1. Properties of Free Radicals
1.1. Superoxide Anion Radical (O
1. Formation
Radicals are either (1) formed from spin-paired molecules or (2) from other radicals. Radicals are formed from spin-paired molecules through homolysis of weak bonds or electron transfer, also known as reduction. Radicals are formed from other radicals through substitution, addition, and elimination reactions.

Homolysis of a bromine molecule producing two bromine radicals Radical formation from spin-paired molecules
Homolysis

Homolysis of dibenzoyl peroxide producing two benzoyloxy radicals Homolysis makes two new radicals from a spin-paired molecule by breaking a covalent bond, leaving each of the fragments with one of the electrons in the bond.[3] Because breaking a chemical bond requires energy, homolysis occurs under the addition of heat or light. The bond dissociation energy associated with homolysis depends on the stability of a given compound, and some weak bonds are able to homolyze at relatively lower temperatures. Some homolysis reactions are particularly important because they serve as an initiator for other radical reactions. One such example is the homolysis of halogens, which occurs under light and serves as the driving force for radical halogenation reactions. Another notable reaction is the homolysis of dibenzoyl peroxide, which results in the formation of two benzoyloxy radicals and acts as an initiator for many radical reactions.[4]

Reduction of a ketone to form a ketyl radical Reduction
Radicals can also form when a single electron is added to a spin-paired molecule, resulting in an electron transfer. This reaction, also called reduction, usually takes place with an alkali metal donating an electron to another spin-paired molecule.[5]
Radical formation from other radicals
Abstraction

Radical abstraction between a benzoyloxy radical and hydrogen bromide 
Radical addition of a bromine radical to a substituted alkene Hydrogen abstraction describes when a hydrogen atom is removed from a hydrogen donor molecule (e.g. tin or silicon hydride) with its one electron.[6] Abstraction produces a new radical and a new spin-paired molecule. This is different from homolysis, which results in two radicals from a single spin-paired molecule and doesn’t include a radical as its reactant. Hydrogen abstraction is a fundamental process in radical chemistry because it serves as the final propagation step in many chemical reactions, converting carbon radicals into stable molecules. The figure to the right shows a radical abstraction between a benzoyloxy radical and a hydrogen bromide molecule, resulting in the production of a benzoic acid molecule and a bromine radical.
Addition
Radical addition describes when a radical is added to a spin-paired molecule to form a new radical.[7] The figure on the right shows the addition of a bromine radical to an alkene. Radical addition follows the Anti -Markovnikov rule, where the substituent is added to the less substituted carbon atom.
Elimination
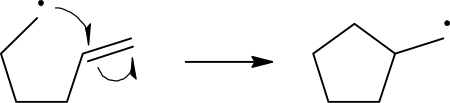
Radical elimination can be viewed as the reverse of radical addition. In radical elimination, an unstable radical compound breaks down into a spin-paired molecule and a new radical compound. Shown below is an example of a radical elimination reaction, where a benzoyloxy radical breaks down into a phenyl radical and a carbon dioxide molecule.[8]
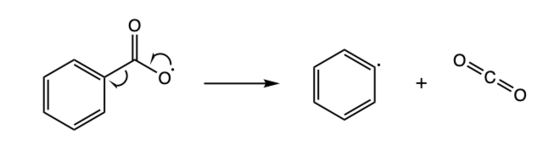
A radical elimination reaction of a benzoyloxy radical 2. Stability
Stability of organic radicals

The radical derived from α-tocopherol Although organic radicals are generally stable intrinsically, practically speaking their existence is only transient because they tend to dimerize. Some are quite long-lived. Generally organic radicals are stabilized by any or all of these factors: presence of electronegativity, delocalization, and steric hindrance.[9] The compound 2,2,6,6-tetramethylpiperidinyloxyl illustrates the combination of all three factors. It is a commercially available solid that, aside from being magnetic, behaves like a normal organic compound.
Electronegativity
Organic radicals are inherently electron deficient thus the greater the electronegativity of the atom on which the unpaired electron resides the less stable the radical.[10] Between carbon, nitrogen, and oxygen, for example, carbon is the most stable and oxygen the least stable. Electronegativity also factors into the stability of carbon atoms of different hybridizations. Greater s-character correlates to higher electronegativity of the carbon atom (due to the close proximity of s orbitals to the nucleus), and the greater the electronegativity the less stable a radical.[10] sp-hybridized carbons (50% s-character) form the least stable radicals compared to sp3-hybridized carbons (25% s-character) which form the most stable radicals.
Delocalization
The delocalization of electrons across the structure of a radical, also known as its ability to form one or more resonance structures, allows for the electron-deficiency to be spread over several atoms, minimizing instability. Delocalization usually occurs in the presence of electron-donating groups, such as hydroxyl groups (−OH), ethers (−OR), adjacent alkenes, and amines (−NH
2
or −NR), or electron-withdrawing groups, such as C=O or C≡N.[3]

Molecular Orbital Diagram of a Radical with an Electron-Donating Group Delocalization effects can also be understood using molecular orbital theory as a lens, more specifically, by examining the intramolecular interaction of the unpaired electron with a donating group’s pair of electrons or the empty π* orbital of an electron-withdrawing group in the form of a molecular orbital diagram. The HOMO of a radical is singly-occupied hence the orbital is aptly referred to as the SOMO, or the Singly-Occupied Molecular Orbital. For an electron-donating group, the SOMO interacts with the lower energy lone pair to form a new lower-energy filled bonding-orbital and a singly-filled new SOMO, higher in energy than the original. While the energy of the unpaired electron has increased, the decrease in energy of the lone pair forming the new bonding orbital outweighs the increase in energy of the new SOMO, resulting in a net decrease of the energy of the molecule. Therefore, electron-donating groups help stabilize radicals.

Molecular orbital diagram of a radical with an electron-withdrawing group With a group that is instead electron-withdrawing, the SOMO then interacts with the empty π* orbital. There are no electrons occupying the higher energy orbital formed, while a new SOMO forms that is lower in energy. This results in a lower energy and higher stability of the radical species. Both donating groups and withdrawing groups stabilize radicals.

The relative stabilities of tertiary, secondary, primary and methyl radicals. Another well-known albeit weaker form of delocalization is hyperconjugation. In radical chemistry, radicals are stabilized by hyperconjugation with adjacent alkyl groups. The donation of sigma (σ) C−H bonds into the partially empty radical orbitals helps to differentiate the stabilities of radicals on tertiary, secondary, and primary carbons. Tertiary carbon radicals have three σ C-H bonds that donate, secondary radicals only two, and primary radicals only one. Therefore, tertiary radicals are the most stable and primary radicals the least stable.
