You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Elsa Marisa Vieira and Version 2 by Lindsay Dong.
Vitamin D is a lipophilic bioactive that plays an important role in bone health. Fortification of beverages, such as milk, fruit juices, teas, and vegetable drinks, could be an efficient strategy to prevent vitamin D deficiency and its associated effects on health.
- vitamin D-fortified beverages
- delivery systems
- stability
- bioavailability
1. Introduction
Vitamin D deficiency (VDD) is one of the most common micronutrient malnutrition disorders globally, affecting both developed and developing countries [1]. Its worldwide prevalence is up to 1 billion [2], a reason why some scautholars have pointed to VDD as a pandemic situation [3][4][5][3,4,5]. As exposure to UV rays is the main natural source of vitamin D3 (VD3), low sunlight exposure, due to factors such as seasonality, lifestyle, and use of sunscreens, greatly contribute to the prevalence of VDD [6][7][8][6,7,8]. Another contributing factor is vitamin D ingestion below the recommended dietary value (RDA), i.e., 10 µg per day for infants below 12 months, 15 µg per day for adults and 20 µg per day for the elderly above 70 years old [9]. Satisfying the RDA of vitamin D is a challenge given the limited availability of food sources of this nutrient [8].
Chemically, vitamin D is a lipophilic compound with two prevalent forms, ergocalciferol (vitamin D2, VD2), which is synthesized by ultraviolet radiation from plants, and cholecalciferol (VD3), which is formed in human skin and is present in animal foods [10]. Their chemical forms are illustrated in Figure 1; VD2 has a double bond between carbons 22 and 23 and a methyl group in its side chain, unlike VD3 [11].
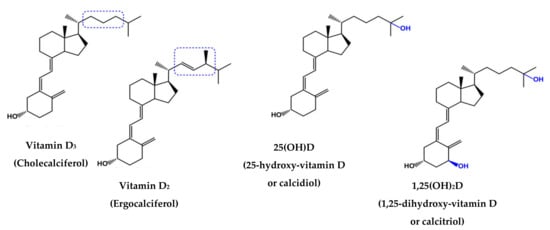
Figure 1.
Chemical structures of vitamins D3, D2, 25(OH)D and 1,25(OH)
2
2. Examples of Vitamin D-Fortified Beverages
Several beverages have been fortified with vitamin D, such as milk, fruit juices, vegetable drinks and tea. Table 1 presents some examples of these beverages and summarizes the information regarding the fortification level applied and the main effects of processing (e.g., temperature, humidity, pH, and ionic strength) and storage conditions on the stability and bioaccessibility of vitamin D in the formulated products.
Table 1.
Fortification approaches of vitamin D in different types of beverages.
| Fortified Beverage | Country | Formulation | Fortification Level | Processing | Vitamin Stability and Bioaccessibility |
Effects on Sensory Properties |
Effects on Health | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HTST 2% fat milk | USA | Water dispersible VD3 |
250 IU/240 mL | HTST (73 °C for 15 s) and storage at 4 °C for 21, 42 and 60 days | Tolerate HTST No loss of VD3 during storage at 4 °C |
No significant changes in composition and sensory attributes |
NE | [13] | [34] | ||||||
| UHT 2% fat chocolate milk |
USA | Water dispersible VD3 |
100 IU/240 mL | UHT (138 °C for 2 s) and storage at 4 °C for 21, 42 and 60 days | Tolerate UHT No loss of VD3 during storage at 4 °C |
No significant changes in composition and sensory attributes |
NE | [13] | [34] | ||||||
| Milk | Iran | ND | 100 IU/200 mL | NE | NE | Lower acceptance compared to orange juice |
↑[25(OH)D] serum levels |
[14] | [35] | ||||||
