Global rise of infections and deaths caused by drug-resistant bacterial pathogens are among the unmet medical needs. In an age of drying pipeline of novel antibiotics to treat bacterial infections, antimicrobial peptides (AMPs) are proven to be valid therapeutics modalities. Direct in vivo applications of many AMPs could be challenging; however, works are demonstrating encouraging results for some of them. In this review article, we discussed 3-D structures of potent AMPs e.g., polymyxin, thanatin, MSI, protegrin, OMPTA in complex with bacterial targets and their mode of actions. Studies on human peptide LL37 and de novo-designed peptides are also discussed. We have focused on AMPs which are effective against drug-resistant Gram-negative bacteria. Since treatment options for the infections caused by super bugs of Gram-negative bacteria are now extremely limited. We also summarize some of the pertinent challenges in the field of clinical trials of AMPs.
- antibiotics
- multidrug resistant (MDR) bacteria
- MDR Gram negative bacteria
- antimicrobial peptides (AMPs)
1. Introduction
2. Polymyxins
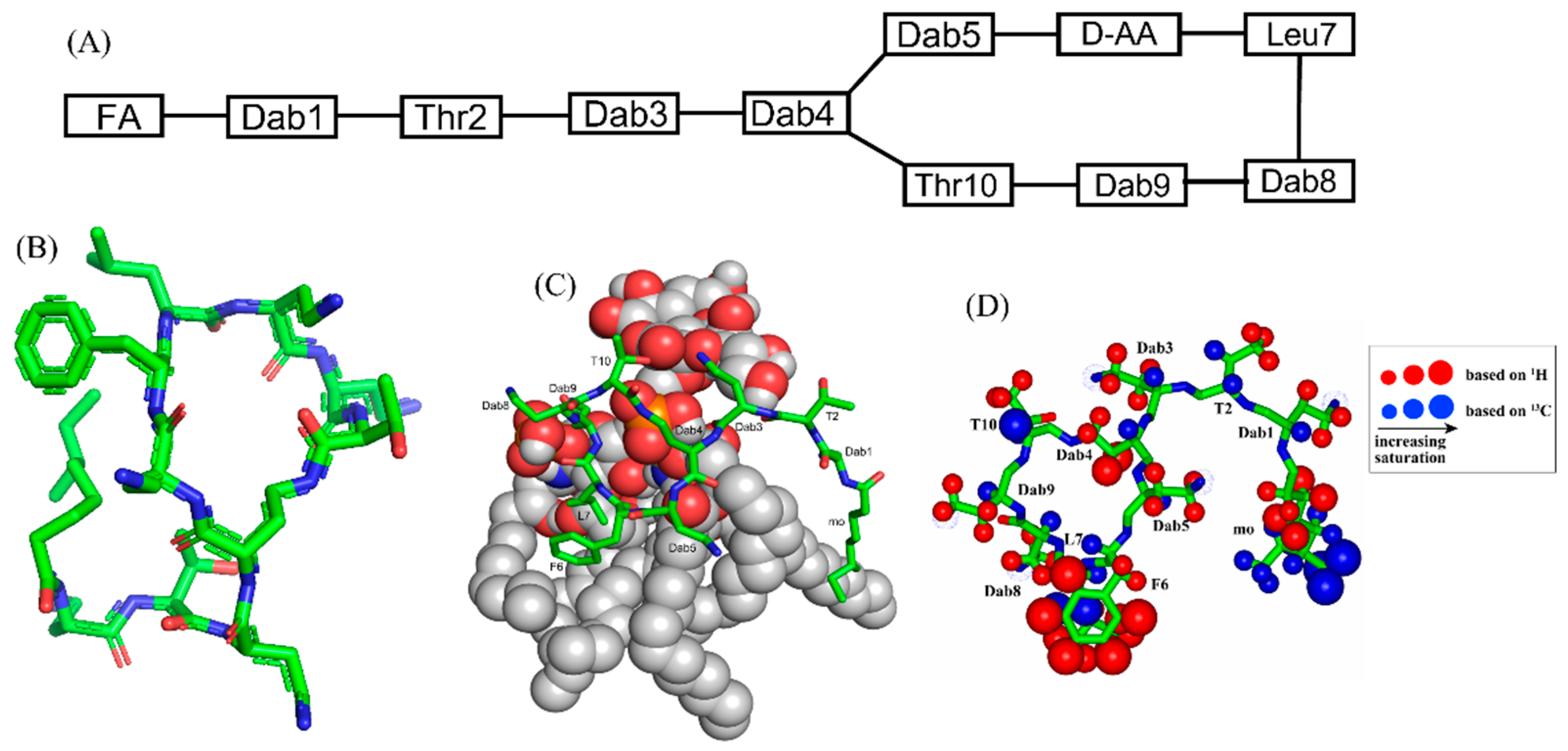
Polymyxins are a family of cyclic lipopeptides isolated as early as 1947 from spore-forming Gram-positive bacteria Bacillus polymyxa [51]. Polymyxins are produced from non-ribosomal peptide synthetase system (NRPS) employing enzymes PmxA, PmxB, and PmxC [52]. The chemical structures of polymyxins, polymyxin B (B1, B2), and polymyxin E (colistin), vary slightly from each other (Figure 1A, Table 1). The cyclic structures of both polymyxin B (PMB) and polymyxin E (PME) contain four cationic diamino-butyric acid (Dab) at positions 4, 5, 8, and 9 and a polar residue Thr 10 [53]. PMB contains an aromatic residue D-Phe6, whereas the 6th position in PME is substituted with residue D-Leu.