Steric hindrance

Radical form of N-hydroxypiperidine Most simply, the greater the steric hindrance the more difficult it is for reactions to take place, and the radical form is favored by default. For example, compare the hydrogen-abstracted form of N-hydroxypiperidine to the molecule TEMPO.[3] TEMPO, or (2,2,6,6-Tetramethylpiperidin-1-yl)oxyl, is too sterically hindered by the additional methyl groups to react making it stable enough to be sold commercially in its radical form. N-Hydroxypiperidine, however, does not have the four methyl groups to impede the way of a reacting molecule so the structure is unstable.[3]
Facile H-atom donors
The stability of many (or most) organic radicals is not indicated by their isolability but is manifested in their ability to function as donors of H
•−)
The superoxide anion (O2
. This property reflects a weakened bond to hydrogen, usually O−H but sometimes N−H or C−H. This behavior is important because these H
•−) is a reduced form of molecular oxygen created by receiving an electron in a π* antibonding orbital [1]. With only one unpaired electron, superoxide is less radical than O2, and despite its super name, its reactivity with biomolecules is not very sustained [2]. The addition of another electron to O2 donors serve as antioxidants in biology and in commerce. Illustrative is α-tocopherol (vitamin E). The tocopherol radical itself is insufficiently stable for isolation, but the parent molecule is a highly effective hydrogen-atom donor. The C−H bond is weakened in triphenylmethyl (trityl) derivatives. thumb|upright=1.1|2,2,6,6-Tetramethylpiperidinyloxyl is an example of a robust organic radical.
3. Inorganic Radicals
A large variety of inorganic radicals are stable and in fact isolable. Examples include most first-row transition metal complexes. With regard to main group radicals, the most abundant radical in the universe is also the most abundant chemical in the universe, H
•− produces O
. Most main group radicals are not however isolable, despite their intrinsic stability. Hydrogen radicals for example combine eagerly to form H
2−, the peroxide ion, a non-radical (no unpaired electrons) with a weaker oxygen–oxygen bond. The addition of another two electrons to O22− completely eliminates the bond, producing two O2− (oxide ions). In biology, the two-electron reduction product of O2 is H2O2, and the four-electron product is water. It is mostly produced in the mitochondrial electron transport chain in the course of oxidative phosphorylation, which produces adenosine triphosphate (ATP) [3][4]. The superoxide anion can be produced by enzymic or non-enzymic activity, by the direct transfer of electrons to an oxygen molecule [5] or by photochemical means [6]; in biological systems, it is the main precursor of highly reactive species such as HO•, 1O2, CO . Nitric oxide (NO) is well known example of an isolable inorganic radical. Fremy's salt (Potassium nitrosodisulfonate, (KSO
3•−, ONOO•, HOCl and GSSG•− (glutathione disulfide) [1][7]. The enzymes that produce superoxide include oxygenases dependent on cytochrome P450 and xanthine oxidase dependent on lipoxygenase, cyclooxygenase and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [5]. A superoxide radical, as a moderately reactive free radical, can react with another superoxide radical to produce hydrogen peroxide (H )
2O
NO) is a related example. Many thiazyl radicals are known, despite limited extent of π resonance stabilization.[11][12] Many radicals can be envisioned as the products of breaking of covalent bonds by homolysis. The homolytic bond dissociation energies, usually abbreviated as "ΔH °" are a measure of bond strength. Splitting H
2), which can be reduced to water or partially reduced to the extremely reactive hydroxyl radical (HO
into 2 H
•). Dismutation of the superoxide radical can be spontaneous or catalyzed by enzymes known as superoxide dismutases. The formation of HO• is possible by the decomposition of H
, for example, requires a ΔH ° of +435 kJ/mol, while splitting Cl
2O2, catalyzed by transition metal ions in the lower valence state, such as Fe2+ or Cu+ (Fenton reaction), or by the reaction of H2O2 with a superoxide radical (Haber–Weiss reaction); oxidized transitional metals from the Fenton reaction may be re-reduced by O2
into two Cl
Since superoxide is a highly reactive free radical, it can damage molecules (DNA, proteins and lipids) [1]. It may be generated by the immune system to kill invading microorganisms; phagocytes, such as neutrophils, monocytes, macrophages, mast cells and dendritic cells, are mobilized by chemotaxis to the site of bacterial infection and mediate damage through their surface receptors. The phagocytosed bacteria are killed by a process involving O requires a ΔH ° of +243 kJ/mol. For weak bonds, homolysis can be induced thermally. Strong bonds require high energy photons or even flames to induce homolysis.
4. Diradicals
Diradicals are molecules containing two radical centers. Dioxygen (O
2
) is an important example of a stable diradical. Singlet oxygen, the lowest-energy non-radical state of dioxygen, is less stable than the diradical due to Hund's rule of maximum multiplicity. The relative stability of the oxygen diradical is primarily due to the spin-forbidden nature of the triplet-singlet transition required for it to grab electrons, i.e., "oxidize". The diradical state of oxygen also results in its paramagnetic character, which is demonstrated by its attraction to an external magnet.[13] Diradicals can also occur in metal-oxo complexes, lending themselves for studies of spin forbidden reactions in transition metal chemistry.[14] Carbenes in their triplet state can be viewed as diradicals centred on the same atom, while these are usually highly reactive persistent carbenes are known, with N-heterocyclic carbenes being the most common example. Triplet carbenes and nitrenes are diradicals. Their chemical properties are distinct from the properties of their singlet analogues.
5. Occurrence of Radicals
Combustion

Spectrum of the blue flame from a butane torch showing excited molecular radical band emission and Swan bands A familiar radical reaction is combustion. The oxygen molecule is a stable diradical, best represented by
1.2. Hydroxyl Radical (HO
O–O
•)
The hydroxyl radical (HO
. Because spins of the electrons are parallel, this molecule is stable. While the ground state of oxygen is this unreactive spin-unpaired (triplet) diradical, an extremely reactive spin-paired (singlet) state is available. For combustion to occur, the energy barrier between these must be overcome. This barrier can be overcome by heat, requiring high temperatures. The triplet-singlet transition is also "forbidden". This presents an additional barrier to the reaction. It also means molecular oxygen is relatively unreactive at room temperature except in the presence of a catalytic heavy atom such as iron or copper. Combustion consists of various radical chain reactions that the singlet radical can initiate. The flammability of a given material strongly depends on the concentration of radicals that must be obtained before initiation and propagation reactions dominate leading to combustion of the material. Once the combustible material has been consumed, termination reactions again dominate and the flame dies out. As indicated, promotion of propagation or termination reactions alters flammability. For example, because lead itself deactivates radicals in the gasoline-air mixture, tetraethyl lead was once commonly added to gasoline. This prevents the combustion from initiating in an uncontrolled manner or in unburnt residues (engine knocking) or premature ignition (preignition). When a hydrocarbon is burned, a large number of different oxygen radicals are involved. Initially, hydroperoxyl radical (HOO
•) is, chemically, the most reactive free radical formed in vivo. It is formed by the Fenton reaction, in which free iron (Fe2+) reacts with hydrogen peroxide (H2O2), and by the Haber–Weiss reaction of superoxide with ferric iron (Fe3+), producing Fe2+. The reaction is not limited to iron, but it may involve several other ions (Cu2+, Fe3+, Ti4+ and Co3+), which can be recycled by interaction with superoxide anion to form O2 [9]. It is estimated that a cell produces around 50 hydroxyl radicals per second [10]; since hydroxyl radicals have the highest one-electron reduction potential (2310 mV), they can react with anything in living organisms with rate constants from 109 to 1010/M/s [11] and are considered the most harmful free species, since they attack any molecule less than a few nanometers from where they are generated.
