Cancer is an extensive disease and the most common cause of morbidity and mortality worldwide. It is characterized by a deregulation of the cell cycle, which primarily results in a progressive loss of control of cellular growth and differentiation. The repurposing of veterinary antiparasitic drugs for the treatment of cancer is gaining traction, as supported by existing literature. A prominent example is the proposal to implement the use of veterinary antiparasitics such as benzimidazole carbamates and halogenated salicylanilides as novel anticancer drugs. These agents have revealed pronounced anti-tumor activities and gained special attention for “double repositioning”, as they are repurposed for different species and diseases simultaneously, acting via different mechanisms depending on their target. As anticancer agents, these compounds employ several mechanisms, including the inhibition of oncogenic signal transduction pathways of mitochondrial respiration and the inhibition of cellular stress responses.
- drug repurposing
- antiparasitic
- benzimidazole carbamates
- halogenated salicylanilides
- cancer therapy
1. Introduction
2. BZ Carbamates
BZ antiparasitics are a group of heterocyclic aromatic organic compounds that are extensively used in both human and veterinary medicines to inhibit internal parasites. Some important BZ drugs include MZ, albendazole (ABZ), fenbendazole (FZ), flubendazole (FLU), triclabendazole, parbendazole, oxibendazole, and ricobendazole. In the last few years, some of these have been successfully investigated for various types of cancers worldwide.2.1. Mechanism of Action of BZ Carbamates
The molecular mode of action of BZ carbamates involves inhibiting the polymerization of tubulin and facilitating the disruption of microtubules in parasite cells (Figure 1) [7][14]. An in vitro study using the extracts of helminthic and mammalian tubulin has implicated tubulin as the leading molecular target of BZ carbamates [8][15]. Tubulin is pivotal to cell motility, proliferation, and division; the intercellular transport of organelles; the maintenance of cell shape; and the secretion process of cells in all living organisms [9][16]. By blocking microtubule elongation in worms, BZ carbamates perturb glucose uptake in cells. Eventually, the glycogen reserves are exhausted, and their energy management mechanisms are depleted, culminating in the death of the parasites [10][17].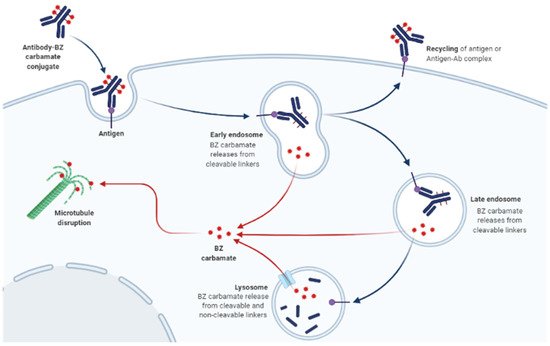
2.2. Anticancer Activity of BZ Carbamates
BZ carbamates are cancer cell-selective, causing minimal cytotoxicity in normal cells but increased cytotoxicity in different tumor cells. Several studies have reported that BZ carbamates inhibit the polymerization of mammalian tubulin in vitro. Whether the same effect would be observed in human cells, and if so, whether such targeted efforts could be effective against tumors, are some questions raised by these reports. Lacey et al. first addressed the activity of BZ carbamates against mouse leukemia cells L1210 in 1985 [11][18]. A more thorough inquiry into the