Brain tissue contains the highest number of perivascular pericytes compared to other organs. Pericytes are known to regulate brain perfusion and to play an important role within the neurovascular unit (NVU). The high phenotypic and functional plasticity of pericytes make this cell type a prime candidate to aid physiological adaptations but also propose pericytes as important modulators in diverse pathologies in the brain. This revisewarch highlights known phenotypes of pericytes in the brain, discusses the diverse markers for brain pericytes, and reviews current in-vitro and in-vivo experimental models to study pericyte function.
- brain
- pericytes
- NVU
- pericyte markers
- breast-to-brain metastasis
- pericyte study models
1. Breast cancer brain metastasis
Breast cancer (BC) remains the leading cause of cancer death and disability-adjusted life-years in women [1] and approximately one in eight women will be diagnosed with breast cancer in their lifetime [2]. Despite significant improvements in diagnosis, treatment, and outcomes, about 6% of all BC cases are diagnosed at the metastatic stage with a poor 5-year survival rate of less than 27% [3]. BC metastasis to the central nervous system (CNS) constitutes a serious complication that is a strong clinical indicator of poor prognosis and can coincide with destructive neurologic complications [4]. CNS lesions comprise 13–30% of all BC metastases [5][6].
BCBM are more likely to occur in women with HER2-enriched BC (30-55%) and Triple-negative tumors (24-46%). [7][8]. In addition, patients with hormone receptor-negative BC were more likely to relapse in the brain in the first 5 years compared to hormone receptor+ BC tumors [9][10].
BCBM can occur in different CNS regions. Rostami et al. reported that 52.2% of BCBM were supratentorial and 24.1% infratentorial, and 14 % of patients had metastases at both locations [11]. Magnetic resonance imaging data suggest that the cerebellum (33%), frontal lobe (26%), and brain stem (5%) are the most common BCBM locations and multiple lesions were observed in 54.2% [11]. Together, these data emphasize a prominent role of the CNS environment for the successful establishment of BCBM lesions. However, little is known about the molecular mechanisms that regulate the formation of BC brain metastases and define the complex cellular microenvironment at BC brain metastatic sites.
2. Brain colonization of breast cancer cells
Brain metastatic colonization is a multistep process that starts at the primary tumor site and includes epithelial to mesenchymal transdifferentiation (EMT) to enable BC cells to detach from the primary BC tumor, penetrate extracellular matrices and invade neighboring tissues. The invasion of blood and lymph vessels signals a major metastatic leap and provides a selective advantage for the evolution of a heterogeneous group of tumor cells that have developed strategies to survive as circulating tumor cells (CTC) in the blood and lymph and, crucially, have the ability to colonize distant organs [12]. The establishment of brain metastatic sites is particularly challenging for CTC and is known to take longer than the colonization of other distant sites such as the lungs or the bones. It requires CTC capable of transitioning through a tightly controlled Blood-Brain-Barrier (BBB) to gain access to the brain microenvironment with its unique extracellular matrix, metabolic and immunological challenges. BC brain metastasizing cells were shown to reside in brain capillaries in close contact with endothelial cells for up to 7 days [13]. The successful completion of this treacherous journey results in the formation of micro-metastatic BCBM lesions that have the potential to progress to macroscopic secondary tumors [14][15].
3. Breast cancer brain metastatic cells and the neurovascular unit (NVU)
The neurovascular unit (NVU) is an anatomically complex functional brain microenvironment that the brain metastatic BC cells encounter first when entering the brain vasculature (Fig. 1). This NVU is a unique vascular niche composed of luminal brain microvascular endothelial cells (BMEC) on a basal lamina (BL) richly surrounded with pericytes on its abluminal side. These vascular structures form intimate contacts with cellular extensions of brain resident cells, including glial cells of astrocytic and oligodendroglial origin, neurons, and microglial cells to create a functional integrated neurovascular barrier critically important for normal brain functions.
Activation of both astrocytes and microglia coincides with the arrival and luminal attachment of CTC, suggesting a role for the NVU in sensing the non-resident (cancer) cells and act as a potential early warning system against metastatic brain colonization even prior to cancer cells crossing the BBB [13][16]. At the same time, the BBB is not just a physical barrier, it also acts as a selective transport interface, secretory body, and metabolic barrier [17].
This revisewarch focuses on pericyte populations at the NVU, their molecular markers, and the roles of these brain pericytes during BC cell colonization of the brain.
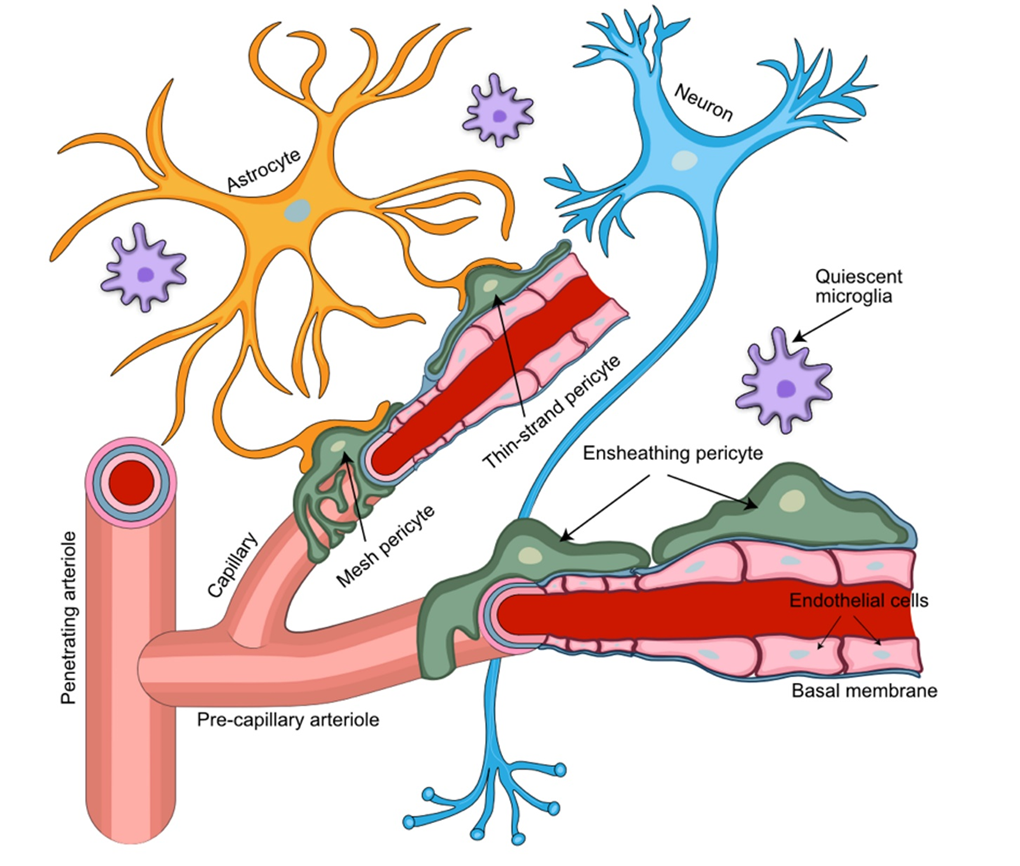
Figure 1. Perivascular arrangement of pericytes among NVU cellular structures. Pericytes extend their ramified processes along the brain vasculature, coming into direct contact with the vascular endothelium and astrocytic end-feet. Pericytes embedded in the capillary basement membrane, together with the endothelial cells of the capillary wall and astrocytic end-feet, form the blood-brain barrier. Different types of pericytes are found in distinct locations of the NVU. Ensheathing pericytes occupy mostly pre-capillary arterioles, while mesh and thin-strand pericytes are found mainly at the capillary portion of the brain microvasculature.
4. Pericytes provide key functional support for the NVU
Pericytes were first identified in 1873 when Charles-Marie Benjamin Rouget described a population of contractile cells surrounding the endothelial cells (EC) of small blood vessels. Originally cells were called “Rouget cells”, but later Zimmermann termed these cells ‘‘pericytes’’ (PC) as this name best describes their location around capillary vessels [18]. The vasculature in the human CNS has the highest density of pericyte coverage, with a 1:1–3:1 EC to pericyte ratio [19]. Brain pericytes are responsible for the integration of endothelial functions with glial/astrocyte functions at the NVU [20], the regulation of cerebral blood flow [21], angiogenesis [22], BBB formation and integrity [23], neuroinflammation [24], and stem cell activity [25].
Pericytes regulate the BBB by reducing the expression of endothelial genes involved with transendothelial permeability and promote astrocyte end-feet polarization [18][19][26]. Crosstalk of pericytes with EC during early vasculogenesis aids in vascular cell development and maturation. The anti-angiogenic potential of pericytes helps with proper stabilization of blood vessels by reducing the proliferation and migration of endothelial cells [27]. PDGFRβ and pericyte-deficient mice exhibit BBB breakdown allowing perivascular IgG accumulation in the hippocampus and cortex in an age-dependent manner [28]. This points to an important function of the pericyte receptor PDGFRβ in the brain endothelial cell differentiation and function.