The hydroxyl radical reacts strongly with most organic and inorganic molecules (DNA, proteins, lipids, amino acids, sugars, vitamins and metals) faster than its speed of generation [12]. These reactions involve the abstraction of hydrogen and the addition and transfer of electrons [1][13]. In saturated compounds, a hydroxyl radical abstracts a hydrogen atom from the weaker C–H bond to produce a free radical [11]. The resulting radicals may react with oxygen and generate other free radicals. Hydroxyl radicals are easily added to double bonds. All mitochondrial enzyme proteins are susceptible to inactivation by HO ) are formed. These then react further to give organic hydroperoxides that break up into hydroxyl radicals (HO
•, while all amino acid residues of proteins can be oxidized by HO• [14]. It is estimated that ·OH is responsible for 60–70% of the tissue damage caused by ionizing radiation [15]. Hydroxyl radicals are also involved in disorders, such as cardiovascular disease [16] and cancer [17].
1.3. Peroxyl Radical (ROO•)
The alkoxyl (RO•) and peroxyl (ROO•) radicals are oxygen-centered organic radicals. They tend to accept electrons and then undergo reduction, having highly positive reduction potentials (1000 to 1600 mV) [18]. Peroxyl and alkoxyl radicals can be generated by the decomposition of alkyl peroxides (ROOH) induced by heat, radiation or a reaction with transition metal ions and other oxidants capable of subtracting hydrogen [18]. They can also be generated by the oxidation of proteins and nucleic acid [19]. These carbon-centered radicals react directly with biological molecules, such as DNA and albumin -SH-groups. They can abstract hydrogen from other molecules that have a lower standard reduction potential, as observed in the propagation phase of lipid peroxidation. The alkyl radical formed by this reaction may react with oxygen to form another peroxyl radical, resulting in a chain reaction. The RO• radicals formed by the reduction of peroxides are significantly more reactive than ROO• but less reactive than •OH [20]. ROO• may diffuse to remote parts of cells. Their half-lives are of the order of seconds, and they are generally less reactive than HOO• when R is an alkyl or an alkenyl group [21]. Some peroxyl radicals cleave, releasing superoxide anion, or react with each other to generate singlet oxygen [1].
1.4. Hydroperoxyl Radical (HO
).
Polymerization
Many polymerization reactions are initiated by radicals. Polymerization involves an initial radical adding to non-radical (usually an alkene) to give new radicals. This process is the basis of the radical chain reaction. The art of polymerization entails the method by which the initiating radical is introduced. For example, methyl methacrylate (MMA) can be polymerized to produce Poly(methyl methacrylate) (PMMA - Plexiglas or Perspex) via a repeating series of radical addition steps: upright=3.35|center|thumb|Radical intermediates in the formation of polymethacrylate (plexiglas or perspex). Newer radical polymerization methods are known as living radical polymerization. Variants include reversible addition-fragmentation chain transfer (RAFT) and atom transfer radical polymerization (ATRP). Being a prevalent radical, O
2
reacts with many organic compounds to generate radicals together with the hydroperoxide radical. Drying oils and alkyd paints harden due to radical crosslinking initiated by oxygen from the atmosphere.
Atmospheric radicals
The most common radical in the lower atmosphere is molecular dioxygen. Photodissociation of source molecules produces other radicals. In the lower atmosphere, important radical are produced by the photodissociation of nitrogen dioxide to an oxygen atom and nitric oxide (see eq. 1.1 below), which plays a key role in smog formation—and the photodissociation of ozone to give the excited oxygen atom O(1D) (see eq. 1.2 below). The net and return reactions are also shown (eq. 1.3 and eq. 1.4, respectively).
-
[math]\ce{ NO2 ->[h \nu] NO + O }[/math] | | [math]\ce{ O + O2 -> O3 }[/math] | | |
, and it is mainly produced by enzyme reactions [24]. The presence of oxidases (urate oxidase, glucose oxidase, D- amino acid oxidase) may lead to the direct synthesis of hydrogen peroxide by the transfer of two electrons to molecular oxygen; these enzymes are found in microsomes, peroxisomes and mitochondria [24]. Hydrogen peroxide is liposoluble and can therefore diffuse through the cell membrane. Being weakly reactive, this non-free-radical cannot readily oxidize most lipids, proteins and nucleic acids. The threat posed by H2O2 lies in its conversion to the hydroxyl radical (HO•) by homolytic fission, induced by UV or by the interaction with transition metal ions (Fenton reaction) [25]. Hydrogen peroxide may produce singlet oxygen through a reaction with a superoxide anion or with HOCl or chloramines in living systems [7]. The direct action of H2O2 involves an attack on the structure of heme proteins with the release of iron, enzyme inactivation and oxidation of DNA, lipids, -SH groups and keto-acids [13].
1.6. Molecular Oxygen (O2••) and Singlet Oxygen (1O2)
In the evolutionary history of the Earth, oxygen appeared two billion years ago, what is called the ‘’Great Oxidation Event’’, by virtue of the photosynthesis of cyanobacteria, which used solar energy to split water [26]. Oxygen, a metabolic by-product, was released into the atmosphere [10], where it formed the ozone (O , thus facilitating ozone depletion (eq. 2.2–eq. 2.4 below).
-
[math]\ce{ CFCS ->[h \nu] Cl^\bullet }[/math] | | (eq. 2.1) |
-
[math]\ce{ Cl^\bullet {}+ O3 -> ClO^\bullet {}+ O2 }[/math] | |
[math]\ce{ O3 ->[h \nu] O + O2 }[/math] | | | (eq. 1.1) |
-
-
[math]\ce{ NO2 + O2 ->[h \nu] NO + O3 }[/math] | | |
-
[math]\ce{ NO + O3 -> NO2 + O2 }[/math] | | | (eq. 1.3) | (eq. 1.4) |
In the upper atmosphere, the photodissociation of normally unreactive chlorofluorocarbons (CFCs) by solar ultraviolet radiation is an important source of radicals (see eq. 1 below). These reactions give the chlorine radical, Cl
•)
HO
, which catalyzes the conversion of ozone to O
2•, usually termed hydroperoxyl radical or perhydroxyl radical, is the simplest form of a peroxyl radical, produced by the protonation of the superoxide anion radical or by the decomposition of hydroperoxide; approximately 0.3% of superoxide present in the cell cytosol exists in the protonated form [22]. The hydroperoxyl radical produces H2O2, which can react with active redox metals, including iron and copper, to trigger Fenton or Haber–Weiss reactions. The hydroperoxyl radical can also extract hydrogen atoms from NADH or glyceraldehyde-3-phosphate dehydrogenase–NADH, forming H2O2 [23]. Its reactions are slower than HO• but competitive with organic peroxyl radicals. The hydroperoxyl radical plays an important role in the chemistry of lipid peroxidation. It is a much stronger oxidant than superoxide anion due to its ability to extract hydrogen atoms from linoleic, linolenic and arachidonic fatty acids, suggesting a role in the initiation of lipid oxidation [1][23].