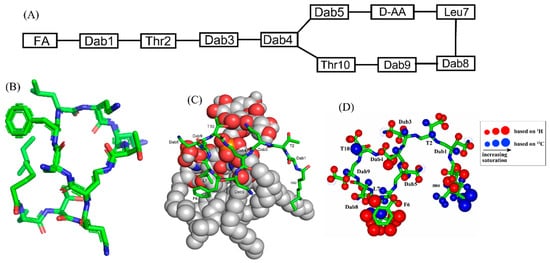
| Compound | FA | Sequence | ||||||
|---|---|---|---|---|---|---|---|---|
| Polymyxin B (PMB) | Methyloctanoyl/methylheptanoyl | Dab1-Thr2-Dab3-cy[Dab4-Dab5-DPhe6-Leu7-Dab8-Dab8-Thr10] | ||||||
| Polymyxin E (PME, Colistin) | Methyloctanoyl/methylheptanoyl | Dab1-Thr2-Dab3-cy[Dab4-Dab5- | DLeu6 | -Leu7-Dab8-Dab8-Thr10] | ||||
| FADD002 | Octanoyl | Dab1-Thr2-Dab3-cy[Dab4-Dab5- | DAda6 | -Leu7-Dab8-Dab8-Thr10] | ||||
| FADD287 | Octanoyl | Dab1-Thr2- | Dap3 | -cy[Dab4-Dab5- | DLeu6 | - | Abu7 | -Dab8-Dab8-Thr10] |
| CA284 | ( | S | )-1-(2-methylpropyl)-piperazine-2-carbonyl+ | Thr1-Dab2-cy[Dab3-Dab4-DPhe-Leu6-Dab7-Dab8-Thr9] | ||||
| SPR206 | (3 | S | )-4-amino-3-(3-chlorophenyl)butanoyl | Thr1-Dab2-cy[Dab3-Dab4-DPhe-Leu6-Dab7-Dab8-Thr9] | ||||
| MicuRx-12 | 3-(2,2-dimethyl-butanoyloxy)-propanoyl (ester bond) | Dab1-Thr2-Dab3-cy[Dab4-Dab5-DPhe6-Leu7-Dab8-Dab8-Thr10] | ||||||
| NAB379 | Octanoyl | Thr1- | DSer2 | -cy[Dab3-Dab4-DPhe5-Leu6-Dab7-Dab8-Thr9] | ||||
| NAB815 | Octanoyl | Dab1-Thr2- | DThr3 | -cy[Dab4-Dab5-DPhe6-Leu7-Abu8-Dab8-Thr10] |
3. β-Sheet AMPs: Protegrins and Thanatin