Pericytes also have critical roles in tumor vasculature. In fibrosarcoma and osteosarcoma mouse models, pericytes were shown to display aberrant PDGF signaling and, similar to mice lacking PDGF-B and its receptor, this coincides with increased blood vessel diameters and reduced endothelial cell junctional circumference in the tumor vasculature [29][30].
Pericytes have stem cell ability and can differentiate into multiple types of mesenchymal precursor cells: fibroblasts, osteoblasts, chondroblasts, adipocytes, vascular smooth muscle cells, and skeletal muscle cell precursors. These cells express stem cell markers such as CD44, CD73, CD90, CD105 [31]. Pericytes in the brain were also shown to have multilineage differentiation potential in vitro capable of differentiating into neural and vascular cells under hypoxic conditions [32]. This identifies the pericyte population as a source of regeneration and plasticity for functional gain of the NVU.
5. Pericyte markers
Improper identification and frequent mix-ups with adjacent cell types, e.g., vascular smooth muscle cells (vSMCs) and juxtavascular microglia, have resulted in conflicting data being published [33]. Hence, the identification of specific pericytic markers is fundamental in better understanding the role of pericytes in normal and tumor environments, including BCBM. Pericytes can be definitively identified by electron microscopy (EM) features such as perivascular location with extensions of their slender ramified cytoplasmic processes along the capillary, an oval nuclear shape with high nucleus-to-cytoplasm ratio, and poorly developed cell organelles [34]. However, such morphological EM studies only provide very limited functional information and are not suitable for the selective isolation of defined populations of microvascular pericytes [35]. The diversity of pericytic phenotypes, the lack of a definitive pan-pericyte marker, and the differences in expression profiles of pericyte-associated genes in isolated pericytes versus in vivo samples are major challenges [27][36].
The markers commonly used to identify brain pericytes include PDGFR-β [37], membrane alanyl aminopeptidase (CD13) [38], alpha-smooth muscle actin (αSMA) [39], neuron-glial antigen 2 (NG2) [40], melanoma cell adhesion molecule (MCAM or CD146) [41] and desmin [42]. The level of expression of a pericyte marker may also fluctuate as these multipotent cells possess self-renewing potential and display high phenotypic plasticity [43]. Hence, a state-of-the-art approach to identify pericytes in tissues must rely on a combination of tissue morphology, counter-labeling of EC and vSMC, and simultaneous staining for two or more pericyte markers [18]. Moreover, accurate identification of pericytes in vitro should also consider origin and co-culture conditions as these cells tend to rapidly differentiate along multiple lineages depending on prevailing regulatory signals introduced with the specific culture conditions and the cellular microenvironment [44].
6. The NVU contains distinct pericyte populations
Several distinct types of pericytes have been described based upon the morphological appearance of their processes. This includes ensheathing, mesh, and thin-strand pericytes (Fig.2) [45]. The ensheathing pericytes cover mostly the pre-capillary portion of brain microvasculature, possess properties of both vSMCs and pericytes, and are considered a transitional form. The mesh and thin-strand pericytes of the capillary bed may be referred to as genuine capillary cells. Confocal imaging of thick coronal mouse brain sections has shown that only ensheathing pericytes demonstrate the level of αSMA expression comparable to vSMC, while αSMA expression was undetectable in both capillary mesh and thin-strand pericytes [36]. Similarly, prominent αSMA staining was exclusively found on relatively large precapillary arterioles [46]. By contrast, nearly all cultured pericytes started to express αSMA by Day 7, which suggests differentiation of pericytes towards an ensheathing subtype in culture in the presence of a serum-containing medium [47]. In spite of the debates on the extent of differences between pericyte subpopulations [21][48], these in-vitro data cast doubt on αSMA as a marker for the in vivo identification of true capillary pericytes.
The expression of CD146, also referred to as melanoma cell adhesion molecule (MCAM) or cell surface glycoprotein MUC18, was shown to be primarily confined to vSMC. High αSMA expression in CD146+ cells in-vitro suggests that CD146 identifies a subpopulations of vSMC, but a role of CD146 as a pericyte marker is questionable [46]. Pericyte expression of NG2 also proved to be inconsistent. While one study suggested that 50-80% of isolated PDGFRβ+ cells were also NG2+ [49], another study demonstrated that prominent NG2 in-situ staining was shown only in cases of focal cortical dysplasia but not in control tissues [50]. These data correlate with weak NG2 staining in human brain pericytes. NG2 is regarded as a plasticity marker with strongest expression at early stages of tissue development but declining later in ontogenesis [51][52]. NG2 might not be an appropriate target for the identification of quiescent human brain pericytes [53]. CD13 has been reported to have high specificity for capillary pericytes [20][38][45][46][54]. In addition, exclusive vascular staining of PDGFRβ may also serve as suitable indicator for the presence of pericytes [20][46][55][56]. Collectively, the most reliable identification of brain pericytes under normal tissue conditions is based on a combination of parameters, including the double-positive staining for CD13 and PDGFRβ, the characterization of “bump-on-a-log” morphology with patterns for cell processes, and the selective counterstaining of vSMCs, EC and astrocytes.
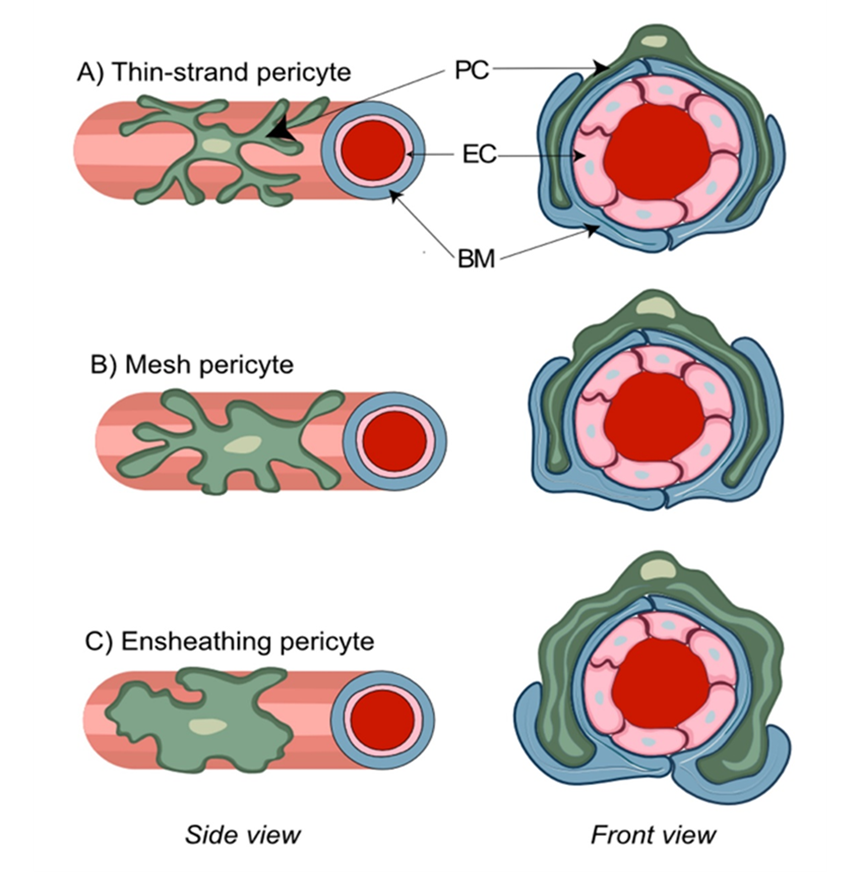
Figure 2. Morphological features of distinct pericyte (PC) subtypes. PC are characterized by a typical “bump on a log” appearance arising from an ovoid protruding nucleus and extending cytoplasmic processes that contact the abluminal side of the vascular basal membrane (BM) and run inside of its duplicature. Thin-strand pericytes (A) possess relatively long thin cytoplasmic processes with a complex branching pattern that sparsely cover brain capillaries. Mesh pericytes (B) have a less complex branching pattern and their thicker and shorter processes provide more comprehensive coverage of brain capillaries. Ensheathing pericytes (C) are characterized by the least complex branching pattern and almost complete coverage of pre-capillary arterioles.
7. The NVU in breast-to-brain metastasis
Among the most common and adverse scenarios associated with BBB disruption are intracranial metastatic lesions [57]. The establishment of brain metastasis is a multistep process eventually leading to successful formation of brain macro-metastases (Fig. 3A). The presence of pre-existing blood vessels for vascular co-option and the close physical contact with the abluminal surface of the blood vessels are critical prerequisites for the survival of cancer cells in perivascular metastatic loci. Importantly, tumor cells have the ability to recruit pericytes to promote angiogenesis and, at the same time, displacing them from their initial vascular niche to enhance leakiness of blood vessels [58]. The underlying mechanism is primarily dependent on paracrine PDGFRβ/ PDGF-BB signaling [59], while pro-angiogenic factors, such as hypoxia-induced factor 1α (HIF1α) and vascular endothelial growth factor (VEGF), determine both vessel co-option and angiogenesis [60][61] (Fig.3B). Enhanced stem cell properties and phenotypical plasticity of pericytes emerge as additional mechanisms by which pericytes may prepare the BC brain metastatic vascular niche to promote metastatic progression. The ability of pericytes to give rise to cancer-associated fibroblasts that promote tumor growth and dissemination was demonstrated in ovarian cancer [62].