antitumor effects of BZ carbamates was carried out; the most promising outcomes of this inquiry are summarized in Table 1. The general pharmacokinetic properties of BZ carbamates are as follows: slow absorption; wide distribution throughout the body; extensive hepatic metabolism; and excretion via urine and feces (Figure 2a). Their common side effects are fever, nausea, vomiting, abdominal discomfort, and hepatotoxicity. The low intestinal absorption rate of BZ carbamates may make it difficult for them to reach concentrations in the systemic circulation effective in treating cancers in humans. Increased bioavailability is necessary to enhance their antitumor effect, making them safe and well tolerable in human and veterinary use.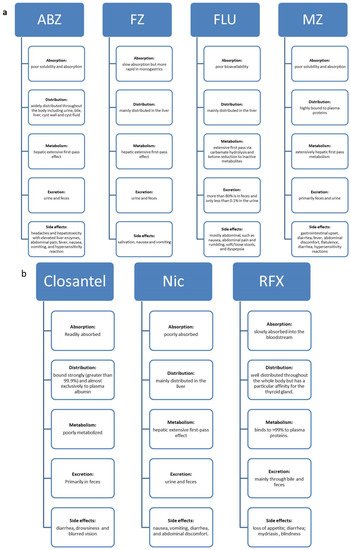
|
Cell Source |
Cell Lines |
Procedure of Study |
Species |
Antiparasitics |
Cancer Type |
Target Pathway |
Reference |
|---|
|
Antiparasitics |
Cancer Type |
Title |
Phase |
Purpose |
Status/Result |
Identifier/Ref |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Human |
Hep G2 and Hep3B |
in vitro |
Mice |
ABZ |
|||||||||||
|
ABZ |
HCC or CRC |
Pilot Study Of Albendazole In Patients With Advanced Malignancy. Effect On Serum Tumor Markers/High Incidence Of Neutropenia |
PS |
Evaluation of anticancer activity HCC |
Stabilization of the disease, but because of neutropenia, treatment was stopped on day 19 Cytotoxicity |
||||||||||
|
Human |
Hep G2 and Hep3B, PLC/PRF/5 and SKHEP-1 |
in vitro |
Mice |
||||||||||||
|
ABZ | ABZ |
Refractory solid tumors |
Phase I Clinical Trial To Determine Maximum Tolerated Dose Of Oral Albendazole In Patients With Advanced Cancer |
1 |
To determine the safety, tolerability, and the maximal tolerated dose. |
HCC |
Tubulin disruption |
To characterize the pharmacokinetics and preliminary evidence of efficacy. | |||||||
|
SKHEP-1 |
in vivo |
||||||||||||||
2400 mg/day from 1200 mg b.d. | Decreased plasma VEGF and 16% patients had a tumor marker response with a fall of at least 50% | ||||||||||||||
|
MZ |
Adreno-cortical carcinoma |
Mebendazole Monotherapy and Long-Term Disease Control in Metastatic Adrenocortical Carcinoma |
CS |
To describe successful long-term tumor control |
Well tolerated, and the associated adverse effects of MZ are minor |
Rat |
HTC, Novikoff |
in vitro |
|||||||
|
MZ |
CC |
Drug Repositioning From Bench To Bedside: Tumour Remission By The Antihelmintic Drug Mebendazole In Refractory Metastatic Colon Cancer |
CS |
Repositioning drugs for use in advanced CC |
No disease-related symptoms were found |
Mice |
|||||||||
|
MZ | Hep1-6 |
Glio- in vitro |
|||||||||||||
blastoma |
Mebendazole In Newly Diagnosed High-Grade Glioma Patients Receiving Temozolomide (Mebendazole) |
1 |
To find the highest dose and the efficiency of MZ to slow the growth of the brain tumor |
Active, not recruiting |
NCT01729260 |
Human |
SW480, SW620, HCT8 and Caco2 |
in vitro |
|||||||
|
MZ | Mice |
Pediatric Gliomas ABZ, RBZ, FLU |
|||||||||||||
A Phase I Study of Mebendazole for the Treatment of Pediatric Gliomas | 1 |
