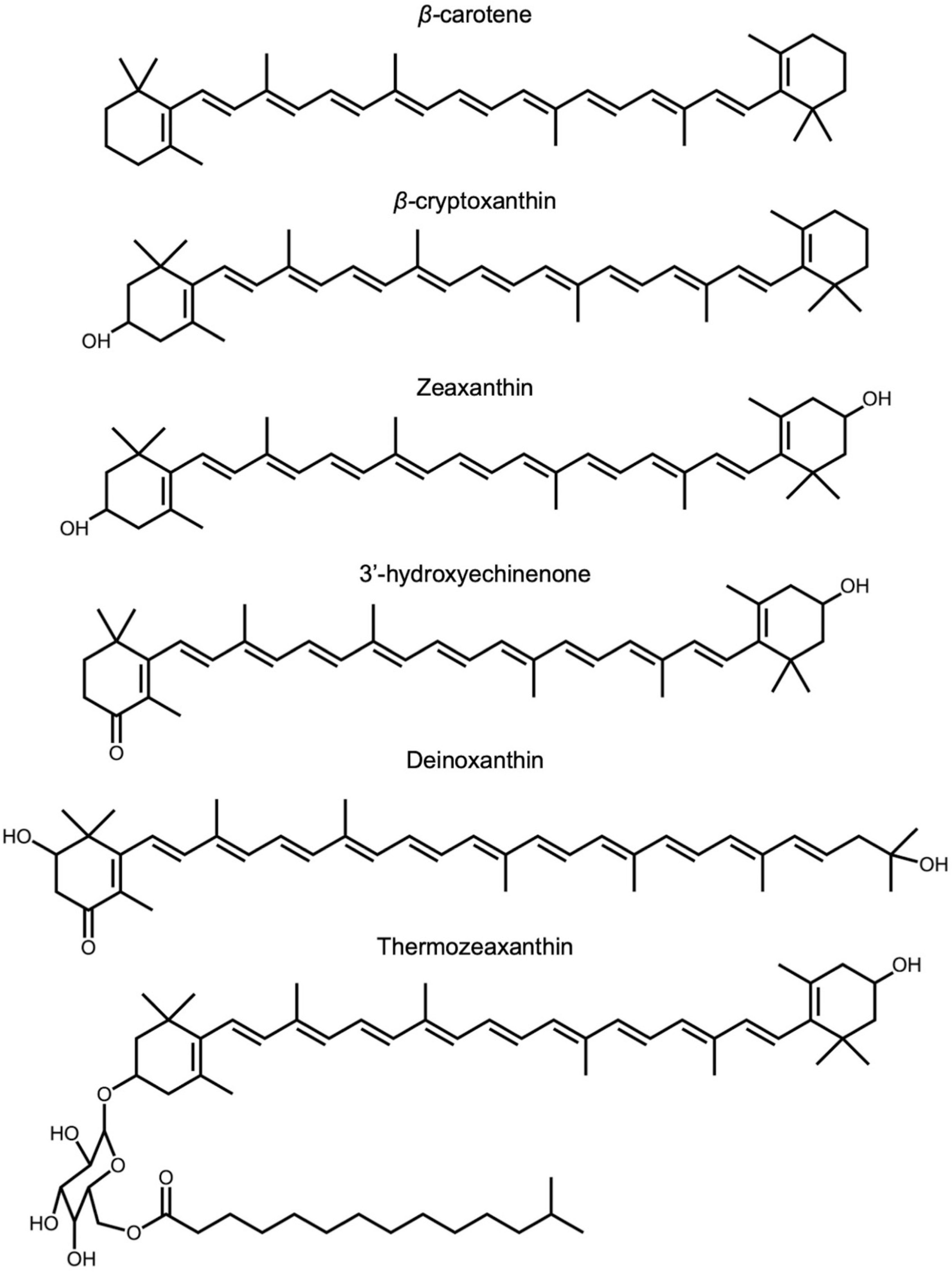
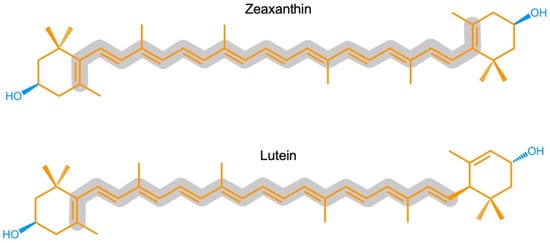
ZeaxaA synthin and lutein are structural isomers with esis is provided of the multiple roles of the carotenoids zeaxanthin possessing a slightly longer system of conjugated double bonds (11) than lutein. Carotenoids also play key roles in light-independent processes, e.g., as gene regulators of human immune function. Carotenoid-derived vitamin A has a well-documented immunoregulatory role and a similar role is emerging for and/or lutein in opposing (i) photodamage in plants, (ii) photodamage to the human eye as well as cognitive dysfunction and a host of human diseases and disorders, and (iii) damage to extremophile microorganisms in the most inhospitable environments on earth. Selected examples are used to examine microenvironments and basic biological structures with which these xanthophylls. Xanthophylls may be especially important in opposing non-resolving inflammation that can trigger a plethora of associated inflammatory diseases, disorders, and dysfunctions. Additionally, lutein and zeaxanthin are emerging as candidates for protecting cognitive function across the human lifespan, including attention, memory, learning, and executive function associate as well as the effect of organisms’ external environments. An overview is presented of the multiple principal mechanisms through which these xanthophylls can directly or indirectly impact organisms’ internal redox (oxidant/antioxidant) balance that provides input into the orchestration of growth, development, and defense in prokaryotic microorganisms, plants, and humans. Research gaps are identified, specifically with respect to the need for further in vivo assessment of mechanisms.