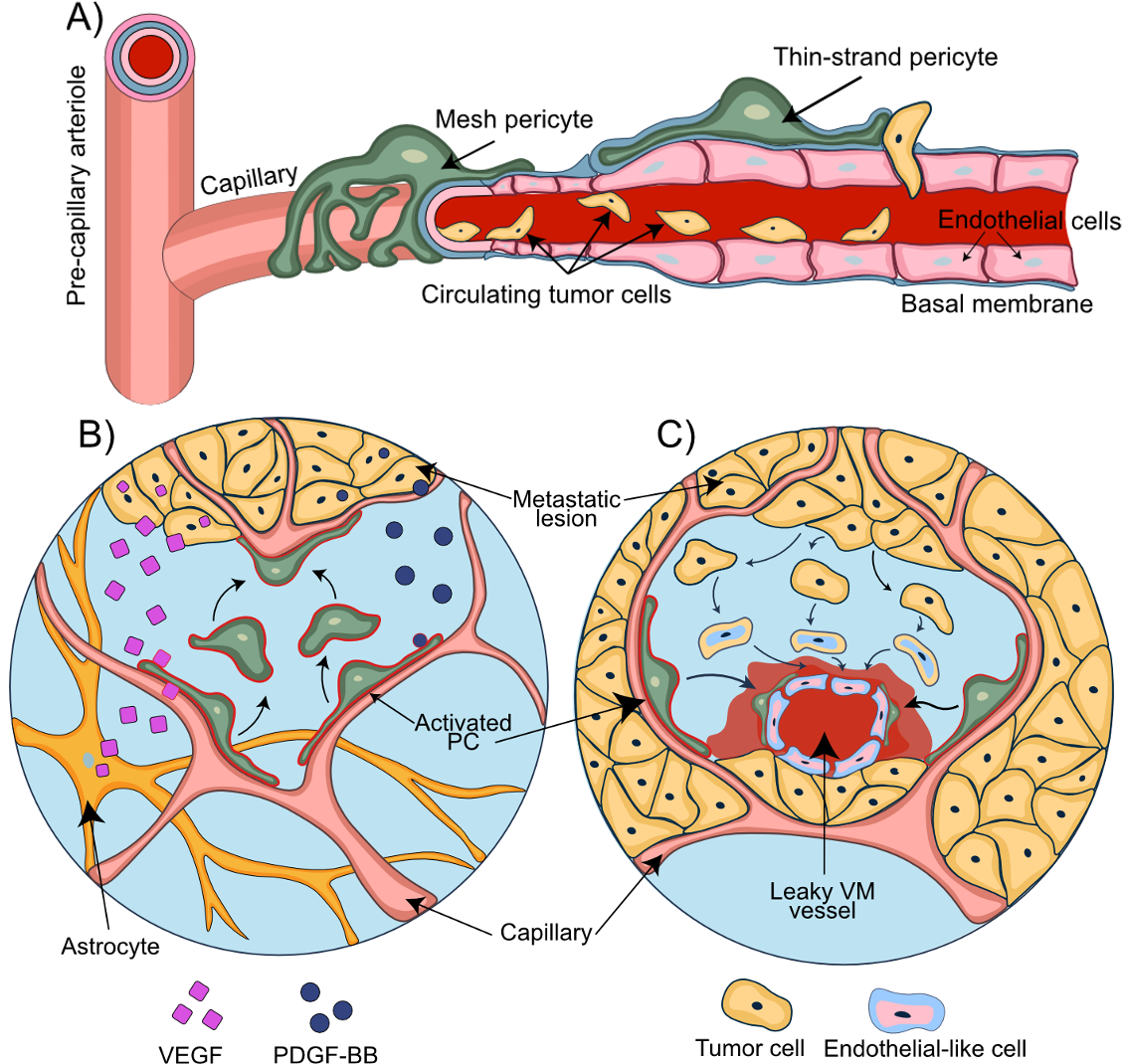
Figure 3. Pericytes support the disruption of the NVU during metastasis. A) A consecutive process of rolling, adhesion, and transendothelial migration of circulating tumor cells results in the extravasation of the metastatic breast cancer cells. B) Tumor and astrocyte secreted VEGF contributes to vessel co-option. PDGF-BB secreted by the metastatic tumor cells mediates displacement of pericytes and their relocation towards the co-opted and newly formed blood vessels. C) Transformation of the tumor cells into endothelial-like cells allows the formation of the vascular mimicry vessels. Pericytes migrate towards these newly formed leaky vascular mimicry vessels and stabilize them.
8. Role of pericytes in the establishment of metastatic lesions
The initial steps of BC cell brain colonization have been described: (1) arrest of BC CTCs in brain capillaries, (2) BC cell passage through the BBB, (3) extravasation of BC cells from capillaries that are surrounded by PDGFRβ+ pericytes, and (4) initial growth of extravasated BC cells in the perivascular niche [13][63] (Fig.4). Upon successful extravasation and growth initiation, the fate of the metastatic cells is determined by the tissue milieu and cellular communication networks at the NVU niche [64].
The intravascular arrest of BC cells at the NVU includes cell-cell interactions of metastatic BC with microvascular EC and this is sufficient to activate astrocytes. Upon extravasation, the formation of micro-metastatic lesions coincides with the appearance of activated astrocytes that accumulate both around and inside of the metastatic foci and form direct contacts with tumor cells [65]. The role of activated astrocytes in this brain metastatic process is largely unknown but may include supportive functions for nutrient transport, ion trafficking across the ECM, and neuronal signaling [13]. Co-culture experiments with astrocytes have demonstrated the ability of astrocyte-derived factors to induce a migratory response in BC cells [16]. Activated astrocytes can secrete potentially oncogenic factors like interleukin 6 (IL-6), transforming growth factor beta (TGFb) and matrix metallopeptidase 9 (MMP-9) [66][67][68]. The astrocyte-derived secretion of the Erb-B2 Receptor Tyrosine Kinase 3 (ErbB3, HER3) ligand neuregulin-1 (NRG-1) induces the proliferation, invasion, and BBB transmigration in Erb-B2 Receptor Tyrosine Kinase 2 (ErbB2, HER2)-positive BC cells [69][70]. BCBM lesions were shown to have higher HER3 activity compared to the corresponding BC primary tumors [71], suggesting HER3 signaling may support metastatic growth. Activated microglia utilize Wnt signaling to promote the invasion and colonization potential of BC cells in brain metastatic lesions [72].
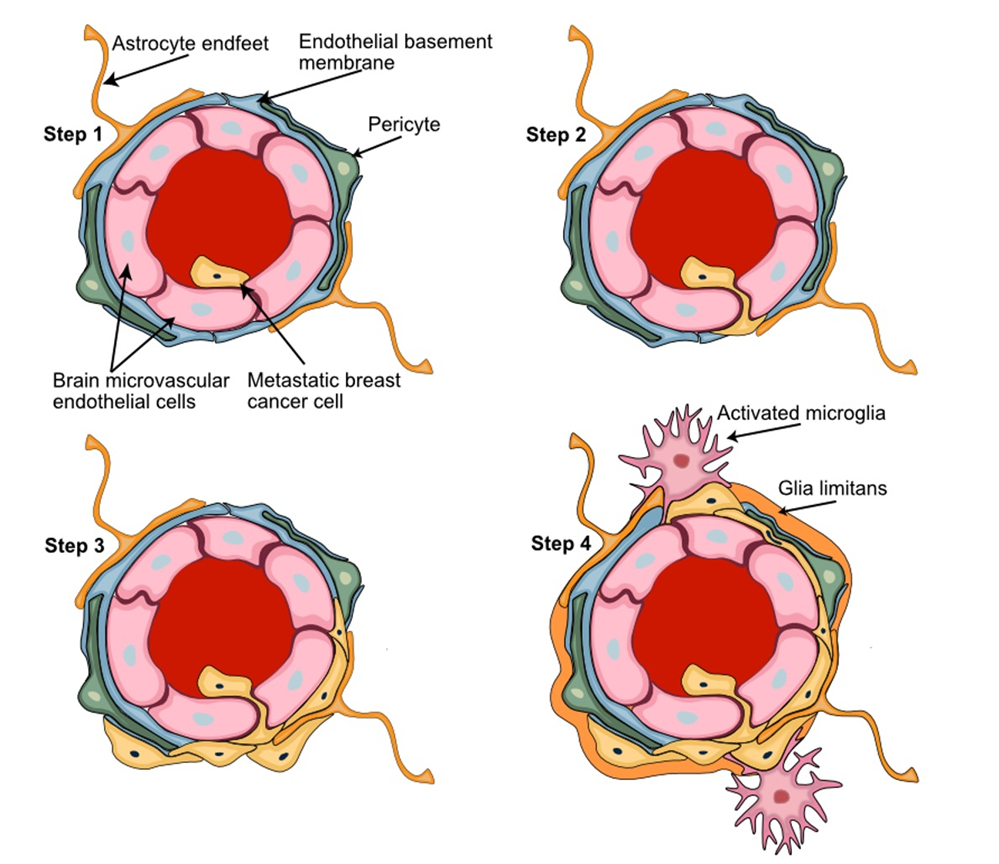
Figure 4. Key steps of the breast-to-brain metastatic process. Step 1) Adhesion of the metastatic circulating breast cancer cells to the brain microvascular endothelial cells; Step 2) BBB passage of metastatic cells leaving the circulation by transendothelial migration; Step 3) Adaptation and proliferation of the metastatic cells in their new perivascular niche; Step 4) Establishment of the tumor microenvironment with metastatic breast cancer cells interacting with the key NVU cellular partners pericytes, astrocytes, and microglia. Astrocyte foot processes associated with the parenchymal basal lamina form the outermost layer of central nervous tissue known as Glia limitans.