β-sheet or β-hairpin AMPs are well-folded even in the absence of bacterial membrane, whereas helical AMPs often lack folded conformations in free solution. The stable β-sheet structures are often supported by inter-strand disulfide bond(s). Protegrins (PGs) constitute 16–18-residue long Arg rich AMPs identified from leucocyte of pig (Figure 2). The β-hairpin structures in PGs are maintained by two antiparallel β-strands with disulfide bonds between residues Cys6-Cys15 and Cys8-Cys13. Broad spectrum antibacterial activity under physiological salt solutions had drawn considerable attention for therapeutic developments of PGs [62][63][68,69]. Where PG-1 represents the well-studied member of the group. PG-1 displayed low minimal inhibitory concentration (MIC), ranging between 0.5 to 5 μM, against several strains of Gram-negative and Gram-positive bacteria [62][63][64][65][68,69,70,71]. Iseganan or IB367 is a synthetic variant of PG-1 developed by IntraBiotics Pharmaceuticals through extensive structure activity studies or SAR [66][72]. IB367 was subjected to several phases of clinical trials targeting number of conditions e.g., oral mucositis in cancer patients, prevention of ventilator associated pneumonia, cystic fibrosis and mycoses [67][68][73,74]. However, the status of these clinical trials of iseganan at present is unknown.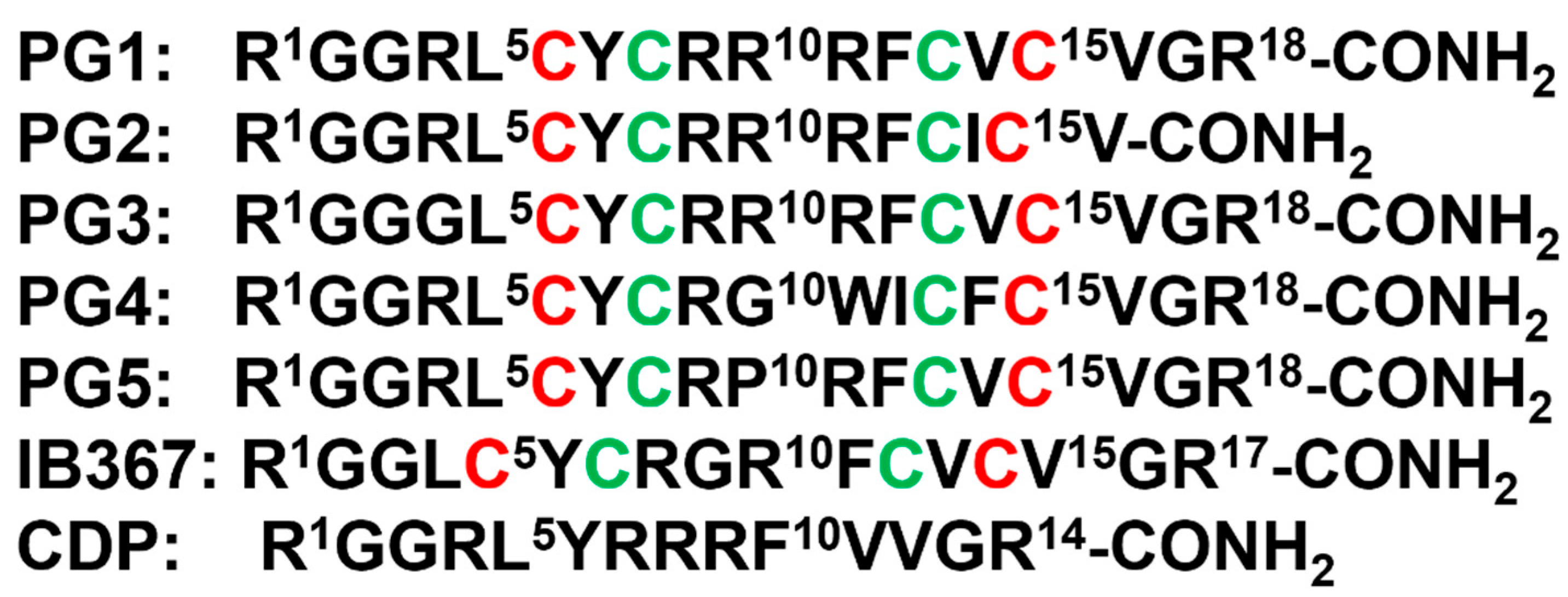

4. Outer Membrane Protein Targeting Antibiotics (OMPTA)
OMPTA defines a new class of peptides or peptide mimetics which may be highly effective in killing MDR Gram-negative bacteria [69][70][71][93,94,95]. As a mode of action, OPMTA binds both to LPS and outer membrane proteins resulting in specific Gram-negative activity. In one of these endeavors, protegin-1 was utilized as a starting template generating series of peptidomimetics