There has been a steady stream of information on the methods and techniques available for detecting harmful algae species. The conventional approaches to identify harmful algal bloom (HAB), such as microscopy and molecular biological methods are mainly laboratory-based and require long assay times, skilled manpower, and pre-enrichment of samples involving various pre-experimental preparations. As an alternative, biosensors with a simple and rapid detection strategy could be an improvement over conventional methods for the detection of toxic algae species. Moreover, recent biosensors that involve the use of nanomaterials to detect HAB are showing further enhanced detection limits with a broader linear range. The improvement is attributed to nanomaterials’ high surface area to volume ratio, excellent biological compatibility with biomolecules, and being capable of amplifying the electrochemical signal.
- harmful algae
- red tide
- biosensor
- HAB detection method
- nanomaterial
- conventional method
1. Introduction
2. Conventional Methods for HAB Identification
The main conventional methods in HAB monitoring and detection is based on light microscopy and counting chamber [26]. Light microscopy can be utilized to do species identification among the various types of HAB, but is not accurate. Whereas counting chamber will be used to estimate the quantity of HAB species found in samples collected. Other approaches in HAB identification can be based on mouse bioassay (MBA) or chromatographic technique. Although, these two mentioned approaches focus more on detecting the HAB toxins content and is thereby unsuitable for early warning system of HAB event but only to monitor the toxicity of the water. Alternatively, many species from HAB, in particular, and phytoplankton in general, can be detected via molecular methods, which are fast and accurate methods capable of simultaneous qualitative detection [27]. Molecular methods can also identify and quantify harmful algae species [28][29]. The biomedical research and diagnostic industries have been designing simplified ways to sample preparation and distribution while utilizing molecular probe technologies to interpret results [30]. For instance, sample preparation and analysis systems that are portable for in situ detection have been designed but no major implementation had occurred [31]. Although other techniques used for the same purpose include DNA microarrays with different molecular probe techniques [32][33], when it comes to identifying phytoplankton, molecular approaches are considered to be quicker and more precise than light microscopy [34]. Several types of molecular methods, including fluorescent in situ hybridization (FISH) of whole-cell, polymerase chain reaction (PCR)-based assays, sandwich hybridization assays, enzyme-linked immunosorbent assay (ELISA) colorimetric whole-cell analysis, monoclonal antibody probes, and DNA microarrays are capable of identifying algae species and detecting toxic algae in routine monitoring programs [24][25][28][35].2.1. Microscopic Analysis