- antioxidant
- carotenoid
- inflammation
- lutein
- photosynthesis
- retina
- ROS
- zeaxanthin
1. Introduction
Carotenoids in a Nutshell
2. Xanthophylls in High-Stress Contexts
Zeaxanthin and lutein are structural isomers with zeaxanthin possessing a slightly longer system of conjugated double bonds (11) than lutein (10; Figure 1).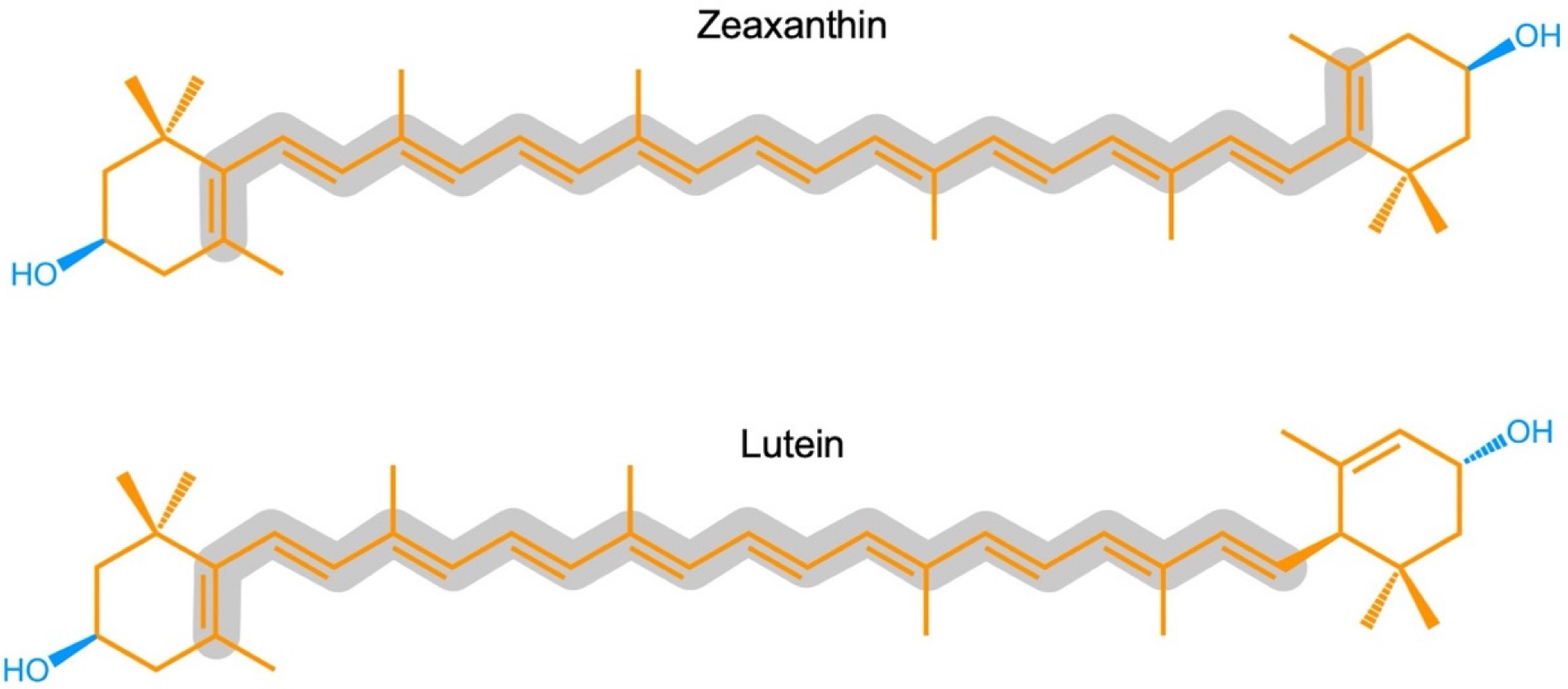
2.1. Zeaxanthin and Lutein in the Human Eye/Retina
Lutein and zeaxanthin are differentially distributed across the human retina (Figure 2). The yellow center of the eye (macula), where the brightest light is received, has the highest overall xanthophyll concentration and the highest ratio of zeaxanthin to lutein [11]. The total xanthophyll concentration is about 1 mM in the macula and declines to less than 10 μΜ in the peripheral regions of the retina [20][23]. In addition to zeaxanthin and lutein, meso-zeaxanthin (a zeaxanthin stereoisomer) is present in the macula and is apparently produced from dietary lutein but not from dietary zeaxanthin [21][24]. A recent study using confocal resonance Raman spectroscopy, validated by biochemical characterization of carotenoid composition, described the variation in the zeaxanthin-to-lutein ratio over short distances using continuous scans of xanthophyll composition across donor retinas [22][25]. The zeaxanthin-to-lutein ratios were 9:1 or greater in the center of the macula; 4:1 at a short distance (200 µm) from the center; and 1:4 just outside the macula (Figure 2; [22][25]). This preferential placement of zeaxanthin where the