from designed libraries. In particular, 14-residue long backbone cyclized β-hairpin peptides were developed with inclusion of conserved L-Pro1 and D-Pro14 dipeptide motif [72][96]. Active peptides were screened from these libraries which specifically inhibited only strains of P. aeruginosa with MIC value as low as 0.008 μg/mL [72][96]. One of the candidate peptides termed murepavadin was tested in clinical trials for potential treatment for pneumonia [73][97]. Murepavadin binds to outer-membrane β-barrel protein LptD one of the components of LPS transport machinery [72][96]. However, the exact site of binding of murepavadin or any other related peptides to LptD is not known. As such, LptD has a large, conserved C-terminal domain embedded in LPS outer membrane and a relatively short variable N-terminal at the periplasmic domain [74][98]. The specific P. aeruginosa killing activity of murepavadin over other Gram-negative bacteria may be conferred by binding with the periplasmic domain of LptD. Recently, the phase II clinical trial of murepavadin was halted due to occurrence of acute kidney disease and further preclinical development of the peptide has been undertaken [75][99]. Darobactin, a seven residue (WNWSKSF) peptide, was first isolated from nematode symbiont bacteria Photorhabdus khanii HGB1456. Non-ribosomally synthesized darobactin contains usual sidechain-sidechain covalent crosslinking between indole ring of W1 and β-carbon of W3, β-carbon of K5 and indole ring of W3 [76][100]. Darobactin can kill several Gram-negative bacteria strains in infection animal model but was