Globally, nations have employed HAB monitoring programs as warning systems. In the last decade, one of the main methods of monitoring and mitigation depends on visually inspecting water discolouration, dead fishes, and laborious cell counts [34]. Samples will be required to be dispatched to a laboratory for testing purposes to examine for the presence of harmful compounds using tried-and-tested procedures, such as light microscopy (LM) [13]. However, heterotrophs and autotrophs with sizes below 5 μm will be extremely difficult to be distinguished under LM [36]. For instance, Pseudo-nitzschia species cannot be identified at the species level via LM [11], whereas the high difficulty is present in species identification among Alexandrium variations. Furthermore, the counting of HAB cells is completed by humans, and this will inevitably result in human error mistakes that could lead to inaccurate results being produced. Thereby, the drawback of LM would be its incapability to discern minor differences between species. Both transmission electron microscopy (TEM) and scanning electron microscopy (SEM) are capable of doing identification of various pico- and nano-sized organisms but will inevitably increase overall expenses and time [27]. Whereas epifluorescence microscopy (EFM) does allow the sizing distinction among heterotrophs and autotrophs. EFM also provides a more accurate enumeration than LM but still holds difficulty in species identification [36]. Optical microscopy with normal light or epifluorescence would be used to identify paralytic shellfish poison (PSP) via Alexandrium (dinoflagellate) and other HABs in marine waters [37]. This approach is highly successful but is restricted by the sheer amount of samples that could take several days to complete analysing high numbers of samples. The examination of harmful algae species from water samples will require a rough estimation of 2 h on average. This will be reflected as an estimation work rate of processing 20 samples per week by one person [11]. Sometimes filtration of water samples is necessary during the sample preparation phase with 4 μm pore size filters and air dry the cells in immersion oil for 2–4 days before being examined under standard LM [36]. These time lags will impede the early warning systems of bloom events, as numerous samples are required from the different sites of the water body to accurately conclude if there will be a possibility of HAB. A simplified diagram is illustrated in Figure 1 to show the flow of microscopy approaches in HAB examination.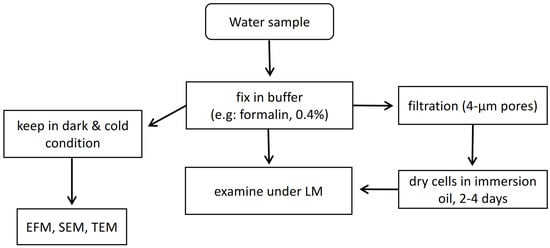
2.2. Fluorescent In Situ Hybridization (FISH)