9. Pericytes and endoplasmatic reticulum (ER) stress – an emerging science
Imbalances in the cellular homeostasis may generate cell stress from aberrant protein folding which leads to ER stress. This causes the activation of the Unfolded Protein Response (UPR) as an adaptive cellular response aimed at restoring homeostasis and cell survival [73]. ER stress may also affect pericyte viability and functions. Cultured retinal pericytes subjected to glucose deprivation or intermittent glucose reduction activate the ER transmembrane protein kinase PERK, which induces autophagy and the expression of VEGF-A and pro-inflammatory monocyte chemoattractant protein-1 (MCP1) [74][75]. Pericytes undergo regulated apoptosis resulting from ER stress-induced by hypoglycemia or fluctuating glucose levels [74][76]. In glioblastoma brain tumor models, the activation of chaperone-mediated autophagy causes brain pericytes to release anti-inflammatory cytokines, such as TGF-β or IL-10, to block anti-tumor immune responses and, instead, promote brain tumor survival [77][78]. Recently, the transmission of ER stress from one cell to another cell was described as a process by which tumor cell-derived extracellular vesicles laden with functional proteins can initiate ER stress in other cells, such as macrophages, brain resident cells, and likely pericytes. This vesicular cargo can contain upstream regulators of UPR that activate UPR stress-related immunosuppressive responses to promote tumor cell survival, metastasis, and angiogenesis [75]. Intercellular transmissible ER stress (TERS) under metabolic stress was also shown to occur in hepatocytes through direct cell-cell contacts [79]. Information is currently lacking on adaptive ER stress responses between metastasizing cancer cells and perivascular pericytes which influence the metastatic process. Emerging TERS research is likely to make ground-breaking discoveries on the multifaceted roles of pericytes in TERS at the NVU and in BCBM as well.
10. Pericytes and metastatic invasion patterns
BM can be distinguished on the basis of their invasion patterns. Berghoff et al. proposed to categorize all metastatic brain lesions into 3 distinct types: well-demarcated, vascular co-option, and diffuse [80]. Another classification was later suggested by Teglasi et al. who distinguished pushing-type, papillary-type, and diffuse invasion patterns [81]. Primary tumors of different origins were shown to form metastatic lesions of distinct morphologies. Breast carcinoma metastases predominantly produced papillary-type metastases (Fig.5A), while pushing- (Fig.5B) and mixed phenotypes were distinctive for colon and lung carcinoma metastases. A prominent feature of pushing-type metastases was the formation of a multicellular PDGFRβ+ pericyte layer embedded within a thickened vascular basement membrane. By contrast, thickening of the pericyte layer was uncommon in papillary-type metastases and restricted to vessels at the metastatic site.
Metastatic growth was accompanied by an increased pericytic expression of Serpin H1 (an enzyme involved in collagen biosynthesis) and αSMA which coincided with elevated levels of collagen in the vessel walls [81]. Hence, pericytes possess the capability to differentiate into other cell types and may be viewed as the main source of the connective tissue in human parenchymal BM.
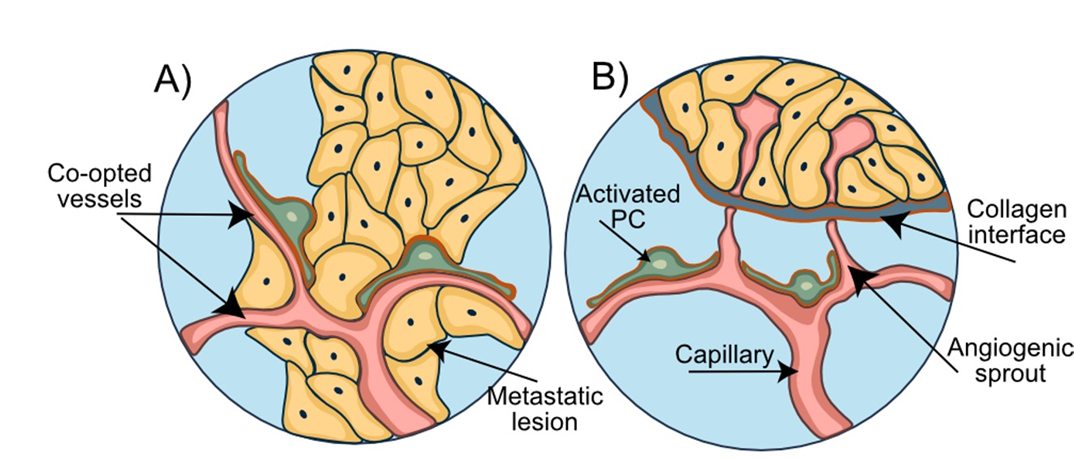
Figure 5. Invasion patterns of breast-to-brain metastasis. A) Breast-to-brain metastases are of the papillary type and characterized by the abundant vessel co-option and close direct contacts between the breast cancer cells, blood vessels, and pericytes. B) Brain metastases of the pushing type, originating for example from colon cancer, show a distinct collagen layer separating the metastatic lesion from the capillaries and surrounding pericytes.
11. In-vitro and in-vivo models in pericyte research
11.1. Pericytes co-culture models
Replicating the functions of pericytes for a functional BBB requires culture conditions which reflect the proper cellular and spatial organization of the NVU. Transwell inserts are most commonly used for in-vitro models of the BBB [82] for studying cell-cell interactions that affect barrier functions [83][84][85] as well as cancer cell migration across the endothelium [86]. (Fig.6).
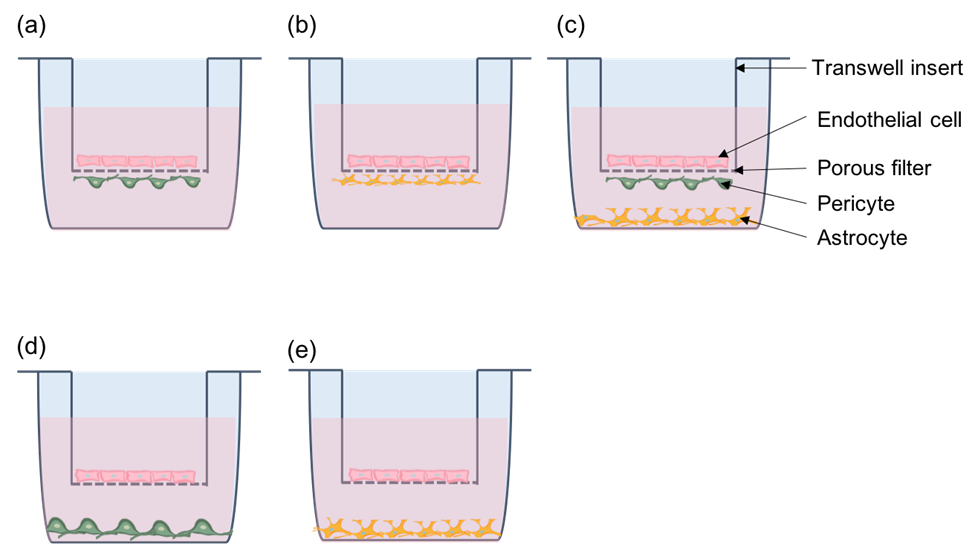
Figure 6. Transwell system for co-culture. (a) Direct contact model with endothelial cells and pericytes. (b) Direct contact model with endothelial cells and astrocytes. (c) Tri-cellular culture model with endothelial cells, pericytes, and astrocytes. Endothelial cells and pericytes are in contact. (d) Non-contact model with endothelial cells and pericytes. (e) Non-contact model with endothelial cells and astrocytes.
11.2. Microfluidics – dynamic in-vitro modeling of the brain microvasculature
Transwell systems are limited by the large fluid-to-cell volume ratio and lack of flow which is a critical parameter in blood vessels and a major contributor of shear stress in EC [87]. Microfluidic devices can overcome these drawbacks by providing a highly controlled platform capable of better mimicking the in vivo microenvironment [88] (Fig.7). Microfluidic devices are promising BBB model systems that provide the in vivo microenvironment more closely and have the potential to be adapted for high-throughput clinical studies [89]. Microfluidic organ-on-a-chip assays are emerging which study intercellular communication in “brain-on–chip” modeled tissue- and organ-specific cellular compartments and are summarized elsewhere [90]. These multicellular brain-on-chip in-vitro models combine capillary flow with multi-cellular brain compartments and allow to monitor cell proliferation and migration in the context of relevant cell connections [91] as well as cell responses from paracrine signaling between compartments [92]. Table 1 summarizes in-vitro models for the BBB.