not effective to tested Gram-positive bacteria. As a mode of action, darobactin directly binds to the outer-membrane protein BamA which is the central component of BamABCDE complex. Binding of darobactin to BamA inhibited chaperon function of the outer membrane protein causing bacterial cell death. BamA/darobactin complex revealed β-sheet like binding of the peptide with the β1-strand of the β-barrel structure of BamA [76][77][100,101].5. MSI Peptides
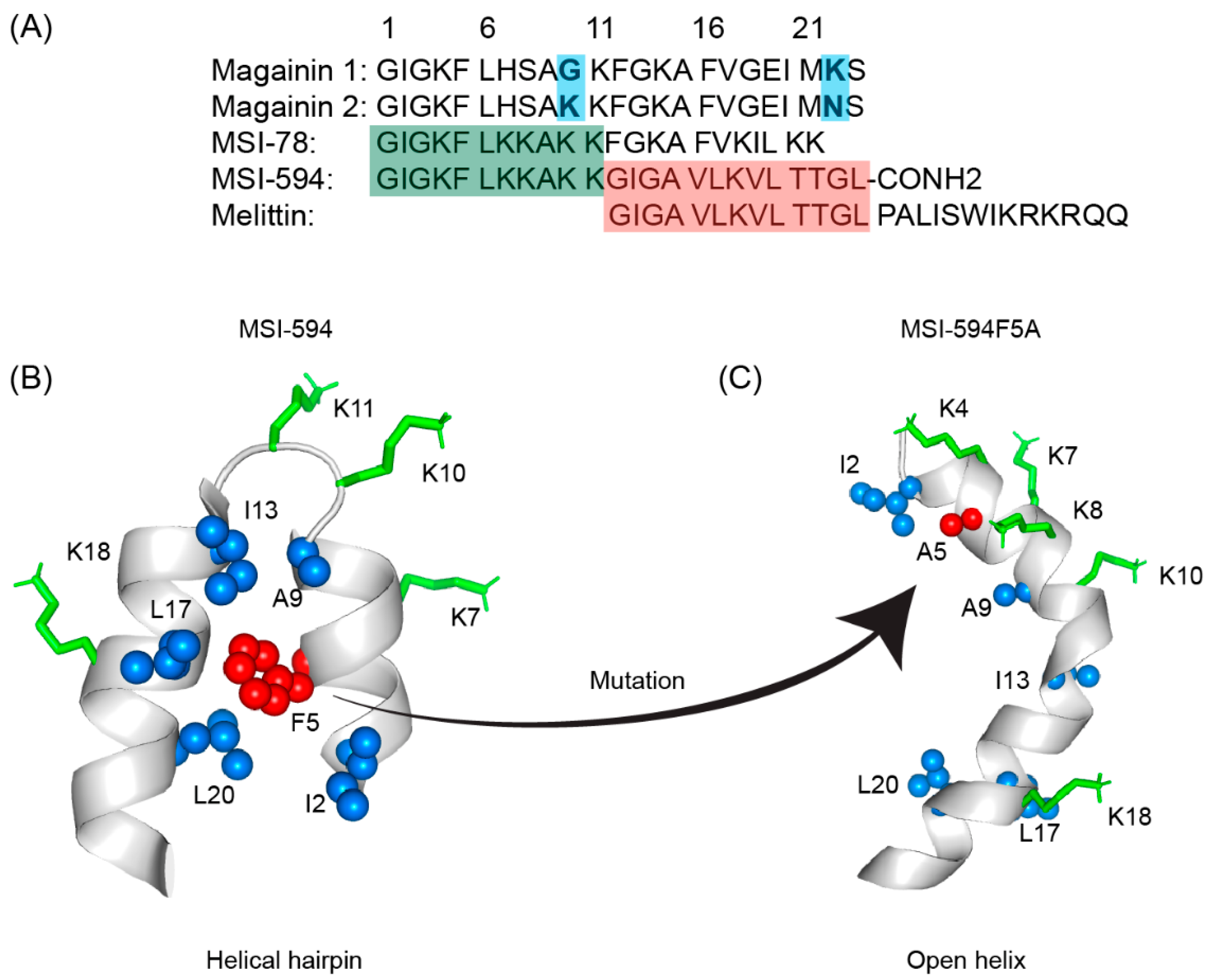
The first AMP isolated from the anuran family is magainin which was discovered from the skin of a female African clawed frog (Xenopus laevis) by Michael Zasloff in 1987 [78][79][102,103]. Members of the magainin family (magainin-1, magainin-2, and PGLa) are cationic peptides which do not assume any preformed secondary structure in free solution but adopt amphipathic α-helical conformations in membranous environments [80][81][104,105]. Magainins are non-hemolytic and non-cytotoxic host defense peptides that however display potent activity against broad range of bacteria, fungi, and protozoa [14][79][14,103]. Magainin family is made up of two closely similar peptides (magainin-1 and magainin-2), each of which has 23 amino acids and differs by two substitutions at 10th and 22nd position of its primary structure (Figure 35A) [78][79][102,103]. It was also found that magainin-2 has comparatively higher activity than magainin-1. Following this discovery, numerous works were done using magainin-2 and its derivatives to understand activity, structure, and mechanism of action. Through extensive SAR