This approach relies on the principle of oligonucleotide such as DNA or PNA as the probe that is attached with a fluorescent marker and required to penetrate through cells to hybridize with the target sequence. The targeted cells will fluoresce and can be visually detected with epifluorescence microscopy (Figure 2). Studies can be found on applying FISH to detecting certain HAB species, but several disadvantages are present. For instance, before observing fluorescently labelled cells, several necessary purification steps including: (I) sample treatment, (II) centrifugation, (III) pipetting, and (IV) washing may lead to the loss of target cells [40]. This will lead to unreliable quantification results unless a gentle filtration method is applied. In addition, the loss of cells from purification steps, the duration from sample collection to reaching a laboratory may gradually decompose the rRNA in the cells [41]. This can reduce the fluorescent intensity of labelled cells. Furthermore, different types of cell walls and membranes can be found in marine phytoplankton and this causes the difficulty in having a universal FISH protocol in fixing all sorts of microalgae cells [42]. For instance, certain modified saline ethanol can allow high permeability for a probe to enter and access target sequence in some microalgal species but does not work well with Alexandrium species, where auto-fluorescence was observed [43]. The background noises can mask over target fluorescent and make results hard to be interpreted.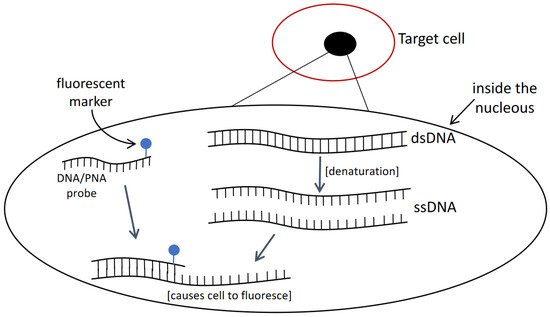
3. Biosensor Methods
Biosensor methods involve a biochemical recognition component that is combined with a signal transducer to detect specific targets. The recognition or bio-receptor component, such as specific probe sequence, antibodies, or enzymes could specifically bind to the target of interest or catalyse a biochemical reaction [45]. The bio-recognition event will then be converted into a quantifiable signal via a transducer (Figure 3). Biosensors are attractive candidates to overcome the limitations of traditional detection quantification methods due to their accuracy, simplicity of use, user-friendly, cost-effective, robustness, low power requirements, as well as their rapid turnaround time, high sensitivity, and versatility [46]. In addition, biosensor has the capacity for miniaturization and in-field application, e.g., portable device, which is suitable to improve monitoring methods by allowing for rapid on-site identification of microbiological pollutants. Biosensor design that could perform multiple targets detection concurrently can be an advantage for reducing the sample required [47].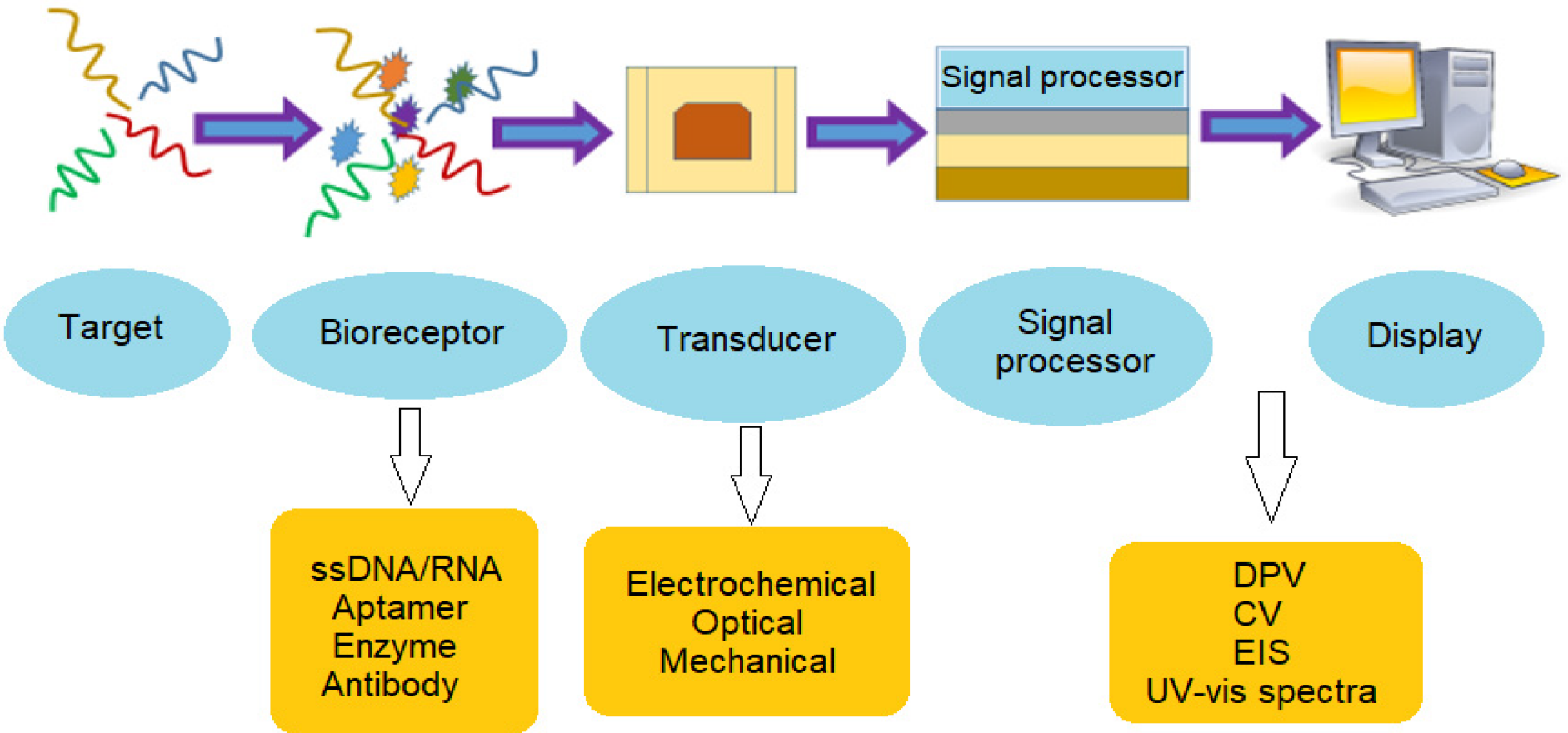
3.1. Electrochemical Biosensor Methods
