According to the United Nations Office on Drugs and Crime (UNODC), new psychoactive substances (NPS) are classified as “substances of abuse, either in a pure form or a preparation, that are not controlled by the 1961 Single Convention on Narcotic Drugs or the 1971 Convention on Psychotropic Substances, but which may pose a public health threat”. Some substances are used as stimulants, in this case, synthetic stimulants include aminoindanes, phenethylamines, piperazines, synthetic cathinones, and tryptamines, of which synthetic cannabinoids and synthetic cathinones are the largest groups. As stimulant drugs, their biological action occurs mainly on the dopamine and serotonin neurotransmitters, and to a lesser extent on epinephrine, which is later responsible for the stimulatory effects of these substances.
- psychoactive substances
- Piperazines
- Aminoindanes
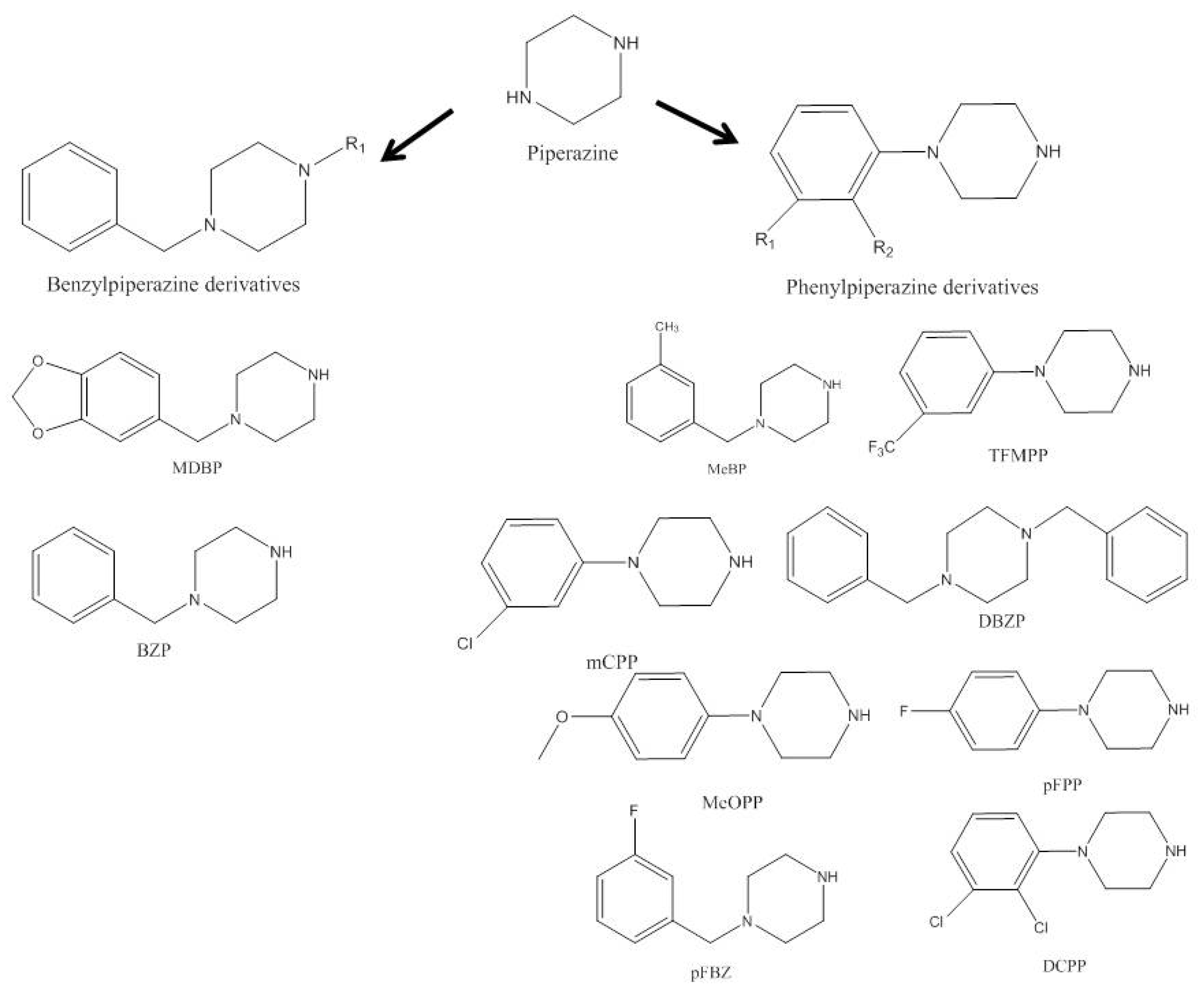
1. Piperazines
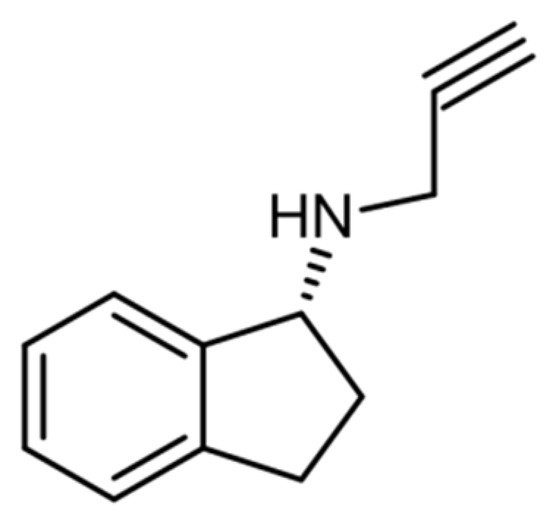
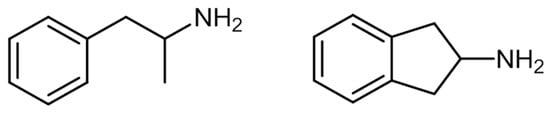
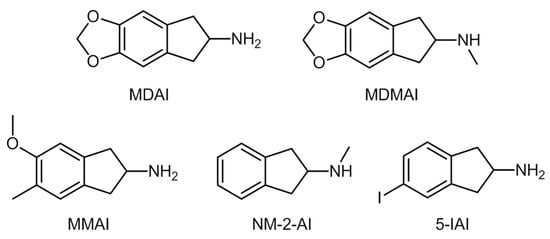
2. Aminoindanes
References
- Arbo, M.D.; Bastos, M.L.; Carmo, H.F. Piperazine compounds as drugs of abuse. Drug Alcohol Depend. 2012, 122, 174–185.
- Souto, C.; Göethel, G.; Peruzzi, C.P.; Cestonaro, L.V.; Garcia, I.; Ávila, D.S.; Eifler-Lima, V.; Carmo, H.; Bastos, M.D.L.; Garcia, S.C.; et al. Piperazine designer drugs elicit toxicity in the alternative in vivo model Caenorhabditis elegans. J. Appl. Toxicol. 2020, 40, 363–372.
