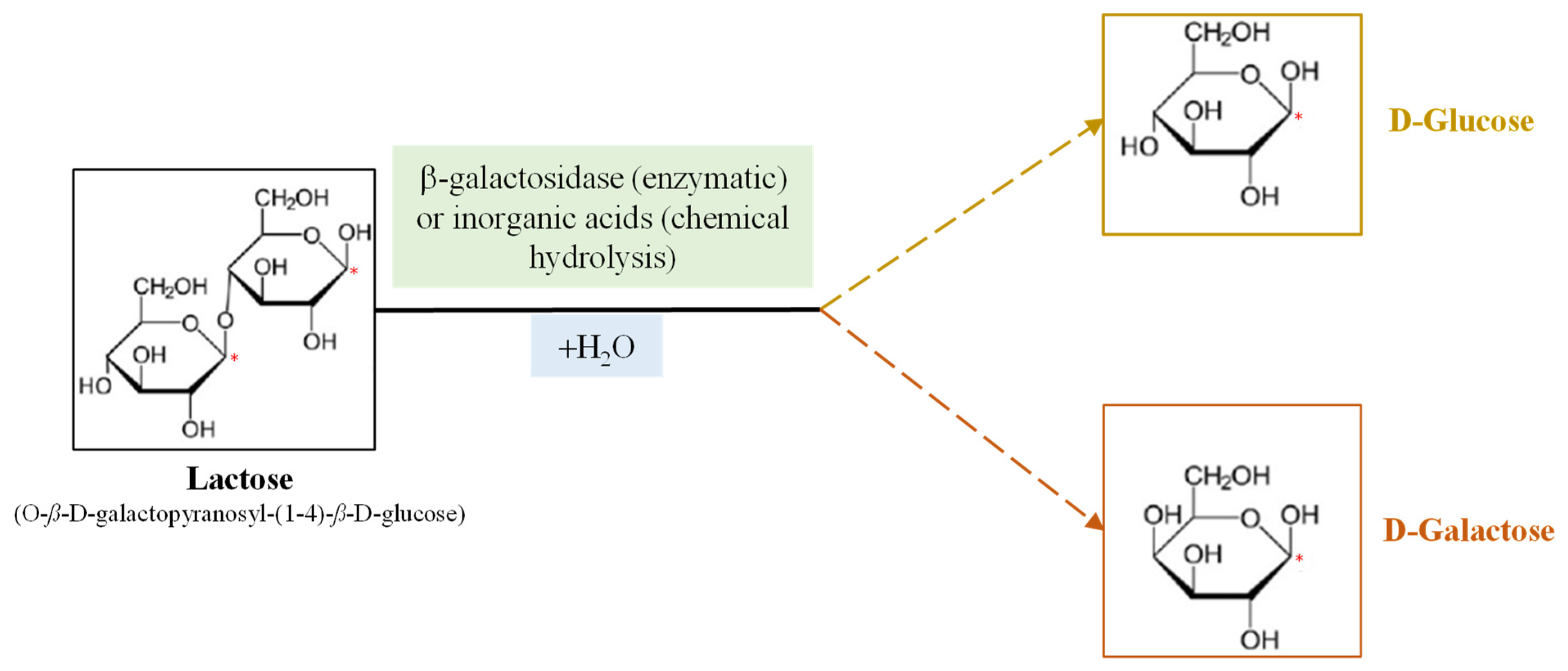
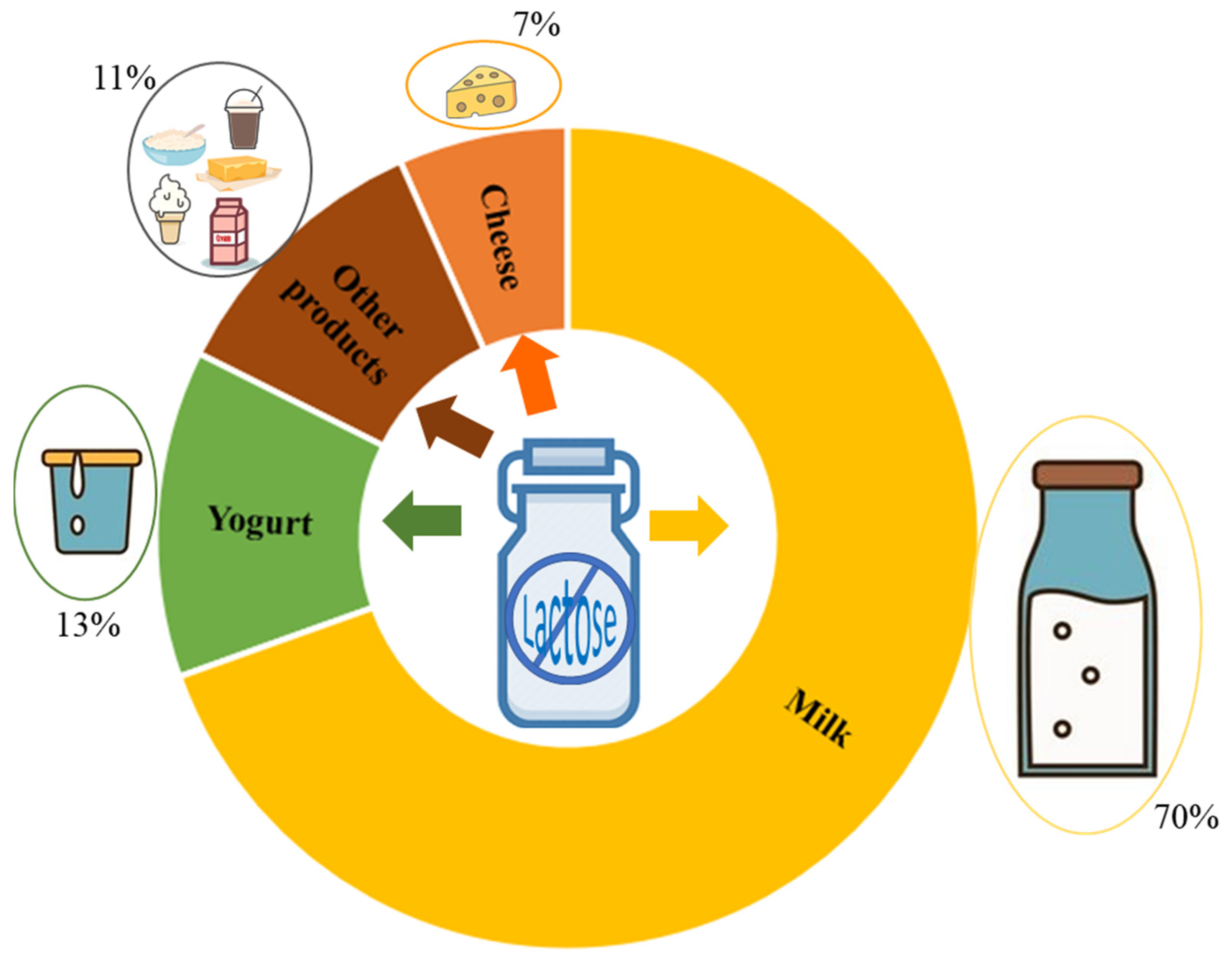
Much attention has recently been paid to β-Galactosidases (β-D-galactoside galactohidrolase; EC 3.2.1.23), commonly known as lactases, due to the lactose intolerance of the human population and the importance of dairy products in the human diet. This enzyme, produced by microorganisms, is being used in the dairy industry for hydrolyzing the lactose found in milk to produce lactose-free milk (LFM). Conventionally, β-galactosidases catalyze the hydrolysis of lactose to produce glucose and galactose in LFM; however, they can also catalyze transgalactosylation reactions that produce a wide range of galactooligosaccharides (GOS), which are functional prebiotic molecules that confer health benefits to human health. In this field, different works aims to identify novel microbial sources of β-galactosidase for removing lactose from milk with the relative GOS production. Lactase extracted from thermophilic microorganisms seems to be more suitable for the transgalactosylation process at relatively high temperatures, as it inhibits microbial contamination. Different immobilization methods, such as adsorption, covalent attachment, chemical aggregation, entrapment and micro-encapsulation, have been used to synthesize lactose-derived oligosaccharides with immobilized β-galactosidases.