The fundamental principle of the electrochemical biosensor is the chemical interactions between immobilized biocomponents and analyte that produces or consume ions or electrons, changing the measurable electrical properties of the solution, such as potential or electric current (Figure 4) [50]. Electrochemical biosensors with increased specificity, stability and sensitivity that are small and easy to fabricate are now accessible [51]. Furthermore, electrochemical biosensors are highly sensitive, portable, relatively inexpensive, and simple to build [52]. Therefore, the electrochemical DNA biosensor can serve as an appropriate HAB monitoring program.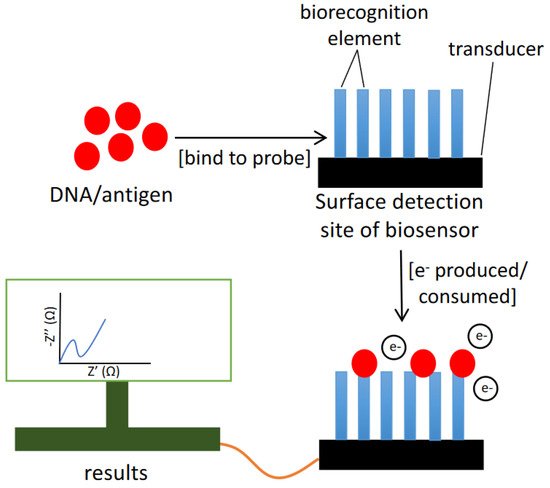
| Reference | Target | Instruments/Methods | Response Time (h) |
Detection Limit (Cells L−1) | Advantages | Drawbacks |
|---|---|---|---|---|---|---|
| [53] | DNA/RNA of microbial pathogens | Rapid PCR-Detect and Hybrid PCR-Detect. | 4–6 | - | Sensitive detection of sample DNA/RNA. | Only capable of detecting single-base mutations from pure culture isolate. |
| [55] | rRNA of toxic algae (toxic dinoflagellate A. Ostenfeldii) | Molecular DNA probes | 7–10 | 5 × 109 | Simplified detection methods | Manual RNA isolation and manipulation of the hybridization steps are required at high temperature system. |
| [54] | Microbial pathogens and Karenia brevis | 8-plex assay of microbes | 3–5 | 1000 | Able to multi-target electrochemical detection of microbial pathogens. | Complex steps in DNA extraction. |
| [23] | rRNA of harmful algae species | Multi-probe chip and a semi-automated rRNA biosensor | ~2 | - | Allows in situ detection and monitoring of toxic algae. | Manual rRNA isolation. |
| [35] | Alexandrium minutum | Multi-probe biosensor (ALGADEC) | ~2 | 25,000 | Almost fully automated device for in situ analysis. | Poor limit detection. |
| [56] | Prymnesium parvum, Gymnodinium catenatum, and Pseudo-nitzschia australis | DIG-enzymatic label assay | 1–2 | - | Simple and easy handling amperometric techniques. | Very poor response for cyclic voltammetry. |
| [57] | Alexandrium species | Surface Plasmon Resonance (SPR) biosensing instrument and peptide nucleic acid probes | >3.5 | - | Cost effective and yield quick result. | Require tubing flushing maintenance. |
3.2. Optical Biosensors
Optical chemical sensors have also been employed to detect HAB [65]. Optical chemical sensors are based on the reaction between biomolecule compounds and analytes, where the optical characteristics are induced by UV-vis absorption, bioluminescence, chemiluminescene, fluorescence, or reflectance. These biosensors have the benefits of being flexible, compact, and resistant to electrical noise (Figure 5) [66]. Furthermore, thanks to the use of optical fibre, it is feasible to construct very compact sensors, making them suited for measurement [64][67].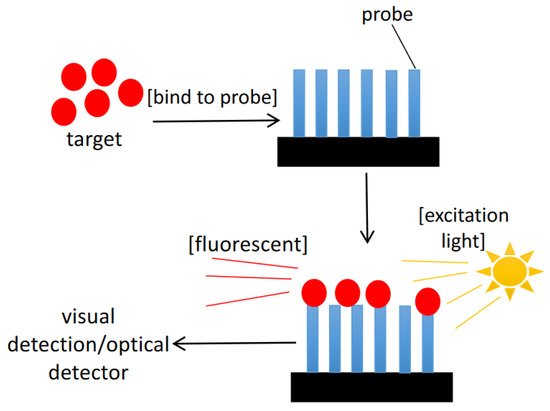
References
- Laabir, M.; Jauzein, C.; Genovesi, B.; Masseret, E.; Cecchi, P.; Collos, Y. Influence of temperature, salinity and irradiance on the growth and cell yield of the harmful red tide dinoflagellate Alexandrium catenella colonizing Mediterranean waters. J. Plankton. Res. 2011, 33, 1550–1563.