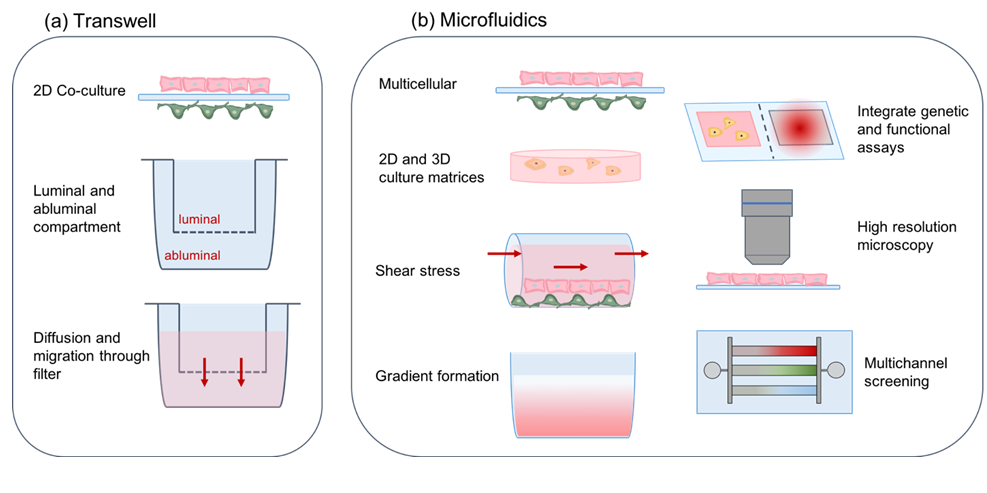
Figure 7. Properties of BBB in-vitro models. (a) Transwell systems utilize a porous filter insert that divides culture wells into luminal and abluminal compartments and allows for diffusion of molecules and cell migration across the filter. (b) Microfluidic devices recapitulate a more complex tissue environment in-vitro by co-culturing cells in 2D and 3D matrices. Parameters including shear stress and gradient formation are tunable to establish a controlled microenvironment. Microfluidics enable integration of sensing systems and high resolution microscopy. Multichannel designs allow samples to be screened in parallel, creating compact medium-throughput systems for simulating biological conditions and enabling drug screening. Brain EC are shown in pink on the luminal side and brain pericytes are marked in green on the abluminal side.
11.3. In-vivo monitoring of pericytes
The high variability in the temporal and spatial occurance of hematogenic brain metastasis imposes great challenges for in-vivo tracing and monitoring of pericyte functions during the initiation and formation of brain metastases. A summary of suitable pericyte in-vivo models is shown in Table 2.
12. Conclusions
A better understanding of the interactions between BC and the NVU as well as the BCBM tumor microenvironment is critical in advancing effective treatments against fatal brain metastases. Newly emerging in-vivo molecular imaging and tissue profiling technologies are expected to reveal detailed gene and/or protein expression patterns at high contextual and spatiotemporal resolution. This will excel outhe researchers' understanding of the diversity and functional roles of pericyte populations in normal and diseased tissues, including the NVU and brain metastases. Putting these spatial tissue data to the test in sophisticated in-vitro devices as well as genetic and tumor animal models will contribute major advances to outhe researchers' understanding of BCBM and be instrumental in the development of new and more efficacious treatment options for breast cancer and other cancer patients with brain metastasis.
|
Table.1: Summary of in-vitro BBB models |
|||
|
Model |
Species |
Cells in co-culture |
Reference |
|
Transwell |
Rat |
Primary cerebral pericytes Primary brain capillary EC Primary cerebral astrocytes |
Nakagawa et al, 2009 |
|
Transwell |
Porcine |
Primary brain EC Primary astrocytes |
Malina et al, 2009 |
|
Transwell |
Porcine |
Primary brain capillary pericytes Primary brain capillary EC |
Thanabalasundaram et al, 2010 |
|
Transwell |
Porcine |
Primary cerebral pericytes Primary brain EC Primary astrocytes |
Thomsen et al, 2015 |
|
Transwell |
Human |
Primary brain vascular pericytes Primary cerebral microvascular EC Cerebral astrocytes |
Hatherell et al, 2011 |
|
Transwell |
Human |
Primary fetal brain pericytes hPSC-derived brain microvascular EC Differentiated neural progenitor cells |
Lippman et al, 2014 |
|
Transwell |
Human |
Primary brain pericytes hPSC-derived pericytes hPSC-derived neural crest stem cells iPSC-derived brain microvascular EC |
Stebbins et al, 2019 |
|
Microfluidic |
Mixed |
iPSC-derived human brain microvascular EC Primary rat astrocytes |
Wang et al, 2016 |
|
Microfluidic |
Human |
Primary brain pericytes iPSC-derived EC Primary brain astrocytes |
Campisi et al, 2018 |
|
Microfluidic |
Human |
Primary placental pericytes Primary brain microvascular EC Primary umbilical vein EC Primary astrocytes |
Lee et al, 2019 |
|
Microfluidic |
Human |
Primary brain pericytes iPSC-derived brain microvascular EC Primary astrocytes |
Park et al, 2019 |
|
Microfluidic |
Human |
Primary brain pericytes iPSC-derived brain microvascular EC Primary astrocytes |
Noorani et al, 2021 |
|
Brain-on-chip |
Human |
Primary brain pericytes, vascular endothelial cells and astrocytes; Human neurons differentiated from neuronal stem cells |
Maoz et al, 2018 |
|
Brain-on-chip |
Human |
Primary brain pericytes, vascular endothelial cells and astrocytes; iPSC-derived cortical neurons and astrocytes |
Brown et al, 2016 |
|
Brain-on-chip |
Human |
Different combinations of human brain and NVU cells in review |
Saliba et al., 2018 |
|
Table.2: Summary of in-vivo models to study pericyte function |
| Model |
|---|
|
Model |
Species |
PC vizualization |
Reference |
|
Cranial window |
Mouse |
Transgenic mice; αSMA promoter to label ensheathing pericytes; 2-Photon microscopy |
Meza-Resillas et al., 2021 |
|
Transcranial imaging |
Mouse |
Neurotrace™ labeling of pericytes; Multimodal optical transcranial imaging |
Arango-Lievano et al, 2020 |
|
Whole brain imaging |
Zebrafish |
Transgenic zebrafish; confocal microscopy |
Bahrami et al, 2018 |
|
Whole mount imaging |
Zebrafish |
pdgfrb promoter transgenic zebrafish; confocal upright fluorescence imaging |
Ando et al., 2016 |
|
Cranial window |
Mouse |
αSMA-mCherry transgenic reporter mice; NeuroTrace 500/525 and TO-PRO-3 PC labeling; optogenetic manipulation of PC in rhodopsin transgenic mice |
Tong at al., 2021 |
| Species | Cells in Co-Culture | Reference | |
|---|---|---|---|
| Transwell | Rat | Primary cerebral pericytes Primary brain capillary EC Primary cerebral astrocytes |
Nakagawa et al., 2009 |
| Transwell | Porcine | Primary brain EC Primary astrocytes |
Malina et al., 2009 |
| Transwell | Porcine | Primary brain capillary pericytes Primary brain capillary EC |
Thanabalasundaram et al., 2010 |
| Transwell | Porcine | Primary cerebral pericytes Primary brain EC Primary astrocytes |
Thomsen et al., 2015 |
| Transwell | Human | Primary brain vascular pericytes Primary cerebral microvascular EC Cerebral astrocytes |
Hatherell et al., 2011 |
| Transwell | Human | Primary fetal brain pericytes hPSC-derived brain microvascular EC Differentiated neural progenitor cells |
Lippman et al., 2014 |
| Transwell | Human | Primary brain pericytes hPSC-derived pericytes hPSC-derived neural crest stem cells iPSC-derived brain microvascular EC |
Stebbins et al., 2019 |
| Microfluidic | Mixed | iPSC-derived human brain microvascular EC Primary rat astrocytes |
Wang et al., 2016 |
| Microfluidic | Human | Primary brain pericytes iPSC-derived EC Primary brain astrocytes |
Campisi et al., 2018 |
| Microfluidic | Human | Primary placental pericytes Primary brain microvascular EC Primary umbilical vein EC Primary astrocytes |
Lee et al., 2019 |
| Microfluidic | Human | Primary brain pericytes iPSC-derived brain microvascular EC Primary astrocytes |
Park et al., 2019 |
| Microfluidic | Human | Primary brain pericytes iPSC-derived brain microvascular EC Primary astrocytes |
Noorani et al., 2021 |
| Brain-on-chip | Human | Primary brain pericytes, vascular endothelial cells and astrocytes Human neurons differentiated from neuronal stem cells |
Maoz et al., 2018 |
| Brain-on-chip | Human | Primary brain pericytes, vascular endothelial cells and astrocytes iPSC-derived cortical neurons and astrocytes |
Brown et al., 2016 |
| Brain-on-chip | Human | Different combinations of human brain and NVU cells in review | Saliba et al., 2018 |
| Model | Species | PC Visualization | Reference |
|---|---|---|---|
| Cranial window | Mouse | Transgenic mice; αSMA promoter to label ensheathing pericytes; 2-Photon microscopy | Meza-Resillas et al., 2021 |
| Transcranial imaging | Mouse | Neurotrace™ labeling of pericytes; multimodal optical transcranial imaging | Arango-Lievano et al., 2020 |
| Whole brain imaging | Zebrafish | Transgenic zebrafish; confocal microscopy | Bahrami et al., 2018 |
| Whole mount imaging | Zebrafish | pdgfrb promoter transgenic zebrafish; confocal upright fluorescence imaging | Ando et al., 2016 |
| Cranial window | Mouse | αSMA-mCherry transgenic reporter mice; NeuroTrace 500/525 and TO-PRO-3 PC labeling; optogenetic manipulation of PC in rhodopsin transgenic mice | Tong at al., 2021 |
References
- Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; Abdulle, A.S.M.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. In JAMA Oncol; 2019; Volume 5, pp. 1749-1768.