analysis, Zasloff and colleagues at Magainin Pharmaceuticals (now Genaera Corporation) synthesized a 22-residue-long cationic MSI-78 peptide that demonstrated higher potency and greater selectivity to microbial cells compared to human red blood cells [80][82][104,106]. MSI-78 exhibited a broad spectrum of potent antimicrobial activities against both Gram-positive and Gram-negative bacteria including the pathogens associated with the diabetic foot infections (DFI) [82][106]. The MIC50 and MIC90 against all organisms tested from DFI were 16 and 32 μg/mL, respectively [83][107]. Although MSI-78 peptide (known as pexiganan or Locilex) showed promising outcomes against several in vitro, in vivo, and pre-clinical studies, the Food and Drug Administration (FDA) of USA finally denied the new drug application (NDA) for its topical administration as a result of a deficit over a traditional antibacterial medication [84][108]. In 2014, Dipexium Pharmaceuticals initiated another phase III clinical trial using pexiganan and ofloxacin (an FDA approved fluoroquinolone antibiotic) in a comparative clinical study for the same use. However, the trial reported a failure in 2017 since the peptide showed unsatisfactory results at sub-inhibitory concentrations against the tested organisms [85][109]. It is noteworthy to mention that the Locilex (pexiganan topical cream 0.8%) received the advisory from European Medicines Agency (EMA) in 2015 for human and clinical use against DFI.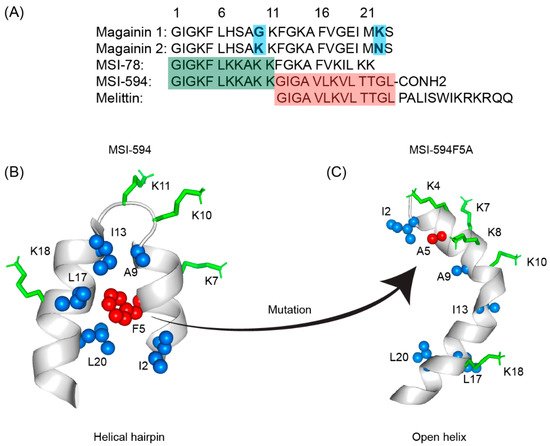
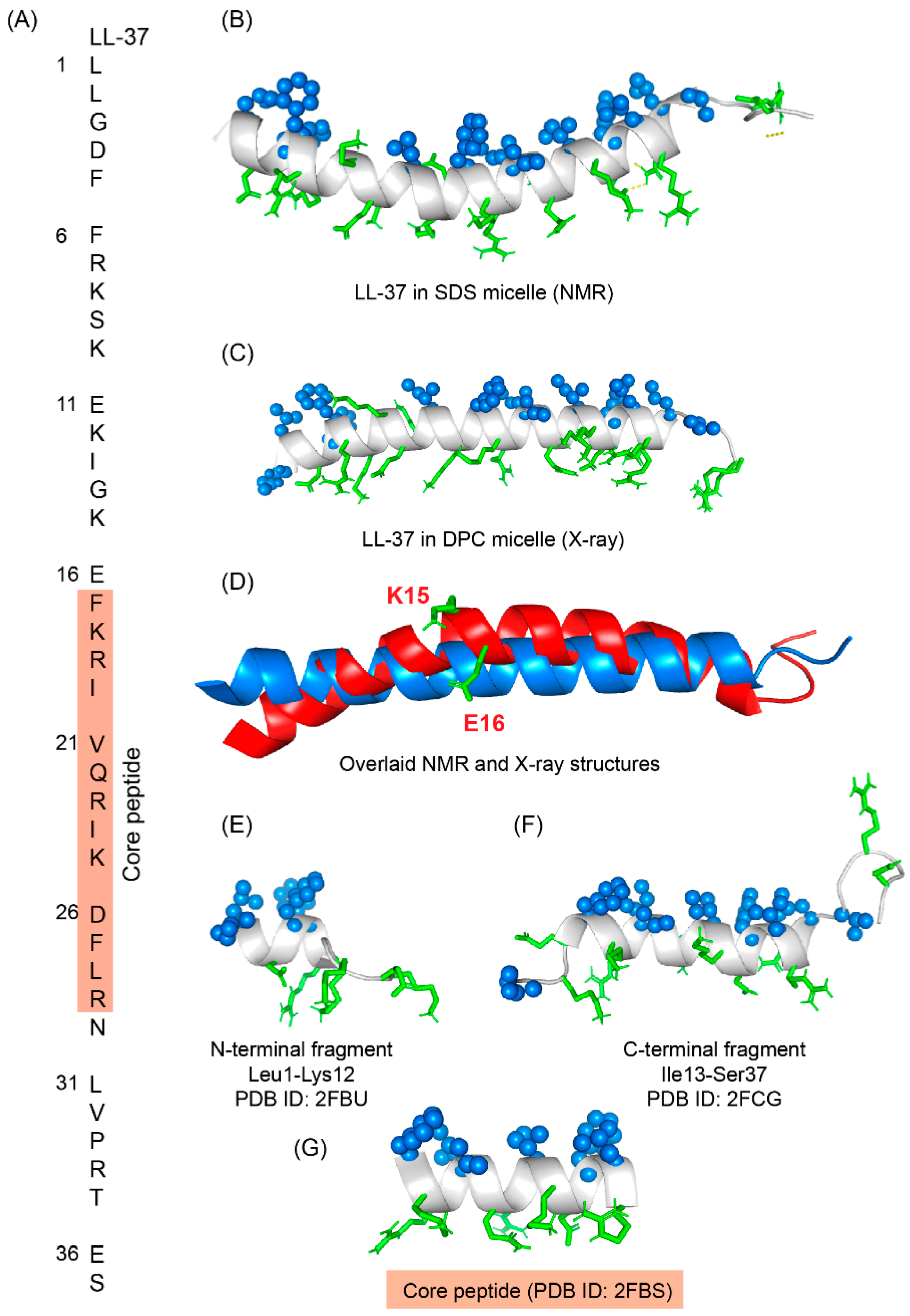
6. LL-37