1.5. Hydrogen Peroxide (H2O2)
Hydrogen peroxide can be generated by the dismutation of O2•− or by the direct reduction of O2
-
[math]\ce{ O {}+ ClO^\bullet -> Cl^\bullet {}+ O2 }[/math] | | (eq. 2.4) |
-
[math]\ce{ 2O3 ->[h \nu] 3O2 }[/math] | | (eq. 2.5) |
Such reactions cause the depletion of the ozone layer, especially since the chlorine radical is free to engage in another reaction chain; consequently, the use of chlorofluorocarbons as refrigerants has been restricted.
In biology

Structure of the deoxyadenosyl radical, a common biosynthetic intermediate.[15] 
An approximate structure of lignin, which constitutes about 30% of plant matter. It is formed by radical reactions. Radicals play important roles in biology. Many of these are necessary for life, such as the intracellular killing of bacteria by phagocytic cells such as granulocytes and macrophages. Radicals are involved in cell signalling processes,[16] known as redox signaling. For example, radical attack of linoleic acid produces a series of 13-hydroxyoctadecadienoic acids and 9-hydroxyoctadecadienoic acids, which may act to regulate localized tissue inflammatory and/or healing responses, pain perception, and the proliferation of malignant cells. Radical attacks on arachidonic acid and docosahexaenoic acid produce a similar but broader array of signaling products.[17] Radicals may also be involved in Parkinson's disease, senile and drug-induced deafness, schizophrenia, and Alzheimer's.[18] The classic free-radical syndrome, the iron-storage disease hemochromatosis, is typically associated with a constellation of free-radical-related symptoms including movement disorder, psychosis, skin pigmentary melanin abnormalities, deafness, arthritis, and diabetes mellitus. The free-radical theory of aging proposes that radicals underlie the aging process itself. Similarly, the process of mitohormesis suggests that repeated exposure to radicals may extend life span. Because radicals are necessary for life, the body has a number of mechanisms to minimize radical-induced damage and to repair damage that occurs, such as the enzymes superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase. In addition, antioxidants play a key role in these defense mechanisms. These are often the three vitamins, vitamin A, vitamin C and vitamin E and polyphenol antioxidants. Furthermore, there is good evidence indicating that bilirubin and uric acid can act as antioxidants to help neutralize certain radicals. Bilirubin comes from the breakdown of red blood cells' contents, while uric acid is a breakdown product of purines. Too much bilirubin, though, can lead to jaundice, which could eventually damage the central nervous system, while too much uric acid causes gout.[19]
Reactive oxygen species
Reactive oxygen species or ROS are species such as superoxide, hydrogen peroxide, and hydroxyl radical, commonly associated with cell damage. ROS form as a natural by-product of the normal metabolism of oxygen and have important roles in cell signaling. Two important oxygen-centered radicals are superoxide and hydroxyl radical. They derive from molecular oxygen under reducing conditions. However, because of their reactivity, these same radicals can participate in unwanted side reactions resulting in cell damage. Excessive amounts of these radicals can lead to cell injury and death, which may contribute to many diseases such as cancer, stroke, myocardial infarction, diabetes and major disorders.[20] Many forms of cancer are thought to be the result of reactions between radicals and DNA, potentially resulting in mutations that can adversely affect the cell cycle and potentially lead to malignancy.[21] Some of the symptoms of aging such as atherosclerosis are also attributed to radical induced oxidation of cholesterol to 7-ketocholesterol.[22] In addition radicals contribute to alcohol-induced liver damage, perhaps more than alcohol itself. Radicals produced by cigarette smoke are implicated in inactivation of alpha 1-antitrypsin in the lung. This process promotes the development of emphysema. Oxybenzone has been found to form radicals in sunlight, and therefore may be associated with cell damage as well. This only occurred when it was combined with other ingredients commonly found in sunscreens, like titanium oxide and octyl methoxycinnamate.[23] ROS attack the polyunsaturated fatty acid, linoleic acid, to form a series of 13-hydroxyoctadecadienoic acid and 9-hydroxyoctadecadienoic acid products that serve as signaling molecules that may trigger responses that counter the tissue injury which caused their formation. ROS attacks other polyunsaturated fatty acids, e.g. arachidonic acid and docosahexaenoic acid, to produce a similar series of signaling products.[24]
6. History and Nomenclature

Moses Gomberg (1866–1947), the founder of radical chemistry Until late in the 20th century the word "radical" was used in chemistry to indicate any connected group of atoms, such as a methyl group or a carboxyl, whether it was part of a larger molecule or a molecule on its own. The qualifier "free" was then needed to specify the unbound case. Following recent nomenclature revisions, a part of a larger molecule is now called a functional group or substituent, and "radical" now implies "free". However, the old nomenclature may still appear in some books. The term radical was already in use when the now obsolete radical theory was developed. Louis-Bernard Guyton de Morveau introduced the phrase "radical" in 1785 and the phrase was employed by Antoine Lavoisier in 1789 in his Traité Élémentaire de Chimie. A radical was then identified as the root base of certain acids (the Latin word "radix" meaning "root"). Historically, the term radical in radical theory was also used for bound parts of the molecule, especially when they remain unchanged in reactions. These are now called functional groups. For example, methyl alcohol was described as consisting of a methyl "radical" and a hydroxyl "radical". Neither are radicals in the modern chemical sense, as they are permanently bound to each other, and have no unpaired, reactive electrons; however, they can be observed as radicals in mass spectrometry when broken apart by irradiation with energetic electrons. In a modern context the first organic (carbon–containing) radical identified was the triphenylmethyl radical, (C6H5)
3) that shields the Earth from the radiation. Oxygen removed ferrous iron (Fe2+) from aqueous environments by forming deposits of insoluble ferric complexes, leaving only traces of soluble iron in sea and river water [10]. Since animals, including humans, need O2, a toxic mutagenic gas for the mitochondria to efficiently produce energy, only advanced antioxidant defenses allow them to survive. In fact, all aerobic organisms, including plants, aerobic bacteria, animals and humans, suffer damage if exposed to higher-than-normal concentrations of O2 [1]. This means that their antioxidant defenses are limited. According to the theory of superoxide toxicity, O2 toxicity is due to excessive superoxide radical formation [2]. From the biological point of view, molecular oxygen, in its diatomic (O2) ground state, is a bi-radical because it contains two unpaired electrons, each of which is located in a different π antibonding orbital. It is indicated as “triplet oxygen” because the spin of these electrons has three possible alignments with an external field [27]. Triplet oxygen, the more abundant form of oxygen, is the common oxygen that is breathed. It carries a “spin restriction” against reacting with most organic molecules. Molecular oxygen is not very reactive because its electrons are in the lowest energy configuration.