- Welz, A.; Koba, M. Piperazine derivatives as dangerous abused compounds. Acta Pharm. 2020, 70, 423–441.
- Zhang, R.H.; Guo, H.Y.; Deng, H.; Li, J.; Quan, Z.S. Piperazine skeleton in the structural modification of natural products: A review. J. Enzyme Inhib. Med. Chem. 2021, 36, 1165–1197.
- Moreno, I.E.D.; da Fonseca, B.M.; Barroso, M.; Costa, S.; Queiroz, J.A.; Gallardo, E. Determination of piperazine-type stimulants in human urine by means of microextraction in packed sorbent and high performance liquid chromatography-diode array detection. J. Pharm. Biomed. Anal. 2012, 61, 93–99.
- Luethi, D.; Liechti, M.E. Designer drugs: Mechanism of action and adverse effects. Arch. Toxicol. 2020, 94, 1085.
- Elliott, S. Current awareness of piperazines: Pharmacology and toxicology. Drug Test. Anal. 2011, 3, 430–438.
- Lau, T.; LeBlanc, R.; Botch-Jones, S. Stability of synthetic piperazines in human whole blood. J. Anal. Toxicol. 2018, 42, 88–98.
- Meyer, M.R.; Peters, F.T. Analytical toxicology of emerging drugs of abuse-An update. Ther. Drug Monit. 2012, 34, 615–621.
- Zapata, F.; Matey, J.M.; Montalvo, G.; García-Ruiz, C. Chemical classification of new psychoactive substances (NPS). Microchem. J. 2021, 163, 105877.