- DeSantis, C.; Ma, J.; Gaudet, M.; Newman, L.; Miller, K.; Goding Sauer, A.; Jemal, A.; Siegel, R. Breast cancer statistics, 2019. CA: a cancer journal for clinicians 2019, 69, doi:10.3322/caac.21583.
- Siegel, R.; Miller, K.; Jemal, A. Cancer statistics, 2018. CA: a cancer journal for clinicians 2018, 68, doi:10.3322/caac.21442.
- Giglio, P.; Gilbert, M. Neurologic complications of cancer and its treatment. Current oncology reports 2010, 12, doi:10.1007/s11912-009-0071-x.
- Nayak, L.; Lee, E.; Wen, P. Epidemiology of brain metastases. Current oncology reports 2012, 14, doi:10.1007/s11912-011-0203-y.
- Saha, A.; Ghosh, S.; Roy, C.; Choudhury, K.; Chakrabarty, B.; Sarkar, R. Demographic and clinical profile of patients with brain metastases: A retrospective study. Asian journal of neurosurgery 2013, 8, doi:10.4103/1793-5482.121688.
- Lin, N.; Amiri-Kordestani, L.; Palmieri, D.; Liewehr, D.; Steeg, P. CNS metastases in breast cancer: old challenge, new frontiers. Clinical cancer research : an official journal of the American Association for Cancer Research 2013, 19, doi:10.1158/1078-0432.CCR-13-0790.
- Lin, N.; Winer, E. Brain metastases: the HER2 paradigm. Clinical cancer research : an official journal of the American Association for Cancer Research 2007, 13, doi:10.1158/1078-0432.CCR-06-2478.
- Prat, A.; Pineda, E.; Adamo, B.; Galvan, P.; Fernandez, A.; Gaba, L.; Diez, M.; Viladot, M.; Arance, A.; Munoz, M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast (Edinburgh, Scotland) 2015, 24 Suppl 2, doi:10.1016/j.breast.2015.07.008.
- Darlix, A.; Louvel, G.; Fraisse, J.; Jacot, W.; Brain, E.; M, D.; Mouret-Reynier, M.; Goncalves, A.; Dalenc, F.; Delaloge, S.; et al. Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. British journal of cancer 2019, 121, doi:10.1038/s41416-019-0619-y.
- Rostami, R.; Mittal, S.; Rostami, P.; Tavassoli, F.; Jabbari, B. Brain metastasis in breast cancer: a comprehensive literature review. Journal of Neuro-Oncology 2016, 127, 407-414, doi:10.1007/s11060-016-2075-3.
- Nathanson, S.; Krag, D.; Kuerer, H.; Newman, L.; Brown, M.; Kerjaschki, D.; Pereira, E.; Padera, T. Breast cancer metastasis through the lympho-vascular system. Clinical & experimental metastasis 2018, 35, doi:10.1007/s10585-018-9902-1.
- Lorger, M.; Felding-Habermann, B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. The American journal of pathology 2010, 176, doi:10.2353/ajpath.2010.090838.
- Steeg, P. Tumor metastasis: mechanistic insights and clinical challenges. Nature medicine 2006, 12, doi:10.1038/nm1469.
- Custódio-Santos, T.; Videira, M.; Brito, M. Brain metastasization of breast cancer. Biochimica et biophysica acta. Reviews on cancer 2017, 1868, doi:10.1016/j.bbcan.2017.03.004.
- Wang, L.; Cossette, S.; Rarick, K.; Gershan, J.; Dwinell, M.; Harder, D.; Ramchandran, R. Astrocytes directly influence tumor cell invasion and metastasis in vivo. PloS one 2013, 8, doi:10.1371/journal.pone.0080933.
- Rhea, E.M.; Banks, W.A. Role of the Blood-Brain Barrier in Central Nervous System Insulin Resistance. Front. Neurosci. 2019, 13, doi:10.3389/fnins.2019.00521.
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, Physiological, and Pathological Perspectives, Problems, and Promises. Developmental Cell 2011, 21, 193-215, doi:10.1016/j.devcel.2011.07.001.
- Mathiisen, T.M.; Lehre, K.P.; Danbolt, N.C.; Ottersen, O.P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction. Glia 2010, 58, 1094-1103, doi:10.1002/glia.20990.
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes Regulate the Blood-Brain Barrier. Nature 2010, 468, doi:10.1038/nature09522.
- Hall, C.; Reynelln, B.; Hamilton, N.; Mishra, A.; Sutherland, B.; O'Farrell, F.; Buchan, A.; Lauritzen, M.; Attwell, D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014, 508, doi:10.1038/nature13165.
- Ribatti, D.; Nico, B.; Crivellato, E. The role of pericytes in angiogenesis. The International journal of developmental biology 2011, 55, doi:10.1387/ijdb.103167dr.
- Heymans, M.; Figueiredo, R.; Dehouck, L.; Francisco, D.; Sano, Y.; Shimizu, F.; Kanda, T.; Bruggmann, R.; Engelhardt, B.; Winter, P.; et al. Contribution of brain pericytes in blood-brain barrier formation and maintenance: a transcriptomic study of cocultured human endothelial cells derived from hematopoietic stem cells. Fluids and barriers of the CNS 2020, 17, doi:10.1186/s12987-020-00208-1.
- Rustenhoven, J.; Jansson, D.; Smyth, L.; Dragunow, M. Brain Pericytes As Mediators of Neuroinflammation. Trends in pharmacological sciences 2017, 38, doi:10.1016/j.tips.2016.12.001.
- Caporarello, N.; D'Angeli, F.; Cambria, M.; Candido, S.; Giallongo, C.; Salmeri, M.; Lombardo, C.; A, L.; Giurdanella, G.; Anfuso, C.; et al. Pericytes in Microvessels: From "Mural" Function to Brain and Retina Regeneration. International journal of molecular sciences 2019, 20, doi:10.3390/ijms20246351.
- ElAli, A.; Thériault, P.; Rivest, S. The Role of Pericytes in Neurovascular Unit Remodeling in Brain Disorders. International Journal of Molecular Sciences 2014, 15, 6453-6474, doi:10.3390/ijms15046453.
- Sweeney, M.; Ayyadurai, S.; Zlokovic, B. Pericytes of the Neurovascular Unit: Key Functions and Signaling Pathways. Nature neuroscience 2016, 19, doi:10.1038/nn.4288.
- Bell, R.D.; Winkler, E.A.; Sagare, A.P.; Singh, I.; Larue, B.; Deane, R.; Zlokovic, B.V. Pericytes Control Key Neurovascular Functions and Neuronal Phenotype in the Adult Brain and during Brain Aging. Neuron 2010, 68, 409-427, doi:10.1016/j.neuron.2010.09.043.
- Sattiraju, A.; Mintz, A. Pericytes in Glioblastomas: Multifaceted Role Within Tumor Microenvironments and Potential for Therapeutic Interventions. Adv Exp Med Biol 2019, 1147, 65-91, doi:10.1007/978-3-030-16908-4_2.
- Abramsson, A.; Berlin, O.; Papayan, H.; Paulin, D.; Shani, M.; Betsholtz, C. Analysis of mural cell recruitment to tumor vessels. Circulation 2002, 105, 112-117, doi:10.1161/hc0102.101437.
- Zhang, Z.-S.; Zhou, H.-N.; He, S.-S.; Xue, M.-Y.; Li, T.; Liu, L.-M. Research advances in pericyte function and their roles in diseases. Chin J Traumatol 2020, 23, 89-95, doi:10.1016/j.cjtee.2020.02.006.