When the two unpaired electrons from triplet oxygen enter two different orbitals, the result is a powerful oxidant named singlet oxygen (1Δg, 1O2) [28]. The 1Δg state, which is 92 kJmol−1 above the ground state, carries an empty π* orbital where it can accommodate a pair of electrons. This ability gives singlet oxygen strong acidic properties. It is therefore a strong electrophile, which reacts with reagents that have high electron density regions, oxidizing them.
Photosensitizers, such as hematoporphyrins, riboflavin and myoglobin, may form singlet oxygen from triplet oxygen in the presence of light by two basic types of photo- oxidation [6]. In the type I reaction, the photosensitizer absorbs light, enabling the excited triplet to react directly with the substrate; while in the type II reaction, it first interacts with the molecular oxygen ground-state (3O2) to produce 1O2, and the excited triplet returns to its ground state. The speed of the type I or II reaction depends on sensitizer type [6][29] and on the substrate and concentrations of substrate and oxygen in the reaction environment. Additionally, 1O2 is produced in vivo by the activation of eosinophils, macrophages and neutrophils [29] and by the enzyme reactions and activities of different peroxidases [28].
Singlet oxygen is very reactive because the “spin restriction” is removed, allowing the species to react as an electrophilic oxidant [6][30] and making it a potential aggressor when it is produced inside the cell [31]. This is indicated especially by its ability to damage DNA, components of guanine and nucleic acids, leading to toxic and mutagenic effects and tissue damage [29]. It is also involved in the oxidation of cholesterol [32] and proteins with high electron density amino acid residues, such as cysteine, methionine, tryptophan, tyrosine and histidine [14]. Singlet oxygen can also play a role in generating cell signals to modify gene expression [29] and can be used to fight cancer cells and various pathogens such as microbes and viruses [7].
1.7. Ozone (O3)
In the history of the Earth, ozone was formed from O2 by the action of high energy electromagnetic radiation and electrical discharges [10]. It is slightly less reactive than HO C
• and a much stronger oxidizing agent than oxygen [32]. It can form free radicals by oxidizing biological molecules and causes oxidative damage to lipids [33], proteins and nucleic acids [34]. Ozone also plays an important role in inflammatory processes [35].
1.8. Hypochlorous Acid (HOCl)
Hypochlorous acid (HOCl) is a highly reactive species involved in oxidation reactions and chlorination of the protein and lipid components. It is generated by hydrogen peroxide and the chloride anion in a reaction catalyzed by myeloperoxidase in macrophages and neutrophils at sites of inflammation [36]. It can oxidize thiols and other biological molecules, including ascorbate, urate, pyridine nucleotides and tryptophan [36]. HOCl chlorinate compounds such as amines to chloramines, residues of tyrosyl to ring chlorinated products, cholesterol and unsaturated lipids to chlorohydrins and may also chlorinate DNA [37].
1.9. Carbonate Radical Anion (CO3•−)
The carbonate radical anion (CO3•−) may be produced by the radiolysis of aqueous solutions of bicarbonate/carbonate [38]; it can also be formed when •OH reacts with carbonate or bicarbonate ions. Levels of bicarbonate are high (25 mM) in blood plasma, enabling the reaction [39]. Although not as strong an oxidizing agent as the hydroxyl radical, the carbonate radical anion is a strong one-electron oxidant that acts by electron transfer and hydrogen abstraction [19]. It has a much longer half-life than •OH and can therefore spread further and oxidatively modify distant cell targets. A wide variety of biomolecules can be oxidized by CO3•−. Regarded as a major oxidant of proteins and nucleic acids, it oxidizes DNA guanine bases by a one-electron transfer process that leads to the formation of stable guanine oxidation products [40]. The carbonate radical anion has been proposed as a key mediator of oxidative damage derived from peroxynitrite production [19][41], xanthine oxidase turnover and superoxide dismutase activity [42]. It is known to play an important role in the modification of selective amino acids in proteins under conditions of oxidative stress, aging and inflammation [43]. The kinetics of tyrosine nitration in the presence of CO . This species was discovered by Moses Gomberg in 1900. In 1933 Morris S. Kharasch and Frank Mayo proposed that free radicals were responsible for anti-Markovnikov addition of hydrogen bromide to allyl bromide.[25][26] In most fields of chemistry, the historical definition of radicals contends that the molecules have nonzero electron spin. However, in fields including spectroscopy, chemical reaction, and astrochemistry, the definition is slightly different. Gerhard Herzberg, who won the Nobel prize for his research into the electron structure and geometry of radicals, suggested a looser definition of free radicals: "any transient (chemically unstable) species (atom, molecule, or ion)".[27] The main point of his suggestion is that there are many chemically unstable molecules that have zero spin, such as C
2 suggest a specific role of CO
, C
3•− in MnSOD nitration by peroxynitrite [44]. The nitration of tyrosine has been observed in neurodegenerative conditions, cardiovascular disorders and diabetes [45].
1.10. Nitric Oxide (NO•)
Nitric oxide (NO•), nitrogen dioxide (NO
, CH
2•) and peroxynitrite (ONOO−), as well as non-radicals such as nitrous acid HNO2 and N2O4 (dinitrogen tetroxide), are included in the collective term reactive nitrogen species (RNS). Nitric oxide or nitrogen monoxide (NO•) is a free radical with a single unpaired electron. The chemical reactivity of NO• is rather limited, and consequently its direct toxicity is less than that of reactive oxygen species (ROS) . However, it reacts with O2•−, producing peroxynitrite anion (ONOO−) [41], a very damaging species for proteins, lipids and DNA [46]. Nitric oxide also reacts with molecular oxygen and nitrogen to form nitrogen dioxide or dinitrogen trioxide, both toxic oxidizing and nitrosating agents [41]. Nitric oxide is generated in biological tissues by specific nitric oxide synthases [47], through the reaction of H2O2 with arginine [48] or through the decomposition of S-nitroso thiols in the presence of metal ions [49].
Nitric oxide is soluble in water and fat, and it therefore diffuses readily through the cytoplasm and plasma membrane. If human blood plasma is exposed to NO•, ascorbic acid and uric acid concentrations become depleted and lipid peroxidation is triggered [1]. Nitric oxide-derived species in cell membranes and lipoproteins react quickly with fatty acids and lipid peroxyl radicals during lipid oxidation, generating oxidized and nitrated products of free lipids and esterified cholesterol [50]. Nitric oxide is also involved in many physiological processes, such as neuro-transmission, relaxation of smooth muscle, vasodilation and regulation of blood pressure, gene expression, defense mechanisms, cell function and regulation of inflammatory and immune mechanisms, as well as in pathological processes such as neurodegenerative disorders and heart diseases [51].