brightest light is received indicates a unique role of zeaxanthin in supporting the vision process in the presence of bright light. Still, this finding does not allow an assessment of which one(s) of the multiple possible roles of zeaxanthin is/are at work in this location. Original ideas (starting in 1861) about the function of the xanthophyll-rich macula initially centered on potential improvements in visual acuity and contrast sensitivity with reduced glare sensitivity and light scatter (see [11]). However, the subsequent rise of age-related macular degeneration in the human population shifted the focus of attention to photoprotection (see review [11]). Nevertheless, both principal roles are still discussed today, and multiple mechanisms are under consideration (see below).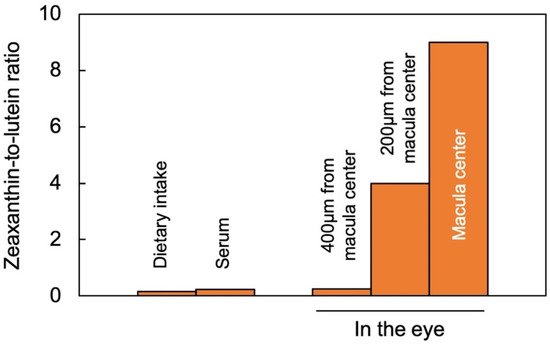
2.2. Zeaxanthin and Lutein in Leaves
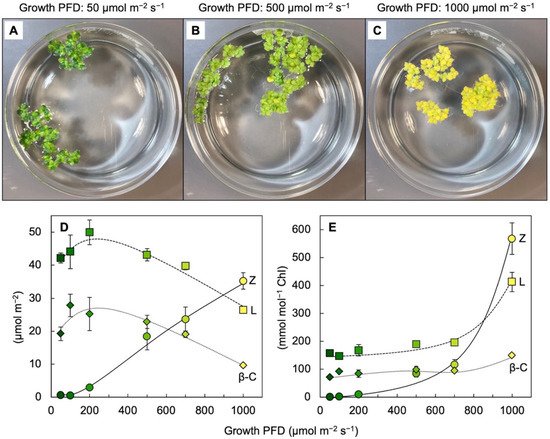
Leaves of plants growing in sunny locations under conditions favorable for growth rapidly form and remove zeaxanthin as the fraction of absorbed light not utilized in photochemistry rises and falls over the course of the day (e.g., [10][24][10,27]). Characterizations of the latter functional features was enabled by the development of portable instruments to measure chlorophyll fluorescence from leaves under field conditions and in the presence of bright light [25][26][28,29]. Zeaxanthin is formed in the presence of excess absorbed light from the di-epoxide violaxanthin via the mono-epoxide antheraxanthin in the xanthophyll cycle [27][28][30,31]. Violaxanthin levels exhibit complementary decreases and increases over the course of the day (e.g., [10][24][10,27]). However, the levels of other ubiquitous leaf carotenoids (lutein, β-carotene, and neoxanthin) do not typically change over the course of the day in sun-exposed habitats [10]. In slow-growing evergreens (that utilize only a low fraction of full sunlight for photochemistry), the ratio of zeaxanthin to lutein can approach unity in full sun at midday, but this ratio is much lower in most plant systems most of the time. Ample zeaxanthin for human nutrition is thus hard to come by when relying on rapidly growing leafy greens, harvested and then stored before consumption. For human nutrition, crops that combine rapid growth and high zeaxanthin levels would be desirable. RWesearchers recently reported on the unusual ability of aquatic floating plants (Lemnaceae, or duckweeds) to simultaneously grow very rapidly and accumulate exceptionally high levels of zeaxanthin [29][30][31][32,33,34]. Figure 3 shows the visual appearance and carotenoid levels for Lemna grown under a wide range of photon flux densities (PFDs) in which plant growth remained high, with plant area doubling every other day. Additionally, zeaxanthin levels continued to rise when absorbed light became increasingly excessive, whereas the levels of chlorophyll, lutein, and β-carotene declined (Figure 3D). The plants grown under the highest light intensity were bright yellow (Figure 3C), still grew very rapidly, and exhibited zeaxanthin-to-lutein ratios as high as ~1.3 (up from ~0.5 under the next lowest growth PFD). Due to the fact that the levels of chlorophyll a + b declined more sharply than those of any of the carotenoids under the highest-growth