- Adam, A.; Mohammad-Noor, N.; Anton, A.; Saleh, E.; Saad, S.; Muhd Shaleh, S.R. Temporal and spatial distribution of harmful algal bloom (HAB) species in coastal waters of Kota Kinabalu, Sabah, Malaysia. Harmful Algae 2011, 10, 495–502.
- Dale, B.; Edwards, M.; Reid, P.C. Climate Change and Harmful Algal Blooms. In Ecology of Harmful Algae; Springer: Berlin/Heidelberg, Germany, 2006; pp. 367–378.
- Handy, S.M.; Demir, E.; Hutchins, D.A.; Portune, K.J.; Whereat, E.B.; Hare, C.E.; Rose, J.M.; Warner, M.; Farestad, M.; Cary, S.C. Using quantitative real-time PCR to study competition and community dynamics among Delaware Inland Bays harmful algae in field and laboratory studies. Harmful Algae 2008, 7, 599–613.
- Bricker, S.B.; Longstaff, B.; Dennison, W.; Jones, A.; Boicourt, K.; Wicks, C.; Woerner, J. Effects of nutrient enrichment in the nation’s estuaries: A decade of change. Harmful Algae 2008, 8, 21–32.
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; Mccarthy, M.J.; Newell, S.E.; Qin, B.; Scott, J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222.
- O’neil, J.; Davis, T.W.; Burford, M.A.; Gobler, C. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334.
- Branch, G.M.; Bustamante, R.H.; Robinson, T.B. Impacts of a ‘black tide’ harmful algal bloom on rocky-shore intertidal communities on the West Coast of South Africa. Harmful Algae 2013, 24, 54–64.
- Zhen, Y.; Mi, T.; Yu, Z. Detection of several harmful algal species by sandwich hybridization integrated with a nuclease protection assay. Harmful Algae 2009, 8, 651–657.
- Meneely, J.P.; Campbell, K.; Greef, C.; Lochhead, M.J.; Elliott, C.T. Development and validation of an ultrasensitive fluorescence planar waveguide biosensor for the detection of paralytic shellfish toxins in marine algae. Biosens. Bioelectron. 2013, 41, 691–697.
- Medlin, L. Molecular tools for monitoring harmful algal blooms. Environ. Sci. Pollut. Res. 2013, 20, 6683–6685.
- Gas, F.; Baus, B.; Pinto, L.; Compere, C.; TanchoU, V.; Quéméneur, E. One step immunochromatographic assay for the rapid detection of Alexandrium minutum. Biosens. Bioelectron. 2010, 25, 1235–1239.
- Dittami, S.M.; Hostyeva, V.; Egge, E.S.; Kegel, J.U.; Eikrem, W.; Edvardsen, B. Seasonal dynamics of harmful algae in outer Oslofjorden monitored by microarray, qPCR, and microscopy. Environ. Sci. Pollut. R. 2013, 20, 1–14.
- Orozco, J.; Medlin, L.K. Review: Advances in electrochemical genosensors-based methods for monitoring blooms of toxic algae. Environ. Sci. Pollut. R. 2013, 20, 6838–6850.
- Turner, J.T. Planktonic marine copepods and harmful algae. Harmful Algae 2014, 32, 81–93.