To determine the safety and efficacy of MZ |
Intestinal cancer |
Tubulin disruption |
Recruiting [ |
NCT01837862 |
|||||||||
|
Human |
HT-29 |
in vitro |
|||||||||||||
|
MZ |
GI Cancer | Mice |
A Clinical Safety and Efficacy Study of Mebendazole on GI Cancer or Cancer of Unknown Origin. (RepoMeb) |
ABZ |
1 |
To determine the safety and efficacy of MZ (ReposMZ) |
CRC |
Terminated (Lack of effect) | Apoptosis |
NCT03628079 | |||||
|
Human |
CEM/dEpoB300 |
in vitro |
Mice |
ABZ |
Leukemia |
Apoptosis |
|||||||||
|
Human |
1A9Pc TX22 |
in vitro |
Mice |
ABZ |
OC |
Apoptosis |
|||||||||
|
Mouse |
EMT6 |
in vitro |
Mice |
FZ |
Mammary carcinoma |
Cytotoxicity |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
H460 and A549 |
in vitro |
nu/nu mice |
FZ |
LC |
microtubule disruption, p53 activation and down regulation of pivotal glycolytic enzymes |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
P493-6 |
in vitro |
SCID mice |
FZ |
Lymphoma |
Tubulin disruption |
|||||||||
|
in vivo |
|||||||||||||||
|
Mice |
EMT6 |
in vitro |
BALB/c Rw mice |
FZ |
Mammary carcinoma |
Tubulin disruption |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
OCI-AML-2 |
in vitro |
SCID mice |
FLU |
Leukaemia and Myeloma |
Tubulin disruption |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
MDA-MB-231, BT-549, SK-BR-3 and MCF-7 |
in vitro |
Mice |
FLU |
BC |
Tubulin disruption |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
TNBC cell lines MDA-MB-231 and MDA-MB-468 |
in vitro |
Mice |
FLU |
BC |
Apoptosis |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
BT474, SK-BR-3, MDA-MB-453, JIMT-1 |
in vitro |
BALB/c mice |
FLU |
BC |
Tubulin disruption |
|||||||||
|
in vivo |
Apoptosis |
||||||||||||||
|
Human |
HCT116, RKO and SW480 |
in vitro |
BALB/c mice |
FLU |
CRC |
Apoptosis |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
H295R and SW-13 |
in vitro |
Mice |
MZ |
Adrenocortical carcinoma |
Apoptosis |
|||||||||
|
Human |
H460, A549, H1299 and WI-38 |
in vitro |
Mice |
MZ |
LC |
Tubulin disruption, Apoptosis |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
HCT 116 and RKO |
in silico |
- |
MZ |
CC |
Tubulin disruption |
|||||||||
|
Human |
DLD-1, HCT-116, HT-29 and SW480 |
in vitro |
Mice |
MZ |
CC |
Tubulin disruption |
|||||||||
|
Human |
ACP-02, ACP-03 and AGP-01 |
in vitro |
Mice |
MZ |
GC |
Tubulin disruption |
|||||||||
|
in vivo |
|||||||||||||||
|
Mouse |
GL261 |
in vitro |
C57BL6 Mice |
MZ | |||||||||||
Human | |||||||||||||||
SKOV3 and CP70 | |||||||||||||||
in vitro | |||||||||||||||
SCID mice | |||||||||||||||
Nic | |||||||||||||||
OC | |||||||||||||||
Induction of metabolic shift to glycolysis | |||||||||||||||
[ | |||||||||||||||
] | |||||||||||||||
[ | |||||||||||||||
] | |||||||||||||||
in vivo | |||||||||||||||
Human | |||||||||||||||
OVCAR-3, SKOV-3 and A2780 | |||||||||||||||
in vitro | |||||||||||||||
NOD/ | SCID mice |
Nic | |||||||||||||
|
Cancer of Unknown Origin |
OC | Inhibition of CP70sps and primary OTICs | |||||||||||||
|
in vivo |
|||||||||||||||
|
Human |
SKOV3.ip1 |
in vitro |
Mice |
Nic |
OC |