- European Monitoring Centre for Drugs and Drug Addiction. Benzylpiperazine (BZP) and other Piperazines Drug Profile. Available online: https://www.emcdda.europa.eu/publications/drug-profiles/bzp_en (accessed on 4 December 2021).
- Moreno, I.E.D.; da Fonseca, B.M.; Magalhães, A.R.; Geraldes, V.S.; Queiroz, J.A.; Barroso, M.; Costa, S.; Gallardo, E. Rapid determination of piperazine-type stimulants in human urine by microextraction in packed sorbent after method optimization using a multivariate approach. J. Chromatogr. A 2012, 1222, 116–120.
- Byrska, B.; Zuba, D.; Stanaszek, R. Determination of piperazine derivatives in “legal highs”. Probl. Forensic Sci. 2010, LXXXI, 101–113.
- European Monitoring Centre for Drugs and Drug Addiction. New Psychoactive Substances in Prison. Available online: https://www.emcdda.europa.eu/system/files/publications/8869/nps-in-prison.pdf (accessed on 8 December 2021).
- Darke, S.; Duflou, J.; Peacock, A.; Farrell, M.; Lappin, J. Characteristics and circumstances of death related to new psychoactive stimulants and hallucinogens in Australia. Drug Alcohol Depend. 2019, 204, 107556.
- Lee, H.; Wang, G.Y.; Curley, L.E.; Sollers, J.J.; Kydd, R.R.; Kirk, I.J.; Russell, B.R. Acute effects of BZP, TFMPP and the combination of BZP and TFMPP in comparison to dexamphetamine on an auditory oddball task using electroencephalography: A single-dose study. Psychopharmacology 2016, 233, 863–871.
- Gaillard, Y.P.; Cuquel, A.C.; Boucher, A.; Romeuf, L.; Bevalot, F.; Prevosto, J.M.; Menard, J.M. A Fatality Following Ingestion of the Designer Drug Meta-Chlorophenylpiperazine (mCPP) in an Asthmatic-HPLC-MS/MS Detection in Biofluids and Hair. J. Forensic Sci. 2013, 58, 263–269.
- Dias da Silva, D.; Silva, M.J.; Moreira, P.; Martins, M.J.; Valente, M.J.; Carvalho, F.; Bastos, M.L.; Carmo, H. In vitro hepatotoxicity of ‘Legal X’: The combination of 1-benzylpiperazine (BZP) and 1-(m-trifluoromethylphenyl)piperazine (TFMPP) triggers oxidative stress, mitochondrial impairment and apoptosis. Arch. Toxicol. 2017, 91, 1413–1430.
- Cohen, B.M.Z.; Butler, R. BZP-party pills: A review of research on benzylpiperazine as a recreational drug. Int. J. Drug Policy 2011, 22, 95–101.
- Antia, U.; Tingle, M.D.; Russell, B.R. Validation of an LC-MS method for the detection and quantification of BZP and TFMPP and their hydroxylated metabolites in human plasma and its application to the pharmacokinetic study of TFMPP in humans. J. Forensic Sci. 2010, 55, 1311–1318.
- Antia, U.; Lee, H.S.; Kydd, R.R.; Tingle, M.D.; Russell, B.R. Pharmacokinetics of “party pill” drug N-benzylpiperazine (BZP) in healthy human participants. Forensic Sci. Int. 2009, 186, 63–67.
- Gijsman, H.J.; Van Gerven, J.M.A.; Tieleman, M.C.; Schoemaker, R.C.; Pieters, M.S.M.; Ferrari, M.D.; Cohen, A.F.; Van Kempen, G.M.J. Pharmacokinetic and pharmacodynamic profile of oral and intravenous meta-chlorophenylpiperazine in healthy volunteers. J. Clin. Psychopharmacol. 1998, 18, 289–295.
- Kersten, B.P.; McLaughlin, M.E. Toxicology and Management of Novel Psychoactive Drugs. J. Pharm. Pract. 2015, 28, 50–60.
- Hondebrink, L.; Zwartsen, A.; Westerink, R.H.S. Effect fingerprinting of new psychoactive substances (NPS): What can we learn from in vitro data? Pharmacol. Ther. 2018, 182, 193–224.
- Gee, P.; Schep, L. 1-Benzylpiperazine and other Piperazine-based Derivatives. In Novel Psychoactive Substance; Dargan, P.I., Wood, D.M., Eds.; Academic Press: London, UK, 2013; pp. 179–209.