| Milk | India | VD3 Spray Drying | 600 IU or 1000 IU/200 mL | NE | Stability loss <10% after 12 weeks of storage period |
NE | ↑[25(OH)D] serum levels |
[15] | [36] | ||||||
| UHT 3% and 8.5% fat milk | India | VD2-protein complexes (NaCas-VD, SNaCas-VD, RNaCas-VD and RSNaCas-VD) | 500 IU/L | Pasteurization (63 °C/30 min), boiling and sterilization (121 °C for 15 min at 15 psi) |
Higher stability during storage at −20 °C, followed by 4 °C and 37 °C |
NE | NE | [16] | [37] | ||||||
| Cow and buffalo milk | India | VD2 Encapsulation |
600 IU/L | Pasteurization (63 °C/30 min), boiling and sterilization (121 °C for 15 min at 15 psi) |
Stable during pasteurization, boiling, sterilization, packaging, and storage conditions |
NE | NE | [17] | [38] | ||||||
| “Lassi” milk-based beverage | India | VD3-NLC | 400 IU/100 mL | Environmental stress conditions of temperature and humidity, pH, and ionic strength |
High physicochemical stability against temperature, pH, and ionic strength |
No significant changes in composition and sensory attributes |
NE | [18] | [39] | ||||||
| Goat milk kefir | Indonesia | VD3 | 42 IU/100 mL | Pasteurization at 72 °C for 15 s and cooling to 25 °C Different times of fermentation tested: 0, 6, 12, 18, and 24 h |
The highest level of VD3 was found after 6 h of fermentation |
Higher viscosity after 24 h of fermentation | NE | [19] | [40] | ||||||
| Orange juice and milk | USA | VD3 | 1000 IU/240 mL | NE | No loss of VD3 during 30 days of storage at 4 °C. The fat content of milk did not affect the bioavailability of VD3 |
NE | ↑[25(OH)D] serum levels |
[20] | [41] | ||||||
| Orange juice | USA | Water dispersible VD3 or VD2 |
1000 IU VD3 or VD2/240 mL orange juice or capsule |
NE | VD2 and VD3 were equally bioavailable in orange juice and capsules |
NE | ↑[25(OH)D] serum levels |
[21] | [42] | ||||||
| Orange juice | USA | ND | 100 IU/240 mL | NE | NE | NE | ↑[25(OH)D] serum levels |
[22] | [43] | ||||||
| Orange juice | Iran | ND | 100 IU/200 mL | NE | NE | Higher acceptance compared to orange juice |
↑[25(OH)D] serum levels | [14] | [35] | ||||||
| Pear juice | Romania | VD3-gum arabic- chitosan complex Spray drying |
0.002 g/100 mL | NE | No loss of VD3 during 7 days of storage at 4 °C | NE | NE | [23] | [44] | ||||||
| Oat-based beverage | Sweden | VD3 | 23 IU/100 g of liquid | Sterilization at 140 °C for 5 or 20 s | Stability loss of 60% | NE | NE | [24] | [45] | ||||||
| Almond and oat milks | ND | VD3 nanocellulose or TiO | 2 | nanoemulsion | 0.4 wt% | NE | Low bioaccessibility (~20%) of VD3 loaded in VD3-nanocellulose or TiO | 2 | nanoemulsion |
Nanocellulose increased the shear viscosity, while TiO | 2 | particles increased the whiteness of fortified milks | NE | [25] | [46] |
| Rooibos Tea | Canada | Water dispersible VD3 |
10,000 IU/200 mL | NE | ND | No significant changes in composition and sensory attributes High sensorial acceptance |
NE | [26] | [47] |
Legend: not defined (ND); not evaluated (NE); seconds (s); ↑ (increase); nanostructured lipid carrier (NLC); ultra-high temperature (UHT); high-temperature short-time (HTST); sodium caseinate complex (NaCas-VD); succinylated sodium caseinate complex (SNaCas-VD); Reassembled sodium caseinate–vitamin D2 complex (RNaCas-VD); Reassembled succinylated sodium caseinate–vitamin D2 complex (RSNaCas-VD).