- Nakagomi, T.; Kubo, S.; Nakano‐Doi, A.; Sakuma, R.; Lu, S.; Narita, A.; Kawahara, M.; Taguchi, A.; Matsuyama, T. Brain Vascular Pericytes Following Ischemia Have Multipotential Stem Cell Activity to Differentiate Into Neural and Vascular Lineage Cells. STEM CELLS 2015, 33, 1962-1974, doi:10.1002/stem.1977.
- Krueger, M.; Bechmann, I. CNS pericytes: concepts, misconceptions, and a way out. Glia 2010, 58, doi:10.1002/glia.20898.
- Jindatip, D.; Fujiwara, K.; Kouki, T.; Yashiro, T. Transmission and scanning electron microscopy study of the characteristics and morphology of pericytes and novel desmin-immunopositive perivascular cells before and after castration in rat anterior pituitary gland. Anatomical science international 2012, 87, doi:10.1007/s12565-012-0144-z.
- Birbrair, A. Pericyte Biology: Development, Homeostasis, and Disease. Advances in experimental medicine and biology 2018, 1109, doi:10.1007/978-3-030-02601-1_1.
- Grant, R.; Hartmann, D.; Underly, R.; Berthiaume, A.; Bhat, N.; Shih, A. Organizational hierarchy and structural diversity of microvascular pericytes in adult mouse cortex. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2017, 39, doi:10.1177/0271678X17732229.
- Winkler, E.A.; Bell, R.D.; Zlokovic, B.V. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol Neurodegener 2010, 5, 32, doi:10.1186/1750-1326-5-32.
- Alliot, F.; Rutin, J.; Leenen, P.; Pessac, B. Pericytes and periendothelial cells of brain parenchyma vessels co-express aminopeptidase N, aminopeptidase A, and nestin. Journal of neuroscience research 1999, 58.
- Bandopadhyay, R.; Orte, C.; Lawrenson, J.; Reid, A.; De Silva, S.; Allt, G. Contractile proteins in pericytes at the blood-brain and blood-retinal barriers. Journal of neurocytology 2001, 30, doi:10.1023/a:1011965307612.
- Ozerdem, U.; Grako, K.; Dahlin-Huppe, K.; Monosov, E.; Stallcup, W. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Developmental dynamics : an official publication of the American Association of Anatomists 2001, 222, doi:10.1002/dvdy.1200.
- Chen, J.; Luo, Y.; Hui, H.; Cai, T.; Huang, H.; Yang, F.; Feng, J.; Zhang, J.; Yan, X. CD146 coordinates brain endothelial cell-pericyte communication for blood-brain barrier development. Proceedings of the National Academy of Sciences of the United States of America 2017, 114, doi:10.1073/pnas.1710848114.
- Yamamoto, S.; Muramatsu, M.; Azuma, E.; Ikutani, M.; Nagai, Y.; Sagara, H.; Koo, B.; Kita, S.; O'Donnell, E.; Osawa, T.; et al. A subset of cerebrovascular pericytes originates from mature macrophages in the very early phase of vascular development in CNS. Scientific reports 2017, 7, doi:10.1038/s41598-017-03994-1.
- Dore-Duffy, P.; Katychev, A.; Wang, X.; Van Buren, E. CNS microvascular pericytes exhibit multipotential stem cell activity. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2006, 26, doi:10.1038/sj.jcbfm.9600272.
- Dore-Duffy, P. Pericytes: pluripotent cells of the blood brain barrier. Current pharmaceutical design 2008, 14, doi:10.2174/138161208784705469.
- Hartmann, D.; Underly, R.; Grant, R.; Watson, A.; Lindner, V.; Shih, A. Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics 2015, 2, doi:10.1117/1.NPh.2.4.041402.
- Smyth, L.; Rustenhoven, J.; Scotter, E.; Schweder, P.; Faull, R.; Park, T.; Dragunow, M. Markers for Human Brain Pericytes and Smooth Muscle Cells. Journal of chemical neuroanatomy 2018, 92, doi:10.1016/j.jchemneu.2018.06.001.
- Dore-Duffy, P. Isolation and characterization of cerebral microvascular pericytes. Methods in molecular medicine 2003, 89, doi:10.1385/1-59259-419-0:375.
- Hill, R.; Tong, L.; Yuan, P.; Murikinati, S.; Gupta, S.; Grutzendler, J. Regional Blood Flow in the Normal and Ischemic Brain Is Controlled by Arteriolar Smooth Muscle Cell Contractility and Not by Capillary Pericytes. Neuron 2015, 87, doi:10.1016/j.neuron.2015.06.001.
- Dore-Duffy, P.; Esen, N. The Microvascular Pericyte: Approaches to Isolation, Characterization, and Cultivation. Advances in experimental medicine and biology 2018, 1109, doi:10.1007/978-3-030-02601-1_5.
- Garbelli, R.; de Bock, F.; Medici, V.; Rousset, M.; Villani, F.; Boussadia, B.; Arango-Lievano, M.; Jeanneteau, F.; Daneman, R.; Bartolomei, F.; et al. PDGFRβ(+) cells in human and experimental neuro-vascular dysplasia and seizures. Neuroscience 2015, 306, doi:10.1016/j.neuroscience.2015.07.090.
- Schultz, N.; Byman, E.; Fex, M.; Wennström, M. Amylin alters human brain pericyte viability and NG2 expression. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2017, 37, doi:10.1177/0271678X16657093.
- Hughes, S.; Chan-Ling, T. Characterization of smooth muscle cell and pericyte differentiation in the rat retina in vivo. Investigative ophthalmology & visual science 2004, 45, doi:10.1167/iovs.03-1312.
- Park, T.; Feisst, V.; Brooks, A.; Rustenhoven, J.; Monzo, H.; Feng, S.; Mee, E.; Bergin, P.; Oldfield, R.; Graham, E.; et al. Cultured pericytes from human brain show phenotypic and functional differences associated with differential CD90 expression. Scientific reports 2016, 6, doi:10.1038/srep26587.
- Ramsauer, M.; Kunz, J.; Krause, D.; Dermietzel, R. Regulation of a blood-brain barrier-specific enzyme expressed by cerebral pericytes (pericytic aminopeptidase N/pAPN) under cell culture conditions. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 1998, 18, doi:10.1097/00004647-199811000-00014.
- Hutter-Schmid, B.; Humpel, C. Platelet-derived Growth Factor Receptor-beta is Differentially Regulated in Primary Mouse Pericytes and Brain Slices. Current neurovascular research 2016, 13, doi:10.2174/1567202613666160219120411.
- Sagare, A.; Sweeney, M.; Makshanoff, J.; Zlokovic, B. Shedding of soluble platelet-derived growth factor receptor-β from human brain pericytes. Neuroscience letters 2015, 607, doi:10.1016/j.neulet.2015.09.025.
- Obermeier, B.; Daneman, R.; Ransohoff, R. Development, maintenance and disruption of the blood-brain barrier. Nature medicine 2013, 19, doi:10.1038/nm.3407.
- Pieterse, Z.; Sinha, D.; Kaur, P. Pericytes in Metastasis. Advances in experimental medicine and biology 2019, 1147, doi:10.1007/978-3-030-16908-4_5.
- Hosaka, K.; Yang, Y.; Seki, T.; Fischer, C.; Dubey, O.; Fredlund, E.; Hartman, J.; Religa, P.; Morikawa, H.; Ishii, Y.; et al. Pericyte-fibroblast transition promotes tumor growth and metastasis. Proceedings of the National Academy of Sciences of the United States of America 2016, 113, doi:10.1073/pnas.1608384113.
- Aalders, K.; Tryfonidis, K.; Senkus, E.; Cardoso, F. Anti-angiogenic treatment in breast cancer: Facts, successes, failures and future perspectives. Cancer treatment reviews 2017, 53, doi:10.1016/j.ctrv.2016.12.009.
- Bos, R.; Zhong, H.; Hanrahan, C.; Mommers, E.; Semenza, G.; Pinedo, H.; Abeloff, M.; Simons, J.; van Diest, P.; van der Wall, E. Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. Journal of the National Cancer Institute 2001, 93, doi:10.1093/jnci/93.4.309.
- Sinha, D.; Chong, L.; George, J.; Schlüter, H.; Mönchgesang, S.; Mills, S.; Li, J.; Parish, C.; Bowtell, D.; Kaur, P. Pericytes Promote Malignant Ovarian Cancer Progression in Mice and Predict Poor Prognosis in Serous Ovarian Cancer Patients. 2016, doi:10.1158/1078-0432.CCR-15-1931.
- Kienast, Y.; von Baumgarten, L.; Fuhrmann, M.; Klinkert, W.; Goldbrunner, R.; Herms, J.; Winkler, F. Real-time Imaging Reveals the Single Steps of Brain Metastasis Formation. Nature medicine 2010, 16, doi:10.1038/nm.2072.
- Arshad, F.; Wang, L.; Sy, C.; Avraham, S.; Avraham, H. Blood-brain barrier integrity and breast cancer metastasis to the brain. Pathology research international 2010, 2011, doi:10.4061/2011/920509.
- Fitzgerald, D.; Palmieri, D.; Hua, E.; Hargrave, E.; Herring, J.; Qian, Y.; Vega-Valle, E.; Weil, R.; Stark, A.; Vortmeyer, A.; et al. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clinical & experimental metastasis 2008, 25, doi:10.1007/s10585-008-9193-z.