- lactose
- β-galactosidase
- transgalactosylation
- galacto-oligosaccharides (GOS)
1. Introduction
2. Recent Advances in
β
-Galactosidase Immobilization for GOS Production
References
- Ugidos-Rodríguez, S.; Matallana-González, M.C.; Sánchez-Mata, M.C. Lactose malabsorption and intolerance: A review. Food Funct. 2018, 9, 4056–4068.
- Brüssow, H. Nutrition, population growth and disease: A short history of lactose. Environ. Microb. 2013, 15, 2154–2161.
- Robert, K.Y.; Nakatani, Y.; Yanagisawa, M. The role of glycosphingolipid metabolism in the developing brain. J. Lipid Res. 2009, 50, S440–S445.
- Brenkert, A.; Radin, N.S. Synthesis of galactosyl ceramide and glucosyl ceramide by rat brain: Assay procedures and changes with age. Brain Res. 1972, 36, 183–193.
- Nilsson, Å. Sphingolipids in the gut? Which are the important issues? Eur. J. Lipid 2007, 109, 971–976.
- Naim, H.Y.; Sterchi, E.E.; Lentze, M.J. Biosynthesis and maturation of lactase-phlorizin hydrolase in the human small intestinal epithelial cells. Biochem. J. 1987, 241, 427–434.
- Elferink, H.; Bruekers, J.P.J.; Veeneman, G.H.; Boltje, T.J. A comprehensive overview of substrate specificity of glycoside hydrolases and transporters in the small intestine: ‘A gut feeling’. Cell. Mol. Life Sci. 2020, 77, 4799–4826.
- Lule, V.K.; Garg, S.; Tomar, S.K.; Khedkar, C.D.; Nalage, D.N. Food Intolerance: Lactose Intolerance. In Encyclopedia of Food and Health; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 43–48.
- Garballo-Rubio, A.; Soto-Chinchilla, J.; Moreno, A.; Zafra-Gómez, A. Determination of residual lactose in lactose-free cow milk by hydrophilic interaction liquid chromatography (HILIC) coupled to tandem mass spectrometry. J. Food Compos. Anal. 2018, 66, 39–45.
- Suri, S.; Kumar, V.; Prasad, R.; Tanwar, B.; Goyal, A.; Kaur, S.; Gat, Y.; Kumar, A.; Kaur, J.; Singh, D. Considerations for development of lactose-free food. J. Nut. Inter. Met. 2019, 15, 27–34.
- Dekker, P.J.T.; Koenders, D.; Bruins, M.J. Lactose-free dairy products: Market developments, production, nutrition and health benefits. Nutrients 2019, 11, 551.
- Raza, A.; Iqbal, S.; Ullah, A.; Khan, M.I.; Imran, M. Enzymatic conversion of milk lactose to prebiotic galacto-oligosaccharides to produce low lactose yogurt. J. Food Proc.Preserv. 2018, 42, e13586.
- Adhikari, K.; Dooley, L.M.; Chambers IV, E.; Bhumiratana, N. Sensory characteristics of commercial lactose-free milks manufactured in the United States. LWT-Food Sci. Technol. 2010, 43, 113–118.
- Harju, M. Milk sugars and minerals as ingredients. Int. J. Dairy Technol. 2001, 54, 61–63.
- Guerrero, C.; Vera, C.; Acevedo, F.; Illanes, A. Simultaneous synthesis of mixtures of lactulose and galacto-oligosaccharides and their selective fermentation. J. Biotechnol. 2015, 209, 31–40.
- Neto, C.A.C.G.; Silva, N.C.G.; de Oliveira Costa, T.; de Albuquerque, T.L.; Gonçalves, L.R.B.; Fernandez-Lafuente, R.; Rocha, M.V.P. The β-galactosidase immobilization protocol determines its performance as catalysts in the kinetically controlled synthesis of lactulose. Int. J. Biol. Macromol. 2021, 176, 468–478.
