnoli, R.; Oliva, P.; Prencipe, F.; Manfredini, S.; Germano, M.P.; De Luca, L.; Ricci, F.; Corallo, D.; Aveic, S.; Mariotto, E.; et al. Cinnamic acid derivatives linked to arylpiperazines as novel potent inhibitors of tyrosinase activity and melanin synthesis. Eur. J. Med. Chem. 2022, 231, 114147.
- Garcia-Molina, P.; Garcia-Molina, F.; Teruel-Puche, J.A.; Rodriguez-Lopez, J.N.; Garcia-Canovas, F.; Munoz-Munoz, J.L. Considerations about the kinetic mechanism of tyrosinase in its action on monophenols: A review. Mol. Catal. 2022, 518, 112072.
- Mann, T.; Gerwat, W.; Batzer, J.; Eggers, K.; Scherner, C.; Wenck, H.; Stab, F.; Hearing, V.J.; Rohm, K.H.; Kolbe, L. Inhibition of human tyrosinase requires molecular motifs distinctively different from mushroom tyrosinase. J. Investig. Dermatol. 2018, 138, 1601–1608.
- BIOPEP. Available online: https://biochemia.uwm.edu.pl/biopep-uwm/ (accessed on 19 April 2022).
- Kongsompong, S.; E-kobon, T.; Chumnanpuen, P. K-nearest neighbor and random forest-based prediction of putative tyrosinase inhibitory peptides of abalone haliotis diversicolor. Molecules 2021, 26, 3671.
- HELPDOCK. Available online: http://huanglab.phys.hust.edu.cn/hpepdock/ (accessed on 19 April 2022).
- Song, X.; Hu, X.; Zhang, Y.; Pan, J.H.; Gong, D.M.; Zhang, G.W. Inhibitory mechanism of epicatechin gallate in tyrosinase inhibitory interaction, conformational change and computational simulation. Food Funct. 2020, 11, 4892–4902.
- Song, X.; Ni, M.; Zhang, Y.; Zhang, G.; Pan, J.; Gong, D. Comparing the inhibitory abilities of epigallocatechin-3-gallate and gallocatechin gallate against tyrosinase and their combined effects with kojic acid. Food Chem. 2021, 349, 129172.
- Lee, H.R.; Jung, J.M.; Seo, J.Y.; Chang, S.E.; Song, Y.S. Anti-melanogenic property of ginsenoside Rf from Panax ginseng via inhibition of CREB/MITF pathway in melanocytes and ex vivo human skin. J. Ginseng. Res. 2021, 45, 555–564.
- Jian, D.; Jiang, D.J.; Su, J.; Chen, W.; Hu, X.L.; Kuang, Y.H.; Xie, H.F.; Li, J.; Chen, X. Diethylstilbestrol enhances melanogenesis via cAMP-PKA-mediating up-regulation of tyrosinase and MITF in mouse B16 melanoma cells. Steroids 2011, 76, 1297–1304.
- Yuan, X.H.; Yao, C.; Oh, J.H.; Park, C.H.; Tian, Y.D.; Han, M.; Kim, J.E.; Chung, J.H.; Jin, Z.H.; Lee, D.H. Vasoactive intestinal peptide stimulates melanogenesis in B16F10 mouse melanoma cells via CREB/MITF/tyrosinase signaling. Biochem. Bioph. Res. Commun. 2016, 477, 336–342.
- Choi, H.; Yoon, J.H.; Youn, K.; Jun, M. Decursin prevents melanogenesis by suppressing MITF expression through the regulation of PKA/CREB, MAPKs, and PI3K/Akt/GSK-3β cascades. Biomed. Pharmacother. 2022, 147, 112651.
- Zhou, S.Q.; Yotsumoto, H.; Tian, Y.; Sakamoto, K. α-Mangostin suppressed melanogenesis in B16F10 murine melanoma cells through GSK3β and ERK signaling pathway. Biochem. Biophys. Rep. 2021, 26, 100949.
- Shirasugi, I.; Kamada, M.; Matsui, T.; Sakakibara, Y.; Liu, M.C.; Suiko, M. Sulforaphane inhibited melanin synthesis by regulating tyrosinase gene expression in B16 mouse melanoma cells. Biosci. Biotechnol. Biochem. 2020, 74, 579–582.
- Huang, H.C.; Lin, H.; Huang, M.C. The lactoferricin B-derived peptide, LfB17-34, induces melanogenesis in B16F10 cells. Int. J. Mol. Med. 2017, 39, 595–602.
- Oh, G.W.; Ko, S.C.; Heo, S.Y.; Nguyen, V.T.; Kim, G.; Jang, C.H.; Park, W.S.; Choi, I.W.; Qian, Z.J.; Jung, W.K. A novel peptide purified from the fermented microalga Pavlova lutheri attenuates oxidative stress and melanogenesis in B16F10 melanoma cells. Process. Biochem. 2015, 50, 1318–1326.
- Kang, B.; Kim, Y.; Park, T.J.; Kang, H.Y. Dasatinib, a second-generation tyrosine kinase inhibitor, induces melanogenesis via ERK-CREB-MITF-tyrosinase signaling in normal human melanocytes. Biochem. Bioph. Res. Commun. 2020, 523, 1034–1039.
- Huang, H.C.; Liao, C.C.; Peng, C.C.; Lim, J.M.; Siao, J.H.; Wei, C.M.; Chen, C.C.; Wu, C.S.; Chang, T.M. Dihydromyricetin from Ampelopsis grossedentata inhibits melanogenesis through down-regulation of MAPK, PKA and PKC signaling pathways. Chem.-Biol. Interact. 2016, 258, 166–174.
- Fu, T.; Chai, B.W.; Shi, Y.L.; Dang, Y.Y.; Ye, X.Y. Fargesin inhibits melanin synthesis in murine malignant and immortalized melanocytes by regulating PKA/CREB and P38/MAPK signaling pathways. J. Dermatol. Sci. 2019, 94, 213–219.
- Wang, Y.; Lan, Y.H.; Yang, X.C.; Gu, Y.G.; Lu, H.G. TGFβ2 upregulates tyrosinase activity through opsin-3 in human skin melanocytes in vitro. J. Investig. Dermatol. 2021, 141, 2679–2689.
- Park, C.H.; Kim, G.; Lee, Y.; Kim, H.; Song, M.J.; Lee, D.H.; Chung, J.H. A natural compound harmine decreases melanin synthesis through regulation of the DYRK1A/NFATC3 pathway. J. Dermatol. Sci. 2021, 103, 16–24.