- Termini, J.; Neman, J.; Jandial, R. Role of the neural niche in brain metastatic cancer. Cancer research 2014, 74, doi:10.1158/0008-5472.CAN-14-1226.
- Sierra, A.; Price, J.; García-Ramirez, M.; Méndez, O.; López, L.; Fabra, A. Astrocyte-derived cytokines contribute to the metastatic brain specificity of breast cancer cells. Laboratory investigation; a journal of technical methods and pathology 1997, 77.
- Bachelder, R.; Crago, A.; Chung, J.; Wendt, M.; Shaw, L.; Robinson, G.; Mercurio, A. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer research 2001, 61.
- Momeny, M.; Saunus, J.; Marturana, F.; McCart Reed, A.; Black, D.; Sala, G.; Iacobelli, S.; Holland, J.; Yu, D.; Da Silva, L.; et al. Heregulin-HER3-HER2 signaling promotes matrix metalloproteinase-dependent blood-brain-barrier transendothelial migration of human breast cancer cell lines. Oncotarget 2015, 6, doi:10.18632/oncotarget.2846.
- Lacroix-Fralish, M.; Tawfik, V.; Nutile-McMenemy, N.; Harris, B.; Deleo, J. Differential regulation of neuregulin 1 expression by progesterone in astrocytes and neurons. Neuron glia biology 2006, 2, doi:10.1017/S1740925X07000385.
- Da Silva, L.; Simpson, P.T.; Smart, C.E.; Cocciardi, S.; Waddell, N.; Lane, A.; Morrison, B.J.; Vargas, A.C.; Healey, S.; Beesley, J.; et al. HER3 and downstream pathways are involved in colonization of brain metastases from breast cancer. Breast Cancer Res 2010, 12, R46, doi:10.1186/bcr2603.
- Pukrop, T.; Dehghani, F.; Chuang, H.; Lohaus, R.; Bayanga, K.; Heermann, S.; Regen, T.; Van Rossum, D.; Klemm, F.; Schulz, M.; et al. Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia 2010, 58, doi:10.1002/glia.21022.
- McGrath, E.P.; Logue, S.E.; Mnich, K.; Deegan, S.; Jäger, R.; Gorman, A.M.; Samali, A. The Unfolded Protein Response in Breast Cancer. Cancers 2018, 10, doi:10.3390/cancers10100344.
- Ikesugi, K.; Mulhern, M.L.; Madson, C.J.; Hosoya, K.-i.; Terasaki, T.; Kador, P.F.; Shinohara, T. Induction of Endoplasmic Reticulum Stress in Retinal Pericytes by Glucose Deprivation. Current Eye Research 2006, 31, 947-953, doi:10.1080/02713680600966785.
- Jiang, Z.; Zhang, G.; Huang, L.; Yuan, Y.; Wu, C.; Li, Y. Transmissible Endoplasmic Reticulum Stress: A Novel Perspective on Tumor Immunity. Front. Cell Dev. Biol. 2020, 8, 846, doi:10.3389/fcell.2020.00846.
- Zhong, Y.; Wang, J.J.; Zhang, S.X. Intermittent But Not Constant High Glucose Induces ER Stress and Inflammation in Human Retinal Pericytes. In Retinal Degenerative Diseases, LaVail, M.M., Ash, J.D., Anderson, R.E., Hollyfield, J.G., Grimm, C., Eds.; Springer US: Boston, MA, 2012; Volume 723, pp. 285-292.
- Sena, I.F.G.; Paiva, A.E.; Prazeres, P.; Azevedo, P.O.; Lousado, L.; Bhutia, S.K.; Salmina, A.B.; Mintz, A.; Birbrair, A. Glioblastoma‐activated pericytes support tumor growth via immunosuppression. In Cancer Med; 2018; Volume 7, pp. 1232-1239.
- Valdor, R.; García-Bernal, D.; Riquelme, D.; Martinez, C.M.; Moraleda, J.M.; Cuervo, A.M.; Macian, F.; Martinez, S. Glioblastoma ablates pericytes antitumor immune function through aberrant up-regulation of chaperone-mediated autophagy. Proc Natl Acad Sci USA 2019, 116, 20655-20665, doi:10.1073/pnas.1903542116.
- Tirosh, A.; Tuncman, G.; Calay, E.S.; Rathaus, M.; Ron, I.; Tirosh, A.; Yalcin, A.; Lee, Y.G.; Livne, R.; Ron, S.; et al. Intercellular Transmission of Hepatic ER Stress in Obesity Disrupts Systemic Metabolism. Cell Metab 2021, 33, 1716, doi:10.1016/j.cmet.2021.07.005.
- Berghoff, A.; Rajky, O.; Winkler, F.; Bartsch, R.; Furtner, J.; Hainfellner, J.; Goodman, S.; Weller, M.; Schittenhelm, J.; Preusser, M. Invasion patterns in brain metastases of solid cancers. Neuro-oncology 2013, 15, doi:10.1093/neuonc/not112.
- Téglási, V.; Csűry, D.; Dezső, K.; Bugyik, E.; Szabó, V.; Szállási, Z.; Paku, S.; Reiniger, L. Origin and Distribution of Connective Tissue and Pericytes Impacting Vascularization in Brain Metastases With Different Growth Patterns. Journal of neuropathology and experimental neurology 2019, 78, doi:10.1093/jnen/nlz007.
- Abbott, N.J.; Dolman, D.E.M.; Yusof, S.R.; Reichel, A. In vitro models of CNS barriers. AAPS Advances in the Pharmaceutical Sciences Series 2014, 10, 163-197, doi:10.1007/978-1-4614-9105-7_6.
- Hatherell, K.; Couraud, P.-O.; Romero, I.A.; Weksler, B.; Pilkington, G.J. Development of a three-dimensional, all-human in vitro model of the blood–brain barrier using mono-, co-, and tri-cultivation Transwell models. Journal of Neuroscience Methods 2011, 199, 223-229, doi:10.1016/j.jneumeth.2011.05.012.
- Nakagawa, S.; Deli, M.A.; Nakao, S.; Honda, M.; Hayashi, K.; Nakaoke, R.; Kataoka, Y.; Niwa, M. Pericytes from Brain Microvessels Strengthen the Barrier Integrity in Primary Cultures of Rat Brain Endothelial Cells. Cellular and Molecular Neurobiology 2007, 27, 687-694, doi:10.1007/s10571-007-9195-4.
- Thomsen, L.B.; Burkhart, A.; Moos, T. A triple culture model of the blood-brain barrier using porcine brain endothelial cells, astrocytes and pericytes. PLoS ONE 2015, 10, doi:10.1371/journal.pone.0134765.
- Fujimoto, T.; Nakagawa, S.; Morofuji, Y.; Watanabe, D.; Ujifuku, K.; Horie, N.; Izumo, T.; Niwa, M.; Banks, W.A.; Deli, M.A.; et al. Pericytes Suppress Brain Metastasis from Lung Cancer In Vitro. Cellular and Molecular Neurobiology 2020, 40, 113-121, doi:10.1007/s10571-019-00725-0.
- Wang, Y.I.; Abaci, H.E.; Shuler, M.L. Microfluidic blood–brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnology and Bioengineering 2017, 114, 184-194, doi:10.1002/bit.26045.
- Oddo, A.; Peng, B.; Tong, Z.; Wei, Y.; Tong, W.Y.; Thissen, H.; Voelcker, N.H. Advances in Microfluidic Blood-Brain Barrier (BBB) Models. Trends in Biotechnology 2019, doi:10.1016/j.tibtech.2019.04.006.
- Wu, J.; Kumar-Kanojia, A.; Hombach-Klonisch, S.; Klonisch, T.; Lin, F. A radial microfluidic platform for higher throughput chemotaxis studies with individual gradient control. Lab Chip 2018, 18, 3855-3864, doi:10.1039/c8lc00981c.
- Saliba, J.; Daou, A.; Damiati, S.; Saliba, J.; El-Sabban, M.; Mhanna, R. Development of Microplatforms to Mimic the In Vivo Architecture of CNS and PNS Physiology and Their Diseases. Genes (Basel) 2018, 9, doi:10.3390/genes9060285.
- Maoz, B.M.; Herland, A.; FitzGerald, E.A.; Grevesse, T.; Vidoudez, C.; Pacheco, A.R.; Sheehy, S.P.; Park, T.E.; Dauth, S.; Mannix, R.; et al. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat Biotechnol 2018, 36, 865-874, doi:10.1038/nbt.4226.
- Brown, J.A.; Codreanu, S.G.; Shi, M.; Sherrod, S.D.; Markov, D.A.; Neely, M.D.; Britt, C.M.; Hoilett, O.S.; Reiserer, R.S.; Samson, P.C.; et al. Metabolic consequences of inflammatory disruption of the blood-brain barrier in an organ-on-chip model of the human neurovascular unit. J Neuroinflammation 2016, 13, 306, doi:10.1186/s12974-016-0760-y.