- Katz, D.P.; Deruiter, J.; Bhattacharya, D.; Ahuja, M.; Bhattacharya, S.; Clark, C.R.; Suppiramaniam, V.; Dhanasekaran, M. Benzylpiperazine: “A messy drug”. Drug Alcohol Depend. 2016, 164, 1–7.
- Curley, L.E.; Kydd, R.R.; Kirk, I.J.; Russell, B.R. Differential responses to anticipation of reward after an acute dose of the designer drugs benzylpiperazine (BZP) and trifluoromethylphenylpiperazine (TFMPP) alone and in combination using functional magnetic resonance imaging (fMRI). Psychopharmacology 2013, 229, 673–685.
- Simmler, L.D.; Rickli, A.; Schramm, Y.; Hoener, M.C.; Liechti, M.E. Pharmacological profiles of aminoindanes, piperazines, and pipradrol derivatives. Biochem. Pharmacol. 2014, 88, 237–244.
- Musselman, M.E.; Hampton, J.P. “Not for human consumption”: A review of emerging designer drugs. Pharmacotherapy 2014, 34, 745–757.
- Schifano, F.; Orsolini, L.; Duccio Papanti, G.; Corkery, J.M. Novel psychoactive substances of interest for psychiatry. World Psychiatry 2015, 14, 15–26.
- Wilkins, C.; Sweetsur, P. The Impact of the Prohibition of Benzylpiperazine (BZP) ‘Legal Highs’ on the Prevalence of BZP, New Legal Highs and other Drug Use in New Zealand. Available online: https://khepri-node.dev.meta-infra.org/papers/the-impact-of-the-prohibition-of-benzylpiperazine/22819869 (accessed on 5 October 2021).
- Wood, D.M.; Button, J.; Lidder, S.; Ramsey, J.; Holt, D.W.; Dargan, P.I. Dissociative and sympathomimetic toxicity associated with recreational use of 1-(3-trifluoromethylphenyl) piperazine (TFMPP) and 1-benzylpiperzine (BZP). J. Med. Toxicol. 2008, 4, 254–257.
- Smith, C.D.; Robert, S. “Designer drugs”: Update on the management of novel psychoactive substance misuse in the acute care setting. Clin. Med. J. R. Coll. Physicians Lond. 2014, 14, 409–415.
- Costa de Souza e Escada, S. Métodos de Análise de Piperazinas em Fluidos Biológicos. Master’s Thesis, Univesidade de Aveiro, Aveiro, Portugal, 2007.
- Castaneto, M.S.; Barnes, A.J.; Concheiro, M.; Klette, K.L.; Martin, T.A.; Huestis, M.A. Biochip array technology immunoassay performance and quantitative confirmation of designer piperazines for urine workplace drug testing. Anal. Bioanal. Chem. 2015, 407, 4639–4648.
- Arbo, M.D.; Silva, R.; Barbosa, D.J.; da Silva, D.D.; Rossato, L.G.; Bastos, M.D.L.; Carmo, H. Piperazine designer drugs induce toxicity in cardiomyoblast h9c2 cells through mitochondrial impairment. Toxicol. Lett. 2014, 229, 178–189.
- Zwartsen, A.; de Korte, T.; Nacken, P.; de Lange, D.W.; Westerink, R.H.S.; Hondebrink, L. Cardiotoxicity screening of illicit drugs and new psychoactive substances (NPS) in human iPSC-derived cardiomyocytes using microelectrode array (MEA) recordings. J. Mol. Cell. Cardiol. 2019, 136, 102–112.
- Boumrah, Y.; Rosset, M.; Lecompte, Y.; Bouanani, S.; Khimeche, K.; Dahmani, A. Development of a targeted GC/MS screening method and validation of an HPLC/DAD quantification method for piperazines-amphetamines mixtures in seized material. Egypt. J. Forensic Sci. 2014, 4, 90–99.
- Thomas, J.M.; Dourish, C.T.; Tomlinson, J.; Hassan-Smith, Z.; Hansen, P.C.; Higgs, S. The 5-HT2C receptor agonist meta-chlorophenylpiperazine (mCPP) reduces palatable food consumption and BOLD fMRI responses to food images in healthy female volunteers. Psychopharmacology 2018, 235, 257–267.