Inhibition of Wnt/β-catenin Pathway |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
SKOV3 and HO8910 |
in vitro |
Athymic Nude mice |
Nic |
OC |
Mitochondrial Respiration and aerobic glycolysis |
|||||||||
|
in vivo |
|||||||||||||||
|
2 |
|||||||||||||||
|
MZ |
OC, PC and ovarian epithelial cancer |
Study of the Safety, Tolerability and Efficacy of Metabolic Combination Treatments on Cancer (METRICS) |
Human |
A2780ip2, A2780cp20, and SKOV3Trip2 |
in vitro |
SCID mice |
Nic |
OC |
Inhibition of Wnt/β-catenin, mTOR and STAT3 pathways |
||||||
|
in vivo |
|||||||||||||||
|
Human |
Tumorspheres |
in vitro |
Mice |
Nic and its analogs in combination with carboplatin |
OC |
Cytotoxicity |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
HepG2 and QGY7701 |
in vitro |
Mice |
Nic |
HCC |
Apoptosis and suppression of ATF3 expression |
|||||||||
|
Human |
NSCLC, NCI-H1299 and HCT116 |
in vitro |
Mice |
Nic |
LC |
Apoptosis through ROS-mediated p38 MAPK-c-Jun activation |
|||||||||
|
Human |
SK-Hep-1 and Huh7 |
in vitro |
Mice |
Nic |
HCC |
Inhibition of metastasis of HCC, and CD10 |
|||||||||
|
Human |
HCC827, H1650, and H1975 |
in vitro |
Nu/Nu nude mice |
Nic |
LC |
Inhibition of STAT3 phosphorylation |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
A549/DDP |
in vitro |
Mice |
Nic combined with cisplatin (DDP) |
Cisplatin-resistant LC |
Apoptosis and reduction of c-myc protein |
|||||||||
|
Human |
HepG2, QGY-7703 and SMMC-7721 |
in vitro |
Mice |
Nic |
HCC |
Inhibition of cell growth and STAT3 pathway |
|||||||||
|
Human |
Lung adenocarcinoma (549, EKVX, H358, Hop62, H322M, H522, H838, and H23), large cell lung carcinoma (H460, Hop92), NCSLC (H1299, H810) and small cell LC (H82) |
in vitro |
Mice |
Nic |
LC |
Reduction in proliferation and inhibition of S100A4 protein |
|||||||||
|
Human |
U-87 MG |
in vitro |
Mice |
Nic |
Glioblastoma |
Cell toxicity and inhibition of Wnt/β-catenin, PI3K/AKT, MAPK/ERK, and STAT3 |
|||||||||
|
Human |
TS15-88, GSC11 |
in vitro |
Athymic nude mice |
Nic and/or temo-zolomide |
Glioblastoma |
Inhibition of the expression of epithelial-mesenchymal transition-related markers, Zeb1, N-cadherin, and β-catenin |
|||||||||
|
in vivo |
|||||||||||||||
3 | To determine the effectiveness of a regimen of selected metabolic treatments for cancer patients and to perform exploratory analysis on the relationship between the degree of response and changes in biochemical markers |
Not yet recruiting |
NCT02201381 |
||||||||||||
|
MZ |
CC |
Mebendazole as Adjuvant Treatment for Colon Cancer |
3 |
MZ as adjuvant treatment for colon cancer |
Recruiting |
NCT03925662 |
|||||||||
|
Nic |
CC |
A Study of Niclosamide in Patients With Resectable Colon Cancer |
1 |
To determine the maximum tolerated dose (MTD) |
Terminated (low accrual) |
NCT02687009 |
|||||||||
|
Nic |
CRC |
Drug Trial to Investigate the Safety and Efficacy of Niclosamide Tablets in Patients With Metastases of a Colorectal Cancer Progressing After Therapy (Nikolo) |
2 |
To evaluate the safety and efficacy of oral appliqued Nic |
Unknown |
NCT02519582 |
|||||||||