1.11. Nitrogen Dioxide (NO2•)
Unlike nitrous oxide (N2O), nitrogen dioxide (NO2•) can be considered a free radical because the electrons are not paired. It is formed by the reaction of the peroxyl radical and NO in polluted air and smoke [52]. Nitrogen dioxide is a moderately strong oxidant, with reactivity between those of NO• and ONOO− [1]. NO2• reacts with organic molecules at rates ranging from ~104 to 106 M/s, depending on pH [19]. Two NO2• radicals can be dimerized to the highly reactive dinitrogen tetroxide (N2O4). Nitrogen dioxide can affect antioxidant mechanisms, causing the oxidation of ascorbic acid, which leads to lipid peroxidation and free radical production [53].
1.12. Peroxynitrite (ONOO−)
Peroxynitrite (ONOO−) is formed by the reaction of nitric oxide and superoxide anion. It is highly toxic and can react directly with CO2 to form other highly reactive nitroso-peroxo-carboxylates (ONOOCO2−) or peroxynitrous acid (ONOOH), which may undergo further homolysis to form •OH and NO2• or rearrange to form NO3 [54]. Peroxynitrite diffuses readily across cell membranes [9]; it can oxidize lipids, methionine residues and tyrosine in proteins and DNA to nitroguanine [13][46]. It acts as an oxidant in a similar way to the hydroxyl radical [1]. Nitrotyrosine residues are considered markers of cell damage induced by peroxynitrite and have been associated with tissues aging [9][13]. Peroxynitrite causes tissue injury and oxidizes low-density lipoprotein (LDL); it seems to be generated at sites of inflammation [9][53].
1.13. Reactive Sulfur Species
Sulfur is very abundant in nature and in the human body, and it has been implicated in the origin of life [55]. Organic derivatives of sulfur can form thiols (−2), disulfides (−1), sulfenic acids or sulfoxides (0), sulfinic acid (+2) and sulfonic acids (+4). By analogy with ROS and RNS, these compounds are identified as reactive sulfur species (RSS) [56]. Thiols can generate free radicals. In the presence of traces of transition metal ions, thiol compounds are oxidized to thiyl radicals (RS•) and reactive oxygen species. Pro-oxidative action takes place by means of reduction of transition metals such as Fe3+ to Fe2+, leading to the formation of thiyl radicals and the generation of a superoxide radical anion. The biochemistry of thiols, hydrogen sulfide (H2S) and its sulfane sulfur derivatives enables roles in protein structure/folding, cell redox homeostasis, signaling, metal ligation, cell protection, enzymology, metabolism and mitochondrial function [57]. Humans and animals are continuously exposed to many exogenous thiols and related disulfides. Thiol compounds can be found in food, contaminants and products in which sulfur-containing substances are breaking down.
Hydrogen sulfide (H2S) is the hydrogenated sulfur compound with the lowest oxidation state (−2). It is slightly hydrophobic and soluble in lipid membranes, which it crosses rapidly, diffusing between compartments. H2S exerts many physiological activities with potential health benefits [55]. It is mostly synthesized enzymatically, but also non-enzymatically in mammalian tissues [58], and it is produced via different pathways, in mitochondria [59], the kidneys and the brain [60]. Hydrogen sulfide has traditionally been considered toxic to mammals because of its inhibitory effect on cytochrome c oxidase, interrupting oxidative phosphorylation [61]. Since the identification of nitric oxide (NO) and carbon monoxide (CO) as gasotransmitters, H2S has been recognized as the third gasotransmitter [57]. Similar to NO and CO, H2S was considered to regulate various physiological and pathological processes.
Recently, the biological action and signaling of hydrogen sulfide [55] have stimulated interest in species related to H2S and/or as possible mediators/biological effectors derived from it. The biological effects mediated by H2S have mainly been attributed to the persulfidation of proteins, as shown by its vasorelaxant effect mediated by the activation of ATP-sensitive K+ [62]. H2S also blocks the generation of mitochondrial ROS through the induction of p66Shc persulfidation [63] and reduces the advanced toxicity of glycation end products through persulfidation of their receptor [64]. It is involved in inflammatory processes [65], inhibiting leukocyte adherence [66] and in carcinogenesis [67]. Polysulfides protect neurons from oxidative stress through the activation of the Keap1/Nrf2 (Kelch-like ECH associated protein 1/transcription nuclear factor erythroid 2-related factor 2) system and also induce neurite growth [68]. H2S appears to inhibit atherogenesis and platelet aggregation [69], and it has been shown to protect against ischemia-reperfusion damage by the preservation of mitochondrial function [70]. It is stressed that, in biological systems, sulfur species, such as hydrogen sulfide, disulfides, hydropersulfides, dialkyltrisulfides and thiols, are all in dynamic equilibrium [71]. Hydropersulfide, not H2S, has been proposed as a new product of interest in signaling research; in many situations, H2S could be a marker of its presence [72].
2. Generation of Free Radicals
Radicals can be formed by mechanisms other than the addition of a single electron to a non-radical. They can form by homolytic fission, when a covalent bond (C–C, C–H or C–O) is broken and one electron from the bonding pair remains on each atom. These covalent bonds are difficult to break; others are more easily broken, such as disrupted disulfide bonds that generate sulfur radicals [73], whereas the O–O bond in H2O2 is divided by exposing it to ultraviolet light, generating •OH. Sources of free radicals may be endogenous or exogenous. Endogenous sources, generated during normal metabolism, include different cell organelles, such as mitochondria, peroxisomes and endoplasmic reticulum, many enzyme activities, fatty acid metabolism and phagocytic cells [74]. Exogenous sources include radiation X-rays, γ-rays, ultraviolet A, visible light in the presence of a sensitizer, chemical reagents such as heavy or transition metals (e.g., Cd, Hg, Pb, As, metal ions such as Fe2+ and Cu+), HONOO, ozone, N2O2, deoxyosones, ketamine, H2O2, HOCl and HOBr, cooking (smoked meat, used cooking oil), high temperatures, environmental pollutants (aromatic hydrocarbons, pesticides, polychlorinated biphenyls, dioxins and many others), microbial infections, drugs and their metabolites [14][75][76].
2.1. Mitochondria
All the cells of the human body rely on adenosine triphosphate (ATP) to store and transport chemical energy. The body uses molecular oxygen to produce energy via oxidative phosphorylation in mitochondria. Mitochondria generate more than 90% of ATP by oxidative phosphorylation [77], consuming about 85% of the oxygen requirements of the cell to do so. Most of the oxygen is reduced to water, and a small proportion is converted to free radicals. The phosphorylation unit combines oxygen and hydrogen to produce H2O and ATP molecules. The oxidative unit consists mainly of a series of protein complexes in the inner mitochondrial membrane (IMM), known as the respiratory or electron transfer chain (ETC). Hydrogen atoms are known as reducing equivalents. The passage of hydrogen atoms along the respiratory chain is equivalent to the passage of electrons through sequential redox reactions along protein complexes I-IV of the ETC [78], where O2 is reduced to H2O. The production of ATP by oxidative phosphorylation associated with the ETC has an energy loss in the form of electrons [79], which determines the production of free radicals. In eukaryotic organisms, over 90% of ROS are produced by the mitochondrial ETC as a by-product of respiration [80]. A quantity of ROS are also produced by the ETC in the plasma [81], nuclear [82] and endoplasmic reticulum [83] membranes.