- Felsinga, D.E.; Canala, C.E.; Bootha, R.G. Ligand-directed serotonin 5-HT2C receptor desensitization and sensitization. Eur J Pharmacol 2019, 848, 131–139.
- Scotton, W.J.; Hill, L.J.; Williams, A.C.; Barnes, N.M. Serotonin Syndrome: Pathophysiology, Clinical Features, Management, and Potential Future Directions. Int. J. Tryptophan Res. 2019, 12, 1178646919873925.
- Dias-da-Silva, D.; Arbo, M.D.; Valente, M.J.; Bastos, M.L.; Carmo, H. Hepatotoxicity of piperazine designer drugs: Comparison of different in vitro models. Toxicol. Vitr. 2015, 29, 987–996.
- Persona, K.; Polus, A.; Góralska, J.; Gruca, A.; Dembin´ska, A.; Dembin´ska-Kiec´2, D.; Kiec´2, K.; Piekoszewski, W. An In Vitro Study of the Neurotoxic Effects of N-Benzylpiperazine: A Designer Drug of Abuse. Neurotox. Res. 2016, 29, 558–568.
- Parrott, A.C. Mood fluctuation and psychobiological instability: The same core functions are disrupted by novel psychoactive substances and established recreational drugs. Brain Sci. 2018, 8, 43.
- Shelton, R.S. Secondary Beta Phenyl Propyl Amines and Pharmaceutical Compositions Thereof. U.S. Patent No. 2,298,630, 13 October 1942.
- Woodruff, E.H. Amino Compound. U.S. Patent No. 2,293,874, 25 August 1942.
- Manier, S.K.; Felske, C.; Eckstein, N.; Meyer, M. The metabolic fate of the two new psychoactive substances 2-aminoindane and N-methyl-2-aminoindane studied in vitro and in vivo to support drug testing. Drug Test. Anal. 2019, 12, 145–151.
- Levin, N.; Graham, B.E.; Kolloff, H.G. Physiologically active indanamines. J. Org. Chem. 1944, 9, 380–391.
- Witkin, L.B.; Heubner, C.F.; Galdi, F.; O’Keefe, E.; Spitaletta, P.; Plummer, A.J. Pharmacology of 2-amino-indane hydrochloride (Su-8629): A potent non-narcotic analgesic. J. Pharmacol. Exp. Ther. 1961, 133, 400–408.
- Solomons, E.; Sam, J. 2-Aminoindans of pharmacological interest. J. Med. Chem. 1973, 16, 1330–1333.
- Martin, Y.C.; Jarboe, C.H.; Krause, R.A.; Lynn, K.R.; Dunnigan, D.; Holland, J.B. Potential anti-Parkinson drugs designed by receptor mapping. J. Med. Chem. 1973, 16, 147–150.
- The Regents of the University of California Monoamine Oxidase B (MAO-B) Inhibitors. Available online: https://pdcenter.ucsf.edu/monoamine-oxidase-b-mao-b-inhibitors (accessed on 27 November 2021).
- Martin, Y.C.; Holland, J.B.; Jarboe, C.H.; Plotnikoff, N. Discriminant analysis of the relation between physical properties and the inhibition of monoamine oxidase by aminotetralins and aminoindans. J. Med. Chem. 1974, 17, 409–413.
- Youdim, M.B.H.; Gross, A.; Finberg, J.P.M. Rasagiline , a selective and potent inhibitor of mitochondrial monoamine oxidase B. Br. J. Pharmacol. 2001, 132, 500–506.
- Knudsen Gerber, D.S. Selegiline and rasagiline: Twins or distant cousins? Consult Pharm 2011, 26, 48–51.
- Pinterova, N.; Horsley, R.R.; Palenicek, T. Synthetic Aminoindanes: A Summary of Existing Knowledge. Front. Psychiatry 2017, 8, 236.
- Nichols, D.E.; Oberlender, R. Structure-activity-relationships of MDMA and related-compounds—A new class of psychoactive-drugs. Ann. N.Y. Acad. Sci. 1990, 600, 613–625.
- Nichols, D.E.; Hoffman, A.J.; Oberlender, R.A.; Jacob, P., III; Shulgin, A.T. Derivatives of 1-(1,3-benzodioxol-5-yl)-2-butanamine: Representatives of a novel therapeutic class. J. Med. Chem. 1986, 29, 2009–2015.