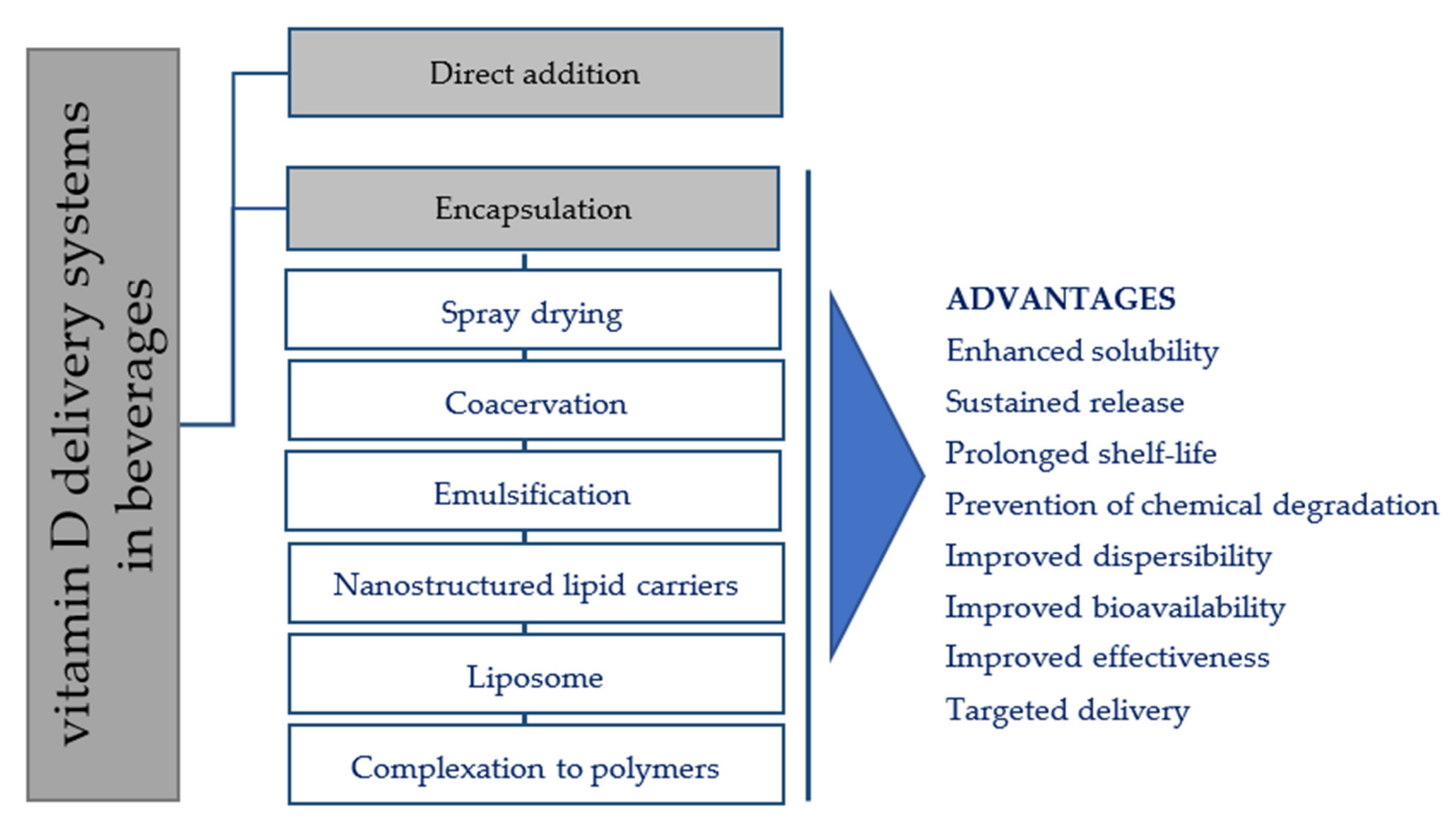
3. Fortification Strategies of Vitamin D in Beverages
The success of vitamin D fortification in beverages is widely dependent on the homogenization, stability, and bioavailability of vitamin D in the matrix. These three aspects should be addressed in the design of fortified beverages. Loss of vitamin D is mainly due to oxidation and isomerization during processing and storage and has been observed in various systems fortified with vitamin D. In addition, the deposition of vitamin D inside the packaging materials and its degradation in aqueous food matrix are the main source of its instability in beverages. This change affects the appearance and taste of formulated beverages, hence affecting their acceptability to customers [27][26]. To face these technological limitations, several techniques have been adopted to improve the delivery of vitamin D in fortified beverages, including direct addition, emulsification, and microencapsulation strategies.3.1. Direct Addition of Vitamin D
The direct addition method is the simplest technique for the fortification of beverages. In this approach, vitamin D is dispersed as fine droplets in water or food-grade organic solvents such as ethanol and then mixed with the beverage to ensure its homogeneous distribution in the matrix [27][26].3.2. Vitamin D Encapsulation Techniques
Encapsulation is the technique of producing capsules via insulation of a bioactive core material in a homogeneous or heterogeneous coating matrix [27][26]. If the particle sizes (PS) of capsules are about 1–5000 μm, they are called microcapsules [10]; colloidal-sized particles with diameters ranging from 10 to 1000 nm are referred as nanocapsules [28][66]. Vitamin D microencapsulation has been widely used in the preparation of fortified beverages and offers several advantages in comparison to the direct addition and emulsification approaches (Figure 2). Some advantages include: (i) higher solubility barrier between vitamin D and the food matrix; (ii) better homogeneity in the dispersion (iii) higher bioavailability of vitamin D though the controlled and targeted release of the encapsulated vitamin; and (iv) improved physiochemical (e.g., moisture, oxidation, pH, temperature, mechanical) and organoleptic characteristics (e.g., appearance, taste, quality of food matrix) of the final products [8][29][8,53]. However, although many of the microencapsulation systems developed for vitamin D delivery look promising, so far, applications have not been found at the industrial level. Spray-drying and coacervation are the two usual methods for microencapsulation of vitamin D in beverage products [30][67]. Other microencapsulation techniques with great potential in the design of vitamin D-fortified beverages include micro/nanoemulsions, spray drying, nanostructured lipid carriers, liposomes, and polymer complexation. Several parameters, namely pH, ionic strength, and heat treatments, can potentially affect these delivery systems [31][68], being essential to test their stability during food processing.
