Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Romualdo Sciorio.
Since the birth of Louise Joy Brown, the first baby conceived via in vitro fertilization, more than 9 million children have been born worldwide using assisted reproductive technologies (ART). In vivo fertilization takes place in the maternal oviduct, where the unique physiological conditions guarantee the healthy development of the embryo. During early embryogenesis, a major wave of epigenetic reprogramming takes place that is crucial for the correct development of the embryo.
- human in vitro fertilization
- assisted reproductive technology
1. Introduction
Over the past 40 years, the use of ART for infertility treatment has been continuously on the rise and has resulted in the birth of more than 9 million children globally [1,2][1][2]. The number of couples facing infertility problems has steadily increased over the last decades, particularly since a growing number of individuals are postponing the desire to have children further into older age. Many of those couples ultimately need in vitro fertilization (IVF) to be able to conceive a baby [3]. Nowadays, nearly 3.3 million ART cycles are performed annually, resulting in over 500,000 deliveries worldwide [1]. ART procedures are considered relatively safe; however, in the last decade, novel concerns have been raised due to increased prevalence of epigenetic errors and imprinting defects in ART-born children [4]. This was first observed in cattle and sheep, where incidence of large offspring syndrome (LOS) increased following transfer of in vitro fertilized embryos [5]. In 2001, Young et al. reported that epigenetic alterations in IGF2R was responsible for LOS following embryo culture in sheep [6]. Epigenetic alterations in various imprinted genes were also observed in preimplantation mouse embryos cultured in M16 or Whitten’s medium [7]. In vivo fertilization takes place in the oviduct, which is a natural environment with optimal physiological conditions including all the metabolic requirements for early embryo development. Even though embryology laboratories try to mimic those natural conditions to the best extent possible, during in vitro fertilization, the embryo is exposed to five or six days of diverse environmental conditions (Figure 1) [8]. Since about 3–5% of children are conceived following ART cycles [1], it is important to determine the potential negative effects of the procedure on the conceived baby. Epidemiological data revealed increased incidence of low and very low birth weight in ART-born babies following fresh embryo replacement [9]. Similar results were recently published by Sunkara et al., who analyzed UK registry data (Human Fertilization and Embryology Authority, HFEA) from 1991 to 2016 including about 117,000 singleton live births following ART. The authors showed that the causes of infertility had a negative impact on preterm birth and low birth weight following fresh embryo transfer [10]. However, the opposite scenario was reported following frozen-thawed embryo transfer (FET) in ART. A large study performed by Terho et al. suggested that FET is linked with higher birth weights and higher risk of large-for-gestational-age [11]. In 2002, a case report [12] was published describing two unrelated patients with Angelman syndrome with sporadic imprinting defects following intracytoplasmic sperm injection (ICSI). A year later, DeBaun et al. reported increased incidence of Beckwith–Wiedemann syndrome with imprinting alterations in H19 and LIT1 in children born after ART [13]. Subsequently, several studies tried to determine possible culprits behind the observed epigenetic errors including controlled ovarian stimulation (COS), in vitro oocyte maturation, intracytoplasmic sperm injection (ICSI), in vitro embryo culture, couple infertility, and more recently, preimplantation embryo manipulation for genetic assessment.