- Ricardi, N.C.; Arenas, L.T.; Benvenutti, E.V.; Hinrichs, R.; Flores, E.E.E.; Hertz, P.F.; Costa, T.M.H. High performance biocatalyst based on β-D-galactosidase immobilized on mesoporous silica/titania/chitosan material. Food Chem. 2021, 359, 129890.
- Guerrero, C.; Vera, C.; Serna, N.; Illanes, A. Immobilization of Aspergillus oryzae β-galactosidase in an agarose matrix functionalized by four different methods and application to the synthesis of lactulose. Bioresour. Technol. 2017, 232, 53–63.
- Liburdi, K.; Benucci, I.; Esti, M. Lysozyme in Wine: An Overview of Current and Future Applications. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1062–1073.
- Panesar, P.S.; Panesar, R.; Singh, R.S.; Kennedy, J.F.; Kumar, H. Review Microbial production, immobilization and applications of β-D-galactosidase. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2006, 81, 530–543.
- Xavier, J.R.; Ramana, K.V.; Sharma, R.K. β-galactosidase: Biotechnological applications in food processing. J. Food Biochem. 2018, 42, e12564.
- Serey, M.; Vera, C.; Guerrero, C.; Illanes, A. Immobilization of Aspergillus oryzae β-galactosidase in cation functionalized agarose matrix and its application in the synthesis of lactulose. Int. J. Biol. Macromol. 2021, 67, 1564–1574.
- Guerrero, C.; Aburto, C.; Suárez, S.; Vera, C.; Illanes, A. Effect of the type of immobilization of β-galactosidase on the yield and selectivity of synthesis of transgalactosylated oligosaccharides. Biocatal. Agric. Biotechnol. 2018, 16, 353–363.
- Eijsink, V.; Bjørk, A.G.; Gåseidnes, S.; Sirevåg, R.; Synstad, B.; van den Burg, B.; Vriend, G. Rational engineering of enzyme stability. J. Biotechnol. 2004, 113, 105–120.
- Rueda, N.; dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R. Improved performance of lipases immobilized on heterofunctional octyl-glyoxyl agarose beads. RSC Adv. 2015, 5, 11212–11222.
- Barbosa, O.; Torres, R.; Ortiz, C.; Berenguer-Murcia, Á.; Rodrigues, R.C.; Fernandez-Lafuente, R. Heterofunctional supports in enzyme immobilization: From traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromolecules 2013, 14, 2433–2462.
- Mateo, C.; Grazu, V.; Palomo, J.M.; Lopez-Gallego, F.; Fernandez-Lafuente, R.; Guisan, J.M. Immobilization of enzymes on heterofunctional epoxy supports. Nat. Protoc. 2007, 2, 1022–1033.
- Urrutia, P.; Bernal, C.; Wilson, L.; Illanes, A. Use of chitosan heterofunctionality for enzyme immobilization: β-galactosidase immobilization for galacto-oligosaccharide synthesis. Int. J. Biol. Macromol. 2018, 116, 182–193.
- Warmerdam, A.; Benjamins, E.; de Leeuw, T.F.; Broekhuis, T.A.; Boom, R.M.; Janssen, A.E.M. Galacto-oligosaccharide production with immobilized β-galactosidase in a packed-bed reactor vs. free β-galactosidase in a batch reactor. Food Bioprod. Process. 2014, 92, 383–392.
- Carević, M.; Ćorović, M.; Mihailović, M.; Banjanac, K.; Milisavljević, A.; Veličković, D.; Bezbradica, D. Galacto-oligosaccharide synthesis using chemically modified β-galactosidase from Aspergillus oryzae immobilised onto macroporous amino resin. Int. Dairy J. 2016, 54, 50–57.
- Osman, A.; Symeou, S.; Trisse, V.; Watson, K.A.; Tzortzis, G.; Charalampopoulos, D. Synthesis of prebiotic galactooligosaccharides from lactose using bifidobacterial β-galactosidase (BbgIV) immobilised on DEAE-Cellulose, Q-Sepharose and amino-ethyl agarose. Biochem. Eng. J. 2014, 82, 188–199.