- Berdalet, E.; Fleming, L.E.; Gowen, R.; Davidson, K.; Hess, P.; Backer, L.C.; Moore, S.K.; Hoagland, P.; Enevoldsen, H. Marine harmful algal blooms, human health and wellbeing: Challenges and opportunities in the 21st century. J. Mar. Biol. Assoc. UK 2015, 96, 61–91.
- Hallegraeff, G.M. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99.
- Ajani, P.; Harwoood, D.T.; Murray, S.A. Recent trends in marine phycotoxins from Australian coastal waters. Mar. Drugs 2017, 15, 33.
- Lucas, B.; Dahlmann, J.; Erler, K.; Gerdts, G.; Wasmund, N.; Hummert, C. Overview of key phytoplankton toxins and their recent occurrence in the North and Baltic Sea. Environ. Toxicol. 2005, 20, 1–17.
- Watkins, S.M.; Reich, A.; Fleming, L.E.; Hammond, R. Neurotoxic Shellfish Poisoning. Mar. Drugs 2008, 6, 431–455.
- Kouakou, C.R.C.; Poder, T.G. Economic impact of harmful algal blooms on human health: A systematic review. J. Water Health 2019, 17, 499–516.
- Teen, L.P.; Gires, U.; Pin, L.C. Harmful Algal Blooms in Malaysian Waters. Sains Malays. 2012, 41, 1509–1515.
- Diercks, S.; Metfies, K.; Medlin, L.K. Development and adaptation of a multiprobe biosensor for the use in a semi-automated device for the detection of toxic algae. Biosens. Bioelectron. 2008, 23, 1527–1533.
- Cox, A.M.; Goodwin, K.D. Sample preparation methods for quantitative detection of DNA by molecular assays and marine biosensors. Mar. Pollut. Bull. 2013, 73, 47–56.
- Liu, F.; Zhang, C.; Wang, Y.; Chen, G. A review of the current and emerging detection methods of marine harmful microalgae. Sci. Total Environ. 2022, 815, 152913.
- Jipanin, S.J.; Shaleh, S.R.M.; Lim, P.T.; Leaw, C.P.; Mustapha, S. The Monitoring of Harmful Algae Blooms in Sabah, Malaysia. J. Phys. Conf. Ser. 2019, 1358, 1–9.
- Scorzetti, G.; Brand, L.; Hitchcock, G.; Rein, K.; Sinigalliano, C.; Fell, J. Multiple simultaneous detection of Harmful Algal Blooms (HABs) through a high throughput bead array technology, with potential use in phytoplankton community analysis. Harmful Algae 2009, 8, 196–211.
- Antonella, P.; Luca, G. The quantitative real-time PCR applications in the monitoring of marine harmful algal bloom (HAB) species. Environ. Sci. Pollut. R. 2012, 20, 1–12.
- Scholin, C. The development and application of molecular probes and novel instrumentation for detection of harmful algae. In Proceedings of the Ocean Development & International Law, Nice, France, 1 October 1998; pp. 367–370.
- Scholin, C.; Doucette, G.; Cembella, A. Prospects for developing automated systems for in situ detection of harmful algae and their toxins. In Real-time Coastal Observing Systems for Marine Ecosystem Dynamics and Harmful Algal Blooms; The United Nations Educational, Scientific and Cultural Organization: Paris, France, 2008; pp. 413–462.
- Metfies, K.; Töbe, K.; Scholin, C.; Medlin, L.K. Laboratory and Field Applications of Ribosomal RNA Probes to Aid the Detection and Monitoring of Harmful Algae. Ecol. Harmful Algae 2006, 189, 311–325.
- Barlaan, E.A.; Furukawa, S.; Takeuchi, K. Detection of bacteria associated with harmful algal blooms from coastal and microcosm environments using electronic microarrays. Environ. Microbiol. 2007, 9, 690–702.