|
Nic |
PC |
Niclosamide and Enzalutamide in Treating Patients With Castration-Resistant, Metastatic PC |
1 |
To determine the side effects and best dose of Nic |
Completed (No result posted) |
NCT02532114 |
|||||||||
|
Nic |
Metastatic PC |
Enzalutamide and Niclosamide in Treating Patients With Recurrent or Metastatic Castration-Resistant PC |
1 |
To determine the best dose and side effects of Nic when given together with enzalutamide |
Recruiting |
NCT03123978 |
|||||||||
|
Recurrent PC |
|||||||||||||||
|
Stage IV PC |
|||||||||||||||
|
Nic |
Metastatic PC |
Abiraterone Acetate, Niclosamide, and Prednisone in Treating Patients With Hormone-Resistant PC |
2 |
To determine the side effects and how well abiraterone acetate, Nic, and prednisone work in treating patients with hormone-resistant PC |
Recruiting |
NCT02807805 |
Brain tumour |
Tubulin disruption |
|||||||
|
in vivo |
Apoptosis |
||||||||||||||
|
Human |
GBM U87-MG, D54, H80, H247, H392, H397, H502 and H566 |
in vitro |
C57BL/6 mice |
MZ |
Brain cancer |
Apoptosis |
|||||||||
|
in vivo |
|||||||||||||||
|
Mouse |
GL261 |
||||||||||||||
|
Human |
D425 MB |
in vivo |
p53 mice |
MZ |
Medullo-blastoma |
Tubulin disruption |
|||||||||
|
Human |
293T and hTERT-RPE1 |
in vitro |
nu/nu athymic mice |
MZ |
Medullo-blastoma |
Hedgehog inhibitor |
|||||||||
|
in vivo |
|||||||||||||||
|
Murine |
CP2 and SP1 |
in vitro |
BALB/c mice |
MZ |
PC |
Tubulin disruption |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
KKU-M213 |
in vitro |
Nude mice |
MZ |
Bile duct Cancer |
Apoptosis |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
PANC-1 |
in vitro |
Mice |
MZ |
Pancreatic cancer |
- |
|||||||||
|
Human |
CAL27 and HCC15 |
in vitro |
Nude mice |
MZ |
Head and neck cancer |
Apoptosis |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
SK-Br-3 |
in vivo |
Mice |
MZ |
BC |
Tubulin disruption |
|||||||||
|
Human |
M-14 and SK-Mel-19 |
in vitro |
Mice |
MZ |
Melanoma |
Tubulin disruption |
|||||||||
|
Human |
MM622, MM540, D08, MM329, D17, and UACC1097 |
in vitro |
Mice |
MZ |
Melanoma |
Tubulin disruption |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
NRASQ61K |
in vitro |
Athymic mice |
MZ |
Melanoma |
Apoptosis |
|||||||||
|
in vivo |
|||||||||||||||
|
in silico |
|||||||||||||||
|
Human |
GL261 |
in vitro |
C57BL/6 mice |
MZ |
Brain cancer |
Tubulin disruption |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
Burkitt’s lymphoma Ramos cells, Hela cells, PANC-1 cells, and HepG2 cells |
in vivo |
Zebra-fish |
Closantel |
Lymphoma, cervical cancer, PC, and LC |
Suppression of antiangiogenesis and Closantel |
|||||||||
|
Human |
Du146 |
in vitro |
Mice |
Nic |
PC |
Inhibition of STAT3 Pathway |
|||||||||
|
Human |
HEK293 cells |
in vitro |
Mice |
Nic |
PC and BC |
Inhibition of Wnt/β-catenin Pathway |
|||||||||
|
Human |
MCF7 and MDA-MB-231 |
in vitro |
NOD/SCID mice |
Nic |
BC |
Apoptosis and downregulation stem pathways |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
MDA-MB-231 |
in vitro |
BALB/c nude mice |
Nic and cisplatin |
BC |