LL-37 is a cationic α-helical host defense peptide of human that belongs to cathelicidin antimicrobial peptide (CAMP) family [88][89][126,127]. LL-37 is produced by many types of epithelial cells, as well as leukocytes such as monocytes, T cells, B cells, and NK cells and mostly stored in the lysosomes of macrophages and polymorphonuclear leukocytes (PMNs) [89][127]. All cathelicidin AMPs are synthesized as preproproteins (18 KDa) containing a highly conserved N-terminal domain (13.5 KDa) and a vastly diverse antimicrobial domain at the C-terminus (4.5 KDa) [89][127]. The N-terminal domain is typically 94–114 amino acids long and shares sequence homology with cathelin, a cysteine protease inhibitor identified in swine neutrophils. The C-terminus of human cathelicidin comprises 37-residue long LL-37 peptide (Figure 46A) [90][128]. The mature, functional form of the peptide is released after proteolytic cleavage of the signal and cathelin domains [90][91][128,129]. In 1995, three scientific groups discovered the LL-37 peptide based on the study of highly conserved “cathelin” domain [89][92][127,130]. Beside the cell proliferation, immunomodulation, and other signaling roles, the cationic LL-37 peptide (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES) shows excellent activity against a broad range of Gram-positive and Gram-negative pathogens [93][94][131,132]. Since the LL-37 peptide possess several promising therapeutic potentials including the induction of angiogenesis, the ProMore Pharma in Poland completed the phase IIb clinical trial of LL-37 for treatment of venous leg ulcers [94][95][132,133].