- Diercks, S.; Gescher, C.; Metfies, K.; Medlin, L.K. Evaluation of locked nucleic acids for signal enhancement of oligonucleotide probes for microalgae immobilised on solid surfaces. J. Appl. Phycol. 2009, 21, 657–668.
- Orozco, J.; Medlin, L.K. Electrochemical performance of a DNA-based sensor device for detecting toxic algae. Sens. Actuators B-Chem. 2011, 153, 71–77.
- Diercks-Horn, S.; Metfies, K.; Jäckel, S.; Medlin, L.K. The ALGADEC device: A semi-automated rRNA biosensor for the detection of toxic algae. Harmful Algae 2011, 10, 395–401.
- Booth, B.C. Estimating Cell Concentration and Biomass of Autotrophic Plankton Using Microscopy. In Handbook of Methods in Aquatic Microbial Ecology; CRC Press: Boca Raton, FL, USA, 2018; pp. 199–205.
- Usup, G.; Pin, L.C.; Ahmad, A.; Teen, L.P. Alexandrium (Dinophyceae) species in Malaysian waters. Harmful Algae 2002, 1, 265–275.
- Lorenzen, C.J. A method for the continuous measurement of in vivo chlorophyll concentration. Deep-Sea Res. 1966, 13, 223.
- Legner, M. Phytoplankton quantity assessment by means of flow cytometry. Mar. Microb. Food Webs 1990, 4, 161.
- Sako, Y.; Hosoi-Tanabe, S.; Uchida, A. Fluorescence in situ hybridization using rrna-targeted probes for simple and rapid identification of the toxic dinoflagellates Alexandrium tamarense and Alexandrium catenella. J. Phycol. 2004, 40, 598–605.
- Miller, P.E.; Scholin, C.A. Identification and enumeration of cultured and wild Pseudo-nitzschia probes and filter-based whole cell hybridization. J. Phycol. 1998, 34, 371–382.
- Medlin, L.K.; Orozco, J. Molecular techniques for the detection of organisms in aquatic environments, with emphasis on harmful algal bloom species. Sensors 2017, 17, 1184.
- Yek, L.H.; Hii, K.S.; Tan, T.H.; Kon, N.F.; Lim, P.T.; Leaw, C.P. Rapid detection of toxic dinoflagellate, Alexandrium minutum (Dinophyceae) using fluorescence in situ hybridization (FISH). Malays. J. Sci. 2013, 32, 1–12.
- Chen, G.; Zhang, C.; Zhang, B.; Wang, G.; Lu, D.; Xu, Z.; Yan, P. Development of a PNA probe for fluorescence in situ hybridization detection of Prorocentrum donghaiense. PLoS ONE 2011, 6, e25527.
- Naresh, V.; Lee, N. Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109.
- Andryukov, B.G.; Besednova, N.N.; Romashko, R.V.; Zaporozhets, T.S.; Efimov, T.A. Label-Free Biosensors for Laboratory-Based Diagnostics of Infections: Current Achievements and New Trends. Biosensors 2020, 10, 11.
- Pilas, J.; Yazici, Y.; Selmer, T.; Keusgen, M.; Schöning, M.J. Application of a Portable Multi-Analyte Biosensor for Organic Acid Determination in Silage. Sensors 2018, 18, 1470.
- Damborský, P.; Švitel, J.; Katrlík, J. Optical biosensors. Essays Biochem. 2016, 60, 91–100.
- Wu, Q.; Zhang, Y.; Yang, Q.; Yuan, N.; Zhang, W. Review of Electrochemical DNA Biosensors for Detecting Food Borne Pathogens. Sensors 2019, 19, 4916.
- Hosu, O.; Selvolini, G.; Cristea, C.; Marrazza, G. Electrochemical Immunosensors for Disease Detection and Diagnosis. Curr. Med. Chem. 2018, 25, 4119–4137.
- Sokolov, A.N.; Roberts, M.E.; Bao, Z. Fabrication Of Low-Cost Electronic Biosensors. Mater. Today 2009, 12, 12–20.