Reactive oxygen species generated as by-products of mitochondrial electron transfer mainly include the superoxide radical anion and hydrogen peroxide. A multielectron reduction of O2 is carried out by protein complexes in the ETC. By virtue of its electron configuration (two unpaired electrons in the outer shell), the oxygen molecule is not very reactive [27] and consequently tends to accept electrons one at a time. If O2 accepts a single electron, the electron must enter an antibonding orbital, producing the superoxide radical O2•−. A two-electron reduction of O2, with the addition of 2H+, generates hydrogen peroxide (H2O2). A one-electron reduction of H2O2 forms a hydroxyl radical (HO•) and a hydroxyl anion HO−. Water is formed after the electron and proton addition to HO•. Although the ETC is a highly efficient system, the redox reactions predispose electron vectors to reactions with molecular oxygen. Thus, up to 2% of electrons leak along the ETC and react directly with oxygen in a one-electron reduction to produce a superoxide (radical anion) instead of a water molecule [84]. About 5% of the oxygen consumed by living organisms can be converted to O2•− by mitochondria under physiological conditions [76]. The production of O2•− in mitochondria is estimated to be approximately 2 to 3 nmol/min per mg of protein [5], confirming its importance as the main source of this radical in living organisms.
Mitochondria are the most significant intracellular source of O2•−. An O2•− concentration 5 to 10 times greater has been estimated in mitochondria than in the nuclear space or the cytosol [80]. Ubiquinone links complex I with III and II with III and is regarded as a major player in the formation of O2•−. The oxidation of ubiquinone proceeds in a set of reactions known as the Q-cycle, and the unstable semiquinone is responsible for O2•− formation [30]. The transfer of electrons from complex I or II dehydrogenase to coenzyme Q or ubiquinone (Q) leads to the formation of a reduced form of coenzyme Q (QH2) that regenerates coenzyme Q via an unstable intermediate semiquinone anion Q•−. The latter transfers electrons to molecular oxygen, leading to the formation of superoxide radical [30]. Since the generation of superoxide is not enzymic, most ROS production will be linked to the higher metabolic rate.
Additionally, mitochondrial superoxide is generated by electron-transfer during fatty acid oxidation, by glycerol-3-phosphate dehydrogenase and other IMM-associated oxidoreductases [85]. The superoxide anion (O2•−) serves as a ROS precursor. Most O2•− is readily metabolized to non-radical H2O2 by superoxide dismutase (SOD) or non-enzyme mechanisms [86]. The subsequent Haber–Weiss reaction of H2O2 and O2•− [87], or Fe2+- (or Cu2+)-driven Fenton cleavage of H2O2 [88], may generate the highly reactive hydroxyl radical (•OH).
The H2O2 produced is in its optimum state for respiration, characterized by a high degree of reduction of the electron carriers and a limiting supply of adenosine diphosphate (ADP) [89]. An additional source of H2O2, not related to breathing, is situated on the external mitochondrial membrane [90], where the oxidative deamination of biogenic amines by monoamine oxidases is associated with the direct two-electron reduction of O2 to H2O2. The hydrogen peroxide produced during the oxidative deamination of catecholamines may be involved in neurodegenerative disorders such as Parkinson’s and Alzheimer’s diseases, presumably through oxidative damage to the mitochondrial membrane [91]. The factors that control the ETC generation of ROS in vivo are not fully understood. Antioxidant enzymes can eliminate ROS. SODs convert O2•− to H2O2, and many enzymes, such as catalase, glutathione peroxidase and peroxiredoxin 3, remove H2O2 [4]. Moreover, the signaling capacity of ROS may be altered by mitochondrial localization. Since ROS are molecules of short duration, the location of their production or signaling site can increase their efficiency.
Conventionally, complex I and complex III, including complex II, are considered the major contributors to ROS production [92]. However, the relative contribution of each site to the total production of O2•− and H2O2 varies from one organ to another and depends on respiration rate and redox state [4][30]. The different sites of ROS production have distinct signaling roles and presumably change under different physiological conditions [92]. It is therefore difficult to pinpoint the specific site of ROS production [93]. Up to eleven distinct mitochondrial sites of production of superoxide and/or hydrogen peroxide linked to substrate catabolism, electron transport and oxidative phosphorylation were recently identified in mammalian mitochondria [4][93]. Sites I [94] and III [30] are considered to generate predominantly or exclusively superoxide. Site II may generate both superoxide and hydrogen peroxide [92]. These sites may also act as sources of mitochondrial redox signal. H2O2 is the primary form of ROS utilized for intracellular signaling. Individual sites of ROS production are implicated in specific pathologies. Parkinson’s disease and longevity are linked to superoxide production from the flavin- and ubiquinone (Q)-binding sites of respiratory complex I [95], ROS from the complex II flavin is linked to Huntington’s disease and cancer [96][97] and ROS from complexes I, II and III and from mitochondrial glycerol phosphate dehydrogenase and matrix dehydrogenases are all invoked in ischemia/reperfusion injury [98][99].
Since most ATP is produced by mitochondria, impaired mitochondrial function is implicated in a variety of health chronic conditions and degenerative diseases [100], many of which can be attributed to excessive mitochondrial production of ROS. However, modest levels of ROS stimulate essential biological processes, such as proliferation, differentiation and immunity [101]. Furthermore, mitohormesis [102], a decrease in the net basal metabolism production of ROS, which increases resistance to oxidative stress [101], may be a way to improve mitochondrial function and resistance to chronic and degenerative diseases. Mitohormesis, a defense mechanism, can therefore promote health and increase longevity through the prevention or delay of diseases [102][103].
2.2. Peroxisomes
In peroxisomes, the respiratory pathway involves the transfer of electrons from various metabolites to oxygen, leading to the formation of H2O2 with the release of free energy in the form of heat [104]. It is not coupled to the production of ATP by oxidative phosphorylation [105]. Other free radicals produced in peroxisomes include O2•−, •OH and NO•. β-oxidation of fatty acids is the main metabolic process producing H2O2 in peroxisomes. However, different peroxisomal enzymes, such as acyl CoA oxidase, d-amino acid oxidase, L-a-hydroxy oxidase, urate oxidase, xanthine oxidase and D-aspartate oxidase, have been shown to produce different ROS [106]. Peroxisome and β-oxidation alterations are involved in many conditions and diseases, such as neurological disorders, and in the development of cancer [107].