3.3. Vitamin D Polymers Complexation
Figure 2. An illustration of various vitamin D delivery approaches used to fortify beverages.
3.3. Vitamin D Polymers Complexation
Several protein-based nanocarriers have been developed as vehicles of vitamin D in fortified beverages. Milk proteins such as casein [16][32][33][34][37,57,58,59], whey [35][60], α-lactoalbumin (αLa) [36][63] and β-lactoglobulin (βLg) [37][38][49,61], have been the most frequently involved proteins to deliver vitamin D in beverages systems. Reassembled casein micelles (rCMs) have been reported to enable the vitamin D fortification of milk products with minimum impact on the functional behaviour of caseins during processing and on sensory attributes of milk products [32][57]. Besides caseins, other bovine milk proteins have been used to entrap VD3 for fortification. For instance, Abbassi et al. [35][60] encapsulated VD3 in whey protein nanoparticles and evaluated its stability in the presence of air for 7 days. The complexation of VD3 with whey proteins proved to increase the stability of the vitamin under light and prolonged storage, compared to the free form. As alternatives to milk proteins, plant-based proteins have been proposed as potential nanovehicles for the delivery of vitamin D in beverages. For instance, the nanocomplexation of VD3 with potato proteins provided significant protection and reduced vitamin losses during pasteurization and simulated shelf-life tests under several different sets of storage conditions [6]. Moreover, results showed that the VD3–potato protein complex may be suitable for the enrichment of clear beverages. Likewise, polysaccharides complexed to proteins have been proposed as promising approaches to deliver vitamin D in beverages systems. Another approach to the vitamin D fortification of beverages is the development of liprotides, i.e., complexes between partially denatured proteins and lipids. In this system, the protein forms a stabilizing shell around a fatty acid micelle core [36][63]. Encapsulating vitamin D by one or more encapsulation techniques is the best approach to achieve the desired stability, bioavailability and dispersibility of vitamin D in fortified beverages. Even though many of the developed carrier systems for vitamin D delivery in fortified beverages seem to be promising, they have not found application at the industrial level so far. Thus, advanced knowledge on the process optimization under industrial conditions should be promoted.3.4. Vitamin D Polymers Complexation
3.3. Vitamin D Polymers Complexation
Several protein-based nanocarriers have been developed as vehicles of vitamin D in fortified beverages. Milk proteins such as casein [16][32][33][34][37,57,58,59], whey [35][60], α-lactoalbumin (αLa) [36][63] and β-lactoglobulin (βLg) [37][38][49,61], have been the most frequently involved proteins to deliver vitamin D in beverages systems. Reassembled casein micelles (rCMs) have been reported to enable the vitamin D fortification of milk products with minimum impact on the functional behaviour of caseins during processing and on sensory attributes of milk products [32][57].4. Stability, Bioaccessibility, and Bioavailability of Vitamin D-Fortified Beverages
The stability of vitamin D, i.e., its ability to tolerate the effects of pH, ionic strength, and temperature changes, is a critical parameter to consider in the formulation of a vitamin D-fortified beverage. The cis-triene configuration of vitamin D (Figure 1) makes it sensitive to isomerization and oxidation. As this phenomenon does not involve the side chain of VD2 and VD3 [39][13], both vitamers have been used for the fortification of beverages. The bioavailability of vitamin D is influenced by three important phenomena: (i) bioaccessibility, i.e., vitamin release from the food matrix after in vitro digestion; (ii) transformation, i.e., chemical, and biochemical conversion; and (iii) absorption, i.e., migration through the mucus layer to the surfaces of the intestinal lumen and uptake by epithelial cells [39][13]. Studies of the bioaccessibility