- Oberlender, R.; Nichols, D.E. (+)-N-methyl-1-(1,3-benzodioxol-5-yl)-2-butanamine as a discriminative stimulus in studies of 3,4-methylenedioxy-methamphetamine-like behavioral activity. J. Pharmacol. Exp. Ther. 1990, 255, 1098–1106.
- European Monitoring Centre for Drugs and Drug Addiction. Action on New Drugs. Available online: https://www.emcdda.europa.eu/html.cfm/index96437EN.html (accessed on 27 November 2021).
- United Nations Office on Drugs and Crime. Early Warning Advisory on New Psychoactive Substances: Aminoindanes. Available online: https://www.unodc.org/LSS/SubstanceGroup/Details/8fd64573-c567-4734-a258-76d1d95dca25 (accessed on 27 November 2021).
- Gill, H.; Gill, B.; Chen-Li, D.; El-Halabi, S.; Rodrigues, N.B.; Cha, D.S.; Lipsitz, O.; Lee, Y.; Rosenblat, J.D.; Majeed, A.; et al. The emerging role of psilocybin and MDMA in the treatment of mental illness. Expert Rev. Neurother. 2020, 20, 1263–1273.
- Leach, B. New Drug to Replace Mephedrone as ‘Legal High’. The Telegraph, 18 April 2010.
- United Nations Office on Drugs and Crime. The challenge of New Psychoactive Substances. Available online: https://www.unodc.org/unodc/en/scientists/the-challenge-of-new-psychoactive-substances---global-smart-programme.html (accessed on 3 November 2021).
- Corkery, J.M.; Elliott, S.; Schifano, F.; Corazza, O.; Ghodse, A.H. MDAI (5,6-methylenedioxy-2-aminoindane; 6,7-dihydro-5H-cyclopentabenzodioxol-6-amine; “sparkle”; ’mindy’) toxicity: A brief overview and update. Hum. Psychopharmacol. Clin. Exp. 2013, 28, 345–355.
- Coppola, M. Is the 5-iodo-2-aminoindan (5-IAI) the new MDMA? J. Addict. Res. Ther. 2012, 3, 1–3.
- Coppola, M.; Mondola, R. 5-Iodo-2-aminoindan (5-IAI): Chemistry, pharmacology, and toxicology of a research chemical producing MDMA-like effects. Toxicol. Lett. 2013, 218, 24–29.
- Kavanagh, P.; Sharma, J.; McNamara, S.; Angelov, D.; McDermott, S.; Mullan, D.; Ryder, S. Head Shop ‘Legal Highs’ Active Constituents Identification Chart (June 2010, Post-Ban). Available online: https://www.drugsandalcohol.ie/13204/ (accessed on 8 December 2021).
- PsychonautWiki MDAI/Summary. Available online: https://psychonautwiki.org/wiki/MDAI/Summary (accessed on 8 December 2021).
- Gross, P.; Smith, R.P. Biologic Activity of Hydroxylamine: A Review. Crit. Rev. Toxicol. 1985, 14, 87–99.
- Páleníček, T.; Lhotková, E.; Žídková, M.; Balíková, M.; Kuchař, M.; Himl, M.; Mikšátková, P.; Čegan, M.; Valeš, K.; Tylš, F.; et al. Emerging toxicity of 5,6-methylenedioxy-2-aminoindane (MDAI): Pharmacokinetics, behaviour, thermoregulation and LD50 in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 69, 49–59.
- Monte, A.P.; Maronalewicka, D.; Cozzi, N.V.; Nichols, D.E. Synthesis and pharmacological examination of benzofuran, indan, and tetralin analogs of 3,4-(methylenedioxy)amphetamine. J. Med. Chem. 1993, 36, 3700–3706.
- Gallagher, C.T.; Assi, S.; Stair, J.L.; Fergus, S.; Corazza, O.; Corkery, J.M.; Schifano, F. 5,6-methylenedioxy-2-aminoindane: From laboratory curiosity to ‘legal high’. Hum. Psychopharmacol. Clin. Exp. 2012, 27, 106–112.
- Elliott, S.; Evans, J. A 3-year review of new psychoactive substances in casework. Forensic Sci. Int. 2014, 243, 55–60.