2.3. Endoplasmic Reticulum
The electron transport chain of the endoplasmic reticulum is the second greatest source of ROS [83]. Catabolism of cell and foreign chemicals by cytochrome P450 includes redox steps and is responsible for the production of ROS in the endoplasmic reticulum. The enzymes of the endoplasmic reticulum that contribute to the formation of ROS include cytochrome P450, b5 enzymes and diamine oxidase [108]. Another important thiol oxidase, Erop1p, catalyzes the transfer of electrons from dithiols to molecular oxygen, resulting in the formation of H2O2 [109]. Other endogenous sources of ROS include the auto-oxidation of adrenalin, reduced riboflavin, inflammation, mental stress, over-exertion, infection, cancer, aging and ischemia [108].
2.4. Role of the Enzyme System
A variety of oxidative enzymes that occur in cells can produce free radicals. Those catalyzing ROS generation include nitric oxide synthases, NADPH oxidase, prostaglandin synthase, xanthine oxidase, lipoxygenases, ribonucleotide reductase, glucose oxidase, myeloperoxidase, cyclooxygenases and cytochrome P450 [14]. A certain quantity of ROS is produced by various oxidases. For example, xanthine oxidase and cytochrome P450 reductase mainly produce the superoxide anion radical, while oxidases of amino acids and glucose mainly generate hydrogen peroxide [110]. In particular, under normal physiologic conditions, xanthine oxidase acts as a dehydrogenase, removing hydrogen from xanthine or hypoxanthine and binding it to nicotinamide adenine dinucleotide (NAD), thus generating the NADH.
Lipoxygenase generates free radicals; it can convert PUFA to hydroperoxides once Fe2+ has been oxidized to Fe3+ [111]. The three major mammalian lipoxygenases are 5-, 12-, and 15-lipoxygenase; they can oxidize arachidonic acid, abundant in the central nervous system, to hydroperoxyeicosatetraenoic acid. In addition, 15-lipoxygenase has been identified in atherosclerotic lesions, suggesting that the enzyme may be involved in the formation of oxidized lipids in vivo [9].
High ROS levels are also generated by immune cells (lymphocytes, granulocytes and phagocytes) which defend the body against invading microorganisms [112]. Macrophages and neutrophils contain NADPH oxidase complex, which, when activated, generates superoxide radicals and hydrogen peroxide. The latter then interacts with intracellular chloride ions to produce hypochlorite, which destroys the pathogen. Patients with chronic granulomatous disease, in which ROS production is drastically reduced by NADPH oxidase complex, are highly sensitive to infections and usually die at an early age [113]. The main enzyme expressed by neutrophils is myeloperoxidase. With heme as a cofactor, it produces hypochlorous acid from hydrogen peroxide and chloride anion [114]. It also oxidizes tyrosine to the tyrosine radical. Hypochlorous acid and the tyrosine radical are both cytotoxic and are used by neutrophils to kill pathogenic organisms [115].
Cytochrome P450 molecules use O2 in their biochemical reactions and generate small amounts of ROS. The amount of ROS produced varies depending on the compound degraded and the cytochrome P450 molecule involved. A molecule particularly active in the production of ROS is cytochrome P450 2E1 [116].
2.5. Role of Metals
The production of free radicals through reactions mediated by transition metals is well established [1][12]. Almost all transition metal ions have the ability to function in various oxidation states. In the active redox state, these ions may act as catalysts in the autoxidation of many biomolecules. In most situations, the oxidation of biomolecules is initiated by the hydroxyl radical (HO•) generated in Fenton and Fenton-like reactions between redox-active transition metal ions and hydrogen peroxide [117]. In biological systems, a two-step reaction may occur in the presence of metal ions, especially free iron, more important because of its abundance in biological material, or copper, leading to the production of hydroxyl radicals. Hydrogen peroxide can produce the hydroxyl radical by removing an electron from the participating metal ion [1]. In the second step, the superoxide radical is involved in regenerating the original metal ions, making them newly available for the reaction with hydrogen peroxide. The two chemical reactions support the role of metals such as iron and copper in creating oxidative stress and cell injury by ROS. Again, the redox state of the transition metal is more important for pro-oxidant activity than its concentration. The ferrous ion (Fe2+) is a stronger pro-oxidant than the ferric ion (Fe3+), which only shows pro-oxidant activity in the presence of a reducing agent, such as ascorbic acid [1]. The pro-oxidant activity of transition metals includes the decomposition of lipid hydroperoxides into free radicals capable of initiating or propagating lipid peroxidation [118]. The metals can decompose hydroperoxides to peroxyl and alkoxyl radicals and greatly accelerate lipid oxidation [53]. The ferric and ferrous ions can both be catalysts in the degradation of lipid hydroperoxides to hydroperoxide-derived free radicals, but the catalytic activity of the ferrous ion is superior to that of the ferric ion. Moreover, the alkoxy radical is more reactive in the abstraction of a labile hydrogen atom than the peroxyl radical [12]. Because of the fundamental contribution of iron to the formation of hydroxyl radicals, any increase in cell concentration of free iron promotes the generation of ROS and oxidative stress [119].
3. Detection of ROS and RNS
The formation of ROS and RNS can be monitored by a variety of procedures, including fluorometric and spectrophotometric methods, chemiluminescence and electron paramagnetic resonance [30]. Many of these methods are based on the redox properties of specific ROS or RNS and are therefore subject to artifacts caused by species of similar reactivity or by reactive intermediates produced by the probe itself [120]. Electron paramagnetic resonance (EPR) spectroscopy has been studied to measure ROS, RNS and their secondary products [121]. The method is very suitable for the direct detection of free radicals at concentrations up to 1 μM. Due to its low sensitivity, EPR can measure ROS directly in vivo. It differs from other methods by virtue of its unique ability to detect free radicals with short and long half-lives, and it can provide information on oxygen/nitrogen radicals and related processes. Since NO is a free diatomic radical, it can be detected directly by EPR, even in tumors [122]. and so on. This definition is more convenient for discussions of transient chemical processes and astrochemistry; therefore researchers in these fields prefer to use this loose definition.[28]
7. Depiction in Chemical Reactions
In chemical equations, radicals are frequently denoted by a dot placed immediately to the right of the atomic symbol or molecular formula as follows:
- [math]\displaystyle{ \mathrm{Cl}_2 \; \xrightarrow{UV} \; 2 {\mathrm{Cl} ^\bullet} }[/math]
Radical reaction mechanisms use single-headed arrows to depict the movement of single electrons:
The homolytic cleavage of the breaking bond is drawn with a 'fish-hook' arrow to distinguish from the usual movement of two electrons depicted by a standard curly arrow. The second electron of the breaking bond also moves to pair up with the attacking radical electron; this is not explicitly indicated in this case. Radicals also take part in radical addition and radical substitution as reactive intermediates. Chain reactions involving radicals can usually be divided into three distinct processes. These are initiation, propagation, and termination.
- Initiation reactions are those that result in a net increase in the number of radicals. They may involve the formation of radicals from stable species as in Reaction 1 above or they may involve reactions of radicals with stable species to form more radicals.
- Propagation reactions are those reactions involving radicals in which the total number of radicals remains the same.
- Termination reactions are those reactions resulting in a net decrease in the number of radicals. Typically two radicals combine to form a more stable species, for example:
- 2 Cl• → Cl2