Apoptosis and inhibition of Akt, ERK, and Src pathways |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
MDA-MB-468 and MCF-7 |
in vitro |
Mice |
Nic |
BC |
Inhibition of cell motility and STAT3 activity |
|||||||||
|
Human |
TNBC MDA-MB-231, MDA-MB-468 and Hs578T |
in vitro |
Athymic nude mice |
Nic |
BC |
Inhibition of Wnt/β-catenin Pathway |
|||||||||
|
in vivo |
|||||||||||||||
|
Mouse |
4T1 |
in vitro |
BALB/c mice |
Nic |
BC |
Apoptosis and suppression of cell migration and invasion |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
MDA-MB-231, MDA-MB-468 and MCF-7 |
in vitro |
|||||||||||||
|
Human |
2LMP, SUM159, HCC1187, and HCC1143 |
in vitro |
NOD/ SCID mice |
Nic |
BC |
Cytotoxicity |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
K562 and KBM5-T315I cells |
in vitro |
NOD mice |
Nic |
Chronic myelogenous leukemia |
Inhibition of FOXM1/β-catenin Pathway |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
HL-60, U937, OCI-AML3, Molm13, MV4-11, and U266 cells |
in vitro |
BALB/c mice |
Nic |
Acute myelogenous leukemia |
Apoptosis and Inhibition of NF-κB pathway |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
MCF7 |
in vitro |
Mice |
Nic |
Adeno-carcinoma |
Inhibition of PI3K-dependent signalling |
|||||||||
|
HCC1954 |
Carcinoma |
||||||||||||||
|
BT-474 |
in vivo |
Ductal Carcinoma |
|||||||||||||
|
MDA-MB-361 and |
Adeno-carcinoma |
||||||||||||||
|
SKBR3 cell |
in silico |
Adeno-carcinoma |
|||||||||||||
|
Human |
HCT116, SW620, and HT29 |
in vivo |
Mice |
Nic |
CC |
Inhibition of STAT3 phosphorylation |
|||||||||
|
Human |
HCT116, SW480, DLD1 and 293 cells |
in vitro |
APC-MIN mice |
Nic |
CC |
Inhibition of Wnt/Snail-mediated EMT |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
HCT116, SW620, LS174T, SW480, and DLD-1 |
in vitro |
NOD/SCID mice |
Nic |
CC |
Inhibition of S100A4-induced metastasis formation |
|||||||||
|
in vivo |
|||||||||||||||
|
in situ |
|||||||||||||||
|
Human |
HT29, HCT116, CaCO2 and MCF-10A |
in vitro |
NOD/SCID mice |
Nic |
CC |
Inhibition of Wnt/β-catenin Pathway |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
HEK293T, U2OS, WIDR, DLD-1, CRC 240, COLO205, CRC57 and HCT116 |
in vitro |
Mice |
Nic |
CC |
Induction of autophagy and inhibition of Wnt/β-catenin Pathway |
|||||||||
|
Human |
SW480 and SW620 |
in vitro |
Mice |
Nic |
CC |
Reduction of Wnt activity |
Human |
LN229, T98G, U87(MG), U138, and U373(MG) |
in vitro |
Rag2−/−Il2rg−/− and SCID/ Beige mice |
Nic |
Glioblastoma |
Cytotoxicity and diminished the pGBMs’ malignant potential |
||
|
in vivo |
|||||||||||||||
|
Human |
C4-2B, LNCaP and DU145 |
in vitro |
Mice |
Nic with enzalutamide |
Enzalutamide resistance PC |
Inhibition of migration, invasion and IL6-Stat3-AR pathway |
|||||||||
|
Human |
LNCaP, VcaP, CWR22Rv1, PC3 and HEK293 |
in vitro |
SCID mice |
Nic with enzalutamide |
Castration-resistant PC |
Inhibition of AR variant and enzalutamide-resistant tumor growth |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
CaLo, HeLa, SiHa, CasKi, DoTc2, ViBo and C-33A |
in vitro |
SCID mice |
Nic |
Cervical cancer |