PFD, carotenoid levels increased relative to chlorophyll, and none more sharply than zeaxanthin (Figure 3E). A considerable portion of this zeaxanthin is presumably dissolved in the phospholipid portion of chloroplast membranes. A role for zeaxanthin, but not lutein, as a membrane-based antioxidant and/or membrane stabilizer was proposed for plants [32][33][35,36]. The sharp increase in the zeaxanthin-to-lutein ratio in duckweed at the highest PFD (Figure 3D) is reminiscent of the dynamics across the human eye described above.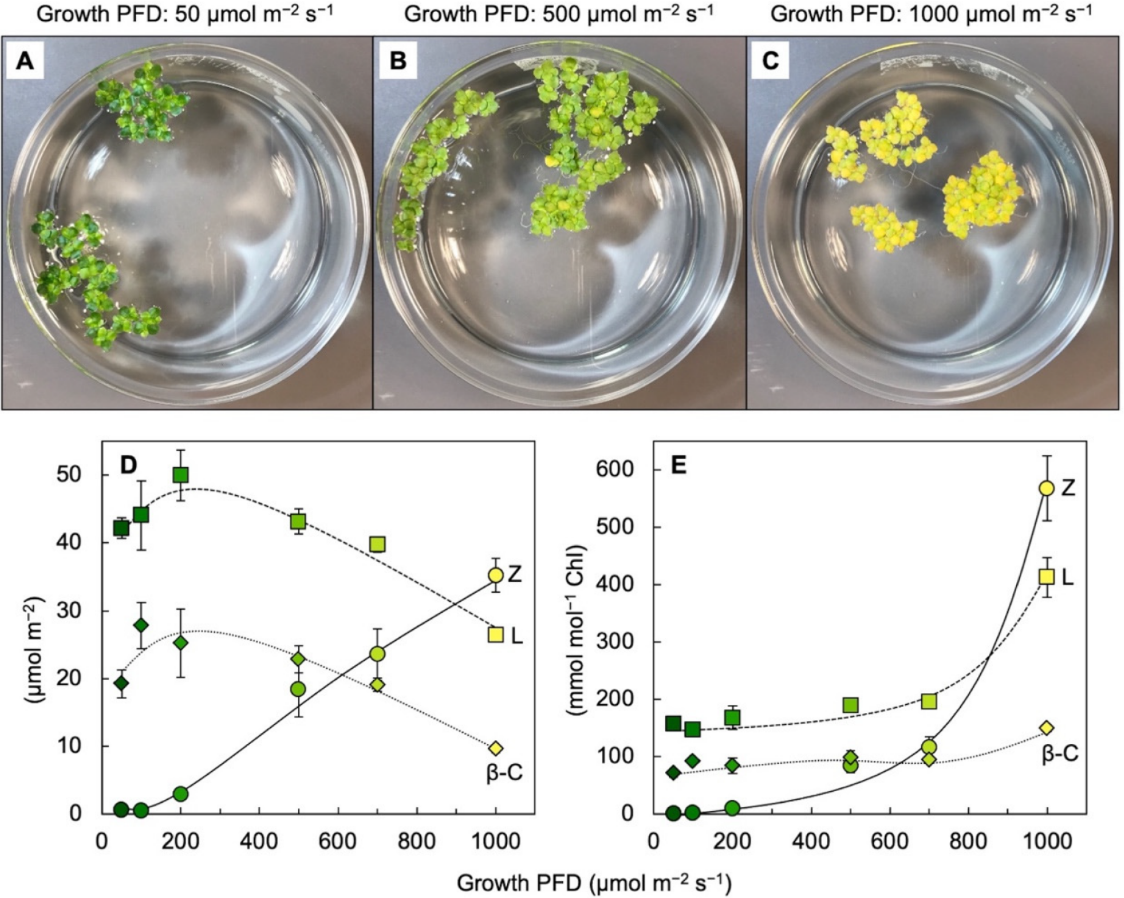
2.3. Zeaxanthin and Related Xanthophylls in Extremophiles
Among the over 1100 naturally occurring carotenoids described, only seven are synthesized de novo by organisms from all three domains of life [1]. The few known carotenoids synthesized by representatives of eukaryotes, bacteria, and archaea include zeaxanthin and its biosynthetic precursors (for a detailed review of carotenoid biosynthetic pathways among the taxa of life, see [37][40]). Zeaxanthin is found not only in light-absorbing/photosynthetic bacteria but also in non-photosynthetic bacteria and archaea. Although the functions of zeaxanthin and related xanthophylls (Figure 4) in these organisms are yet to be elucidated, the environments in which they occur expose these organisms to high levels of stress (visible or ionizing radiation, heat, or salinity). Among eukaryotes, fungi typically do not produce lutein or zeaxanthin but can produce a variety of other carotenoids [38][39][41,42] as well as many other pigments (e.g., melanins, flavins, phenazines, quinones, monascins, violacein, and indigo; [40][43]).