- da Silva, T.S.G.; Souto, E.P.; Barragan, T.C.; Giarola, D.F.; de Moraes, A.C.M.; Kubota, L.T. Electrochemical Biosensors in Point-of-Care Devices: Recent Advances and Future Trends. Chemelectrochem 2017, 4, 778–794.
- Wojciechowski, M.F.; Sanderson, M.J.; Hu, J.M. Evidence on the monophyly of Astragalus (Fabaceae) and its major subgroups based on nuclear ribosomal DNA ITS and chloroplast DNA trnL intron data. Syst. Bot. 1999, 24, 409–437.
- Lagier, M.J.; Fell, J.W.; Goodwin, K.D. Electrochemical detection of harmful algae and other microbial contaminants in coastal waters using hand-held biosensors. Mar. Pollut. Bull. 2007, 54, 757–770.
- Metfies, K.; Huljic, S.; Lange, M.; Medlin, L.K. Electrochemical detection of the toxic dinoflagellate Alexandrium ostenfeldii with a DNA-biosensor. Biosens. Bioelectron. 2005, 20, 1349–1357.
- Orozco, J.; Baudart, J.; Medlin, L.K. Evaluation of probe orientation and effect of the digoxigenin-enzymatic label in a sandwich hybridization format to develop toxic algae biosensors. Harmful Algae 2011, 10, 489–494.
- Bratcher, A.R.; Connell, L.B. The Use of Peptide Nucleic Acids in Surface Plasmon Resonance for Detection of Red Tide Algae. In Molecular Biological Technologies for Ocean Sensing; Humana Press: Totova, NJ, USA, 2012.
- Lucarelli, F.; Tombelli, S.; Minunni, M.; Marrazza, G.; Mascini, M. Electrochemical and piezoelectric DNA biosensors for hybridisation detection. Anal. Chim. Acta 2008, 609, 139–159.
- Mannelli, I.; Lecerf, L.; Guerrouache, M.; Goossens, M.; Millot, M.C.; Canva, M. DNA immobilisation procedures for surface plasmon resonance imaging (SPRI) based microarray systems. Biosens. Bioelectron. 2007, 22, 803–809.
- Liao, J.; Mastali, M.; Li, Y.; Gau, V. Development of an Advanced Electrochemical DNA Biosensor for Bacterial Pathogen Detection. J. Mol. Diagn. 2007, 9, 158–168.
- Fowler, J.M.; Stuart, M.C.; Wong, D.K. Self-assembled layer of thiolated protein G as an immunosensor scaffold. Anal. Chem. 2007, 79, 350–354.
- Aydin, M.; Aydin, E.B.; Sezginturk, M.K. Advances in immunosensor technology. Adv. Clin. Chem. 2020, 102, 1–62.
- Oloketuyi, S.; Mazzega, E.; Zavasnik, J.; Pungjunun, K.; Kalcher, K.; Marco, A.D.; Mehmeti, E. Electrochemical immunosensor functionalized with nanobodies for the detection of the toxic microalgae Alexandrium minutum using glassy carbon electrode modified with gold nanoparticles. Biosens. Bioelectron. 2020, 154, 112052.
- Morgan, C.L.; Newman, D.J.; Price, C. Immunosensors: Technology and opportunities in laboratory medicine. Clin. Chem. 1996, 42, 193–209.
- Stauffer, B.A.; Bowers, H.; Buckley, E.; Davis, T.W. Considerations in Harmful Algal Bloom Research and Monitoring: Perspectives From a Consensus-Building Workshop and Technology Testing. Front. Mar. Sci. 2019, 6, 1–12.
- Lei, Y.; Chen, W.; Mulchandani, A. Microbial biosensors. Anal. Chim. Acta 2006, 568, 200–210.
- Leiner, M.J.; Hartmann, P. Theory and practice in optical pH sensing. Sens. Actuators B-Chem. 1993, 11, 281–289.