Inhibition of mTOR signaling |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
ESO26, FLO-1, KYAE-1, OE33, SK-GT-4, and OE19 |
in vitro |
SCID mice |
Nic |
Esophageal cancer |
Inhibition of Wnt/β-catenin |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
BE3,CE48T/VGH and CE81T/VGH |
in vitro |
Mice |
Nic |
Esophageal cancer |
Inhibition of cell proliferation and STAT3 pathway |
|||||||||
|
Recurrent PC |
|||||||||||||||
|
Stage IV PC | ] | ||||||||||||||
|
Rodent |
CC531 |
in vivo |
|||||||||||||
|
Murine |
MC38 |
in vitro |
APCmin/+ mouse |
Nic-EN and oxyclozanide |
CC |
Mitochondrial uncoupling |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
HCT116 |
in vitro |
|||||||||||||
|
Rodent |
C2C12 |
in vitro |
|||||||||||||
|
in vivo |
Human |
Osteosarcoma cells |
in vitro |
Mouse |
Nic |
Osteosarcoma |
Apoptosis and target multiple signaling pathways |
||||||||
|
in vivo |
|||||||||||||||
|
Human |
NCI-H295R and SW-13 |
in vitro |
Nu+/Nu+ mice |
Nic |
Adrenocortical Carcinoma |
Induction of G1 cell-cycle arrest mitochondrial uncoupling |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
A498 and Caki-1 |
in vitro |
Athymic nude mice |
Nic |
Renal cell carcinoma |
Inhibition of cell proliferation, migration and cell cycle progression |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
SCC4 and SCC25 |
in vitro |
Mice |
Nic |
Oral cancer |
Inhibition of cancer stemness, extracellular matrix remodeling, and metastasis through dysregulation Wnt/β-catenin signaling pathway |
|||||||||
|
Human |
H929, MM1S, U266 and BMSC |
in vitro |
BALB/c nude mice |
RFX |
Multiple myeloma |
Apoptosis and inhibition of DNA synthesis |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
A431 and A375 |
in vitro |
BALB/c nude mice |
RFX |
Skin cancer |
Inhibition of CDK4/6 |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
HCT-116 and HT-29 |
in vitro |
Apcmin/+ mice |
RFX |
CRC |
Inhibition of cell proliferation |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
HCT-116 and DLD1 cells |
in vitro |
BALB/c nude mice |
RFX |
CRC |
Induction of ICD of CRC cells |
|||||||||
|
in vivo |
|||||||||||||||
|
Human |
SGC-7901 and BGC-823, GES-1 |
in vitro |
BALB/c nude mice |
RFX |
GC |
Apoptosis and inhibition of PI3K/Akt/mTOR signaling pathway |
PubMed, Google Scholar, and CTD databases were used to summarize the data for the antitumor effects of BZ carbamates. ABZ—albendazole; BC—breast cancer; CC—colon cancer; CRC—colorectal cancer; EMT—epithelial–mesenchymal transition; FZ—fenbendazole; GC—gastric cancer; HCC—hepatocellular carcinoma; ICD—immunogenic cell death; LC—lung cancer; MZ—Mebendazole; Nic—Niclosamide; Nic-EN—Niclosamide ethanolamine; OC—ovarian cancer; PC—prostate cancer; RBZ—Ricobendazole; RFX—Rafoxanide.
2.3. Anticancer Activity of BZ Carbamates in Clinical Models
NCBI database was used to inquire about the clinical trials on antitumor effects of antiparasitic drugs. ABZ—albendazole; MZ—mebendazole, Nic—niclosamide, CS—clinical study; CC—colon cancer; CRC—colorectal cancer; HCC—hepatocellular carcinoma; PS—pilot Study.