of vitamin D fortification in beverages are still scarce and not well elucidated. However, intervention studies have been conducted indicating that vitamin D-fortified milk and orange juices have good bioavailability in humans. Haham et al. [32][57] incorporated VD3 in casein micelles and observed a high bioavailability of 1% fat milk fortified with 50,000 IU of VD3 in 87 human volunteers. Khadgawat et al. [15][36] reported that 200 mL of milk fortified with 600 IU (15 μg) or 1000 IU (25 μg) of VD3 is effective in improving the 25(OH)D serum levels in children aged 10 to 14 years. Neyestani et al. [14][35] showed that fortified milk (200 mL) with 100 IU VD3 increased the 25(OH)D serum levels of children aged 10 to 12 years. Economos et al. [22][43] observed that a multinutrient-fortified juice improved the vitamin D status in children; Tangpricha et al. [20][41] reported that ingestion of orange juice fortified with 1000 IU of VD3 for 12 weeks can increase serum 25(OH)D concentrations by up to 150% in adults, and Biancuzzo et al. [21][42] found that fortification of orange juice with VD2 or VD3 is as effective as an oral supplement in maintaining vitamin D status.5. Regulation of Vitamin D-Fortified Beverages
Vitamin D (VD2 or VD3, in crystalline, resin or crystal form) is Generally Recognized as Safe (GRAS) at specified maximum levels of safe use for certain beverage products and infant formula [7]. Some vitamin-D fortified beverages are currently sold in several countries, namely USA, Canada, Finland, and Australia (Table 23).| Food (Serving) | USA | Canada | Finland | Australia | ||||
|---|---|---|---|---|---|---|---|---|
| Vitamin D per Serving in μg (1 μg = 40 IU) | ||||||||
| Fluid cow’s milk (250 mL or 1 cup) | 2.5–5.0 | a | 2.5–5.0 | a | 2.5–5.0 | a | 1.25 | b |
| Orange juice with added calcium | b | (125 mL or 1/2 cup) |
1.25 | 1.25 | 1.25 | - | ||
| Plant-based milk (soy, oat, almond) | b | (250 mL or 1 cup) |
1.5–3.0 | 1.5–3.0 | 1.9–3.75 | - | ||
| Malted drink | b | (g powder) | 3.08 | - | - | - | ||
a Mandatory fortification; b Fortification of selected brands.
In USA, the fortification of beverages with vitamin D is regulated by the Food and Drug Administration (FDA) [41][81]; manufacturers are allowed to voluntarily add up to 84 IU/100 g of VD3 to milk, 100 IU/240 mL of VD3 to calcium-fortified fruit juices and fruit juice drinks, 50 IU/100 g of soy beverages, 84 IU/100 g of VD2 to plant-based beverages and 89 IU/100 g of VD2 to plant-based yogurt alternatives. In Europe, the legal policy responsible for vitamin D fortification of beverages is Annex 1 of Regulation (EC) No 1925/2006, amended by the Commission Regulation (EC) No 1170/2009 [42][82].
6. Summary
To date, several beverages have been fortified with vitamin D, such as milk, fruit juices, tea, and vegetable drinks. Encapsulation seems to be an indispensable tool to design vitamin D materials with the desired functionality to deliver vitamin D through beverages, with advantages over the direct addition and emulsification approaches. Some advantages of the encapsulation delivery systems include improved stability against light exposure, chemical and mechanical stress, better homogeneity with the matrix, improved oral bioavailability, and improved organoleptic properties. Although these advantages exist, there are still improvements needed to increase the efficacy of these vitamin D delivery systems in beverages. For example, they should be formulated with natural and safe ingredients, they should not adversely affect the sensory and quality attributes of the developed beverages, they should prevent vitamin D from chemical degradation with a longer shelf-life, and they should provide increased bioavailability. There is limited information on the in vivo bioavailability studies of beverages fortified with vitamin D. Hence, in vivo studies supported with interdisciplinary knowledge should be conducted to ascertain the effectiveness of these delivery systems to develop vitamin D-fortified beverages and address vitamin D deficiency disease.