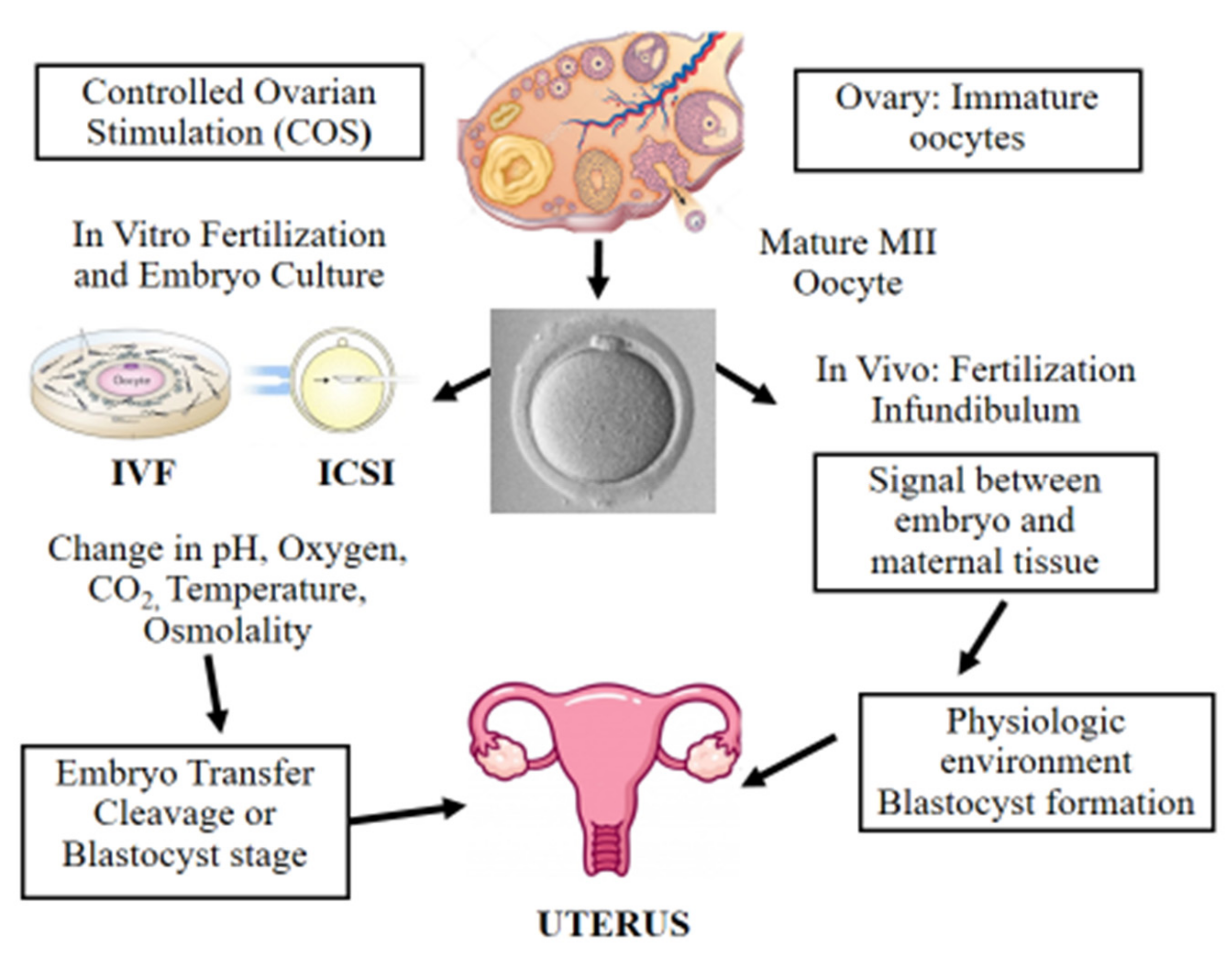
Figure 1. Scheme illustrating in vitro and in vivo fertilization. Controlled ovarian stimulation (COS) is used to promote follicle growth, maturation, and ovulation. ART adopts either IVF or ICSI for fertilization. Following fertilization, the preimplantation embryo is cultured in incubators, where suboptimal culture conditions such as pH, oxygen, temperature, and osmolality may affect its further development. Finally, the in vitro-produced embryo is transferred to the uterus at the cleavage or blastocyst stage. On the other hand, in vivo the female and male gametes interact together and the sperm fertilizes the oocyte in the infundibulum. Next, the developing embryo moves towards the uterus interacting with the female reproductive system in a physiologic and optimal environment.
2. Epigenetics in Development and Imprinted Genes
In 1942, Conrad Waddington highlighted the importance of environmental interactions with genes during early stages of embryo development. Although at that time, only limited information was available about the mechanisms of early embryogenesis, Waddington emphasized the importance of studying features that control embryo development that can mediate the correlations between genotype and phenotype. Waddington introduced the term “Epigenetics”, which he described as the “the branch of biology that studies the causal interactions between genes and their products which bring the phenotype into being” [14]. Epigenetic regulation is essential for normal mammalian development and is described as the study of heritable changes in gene function that are not associated with changes to the DNA sequence itself [15]. In mammals, two waves of epigenetic reprogramming occur during development that reset epigenetic marks in germ cells and preimplantation embryos. During early embryogenesis, epigenetic marks are reprogrammed to prepare the embryo for development; however, parental-specific DNA methylation patterns at imprinted genes are maintained. The second phase occurs during germ cell development when primordial germ cells (PGCs) enter the fetal gonadal ridge. Here, DNA methylation patterns are globally erased including marks at imprinted genes. Parental imprinting marks are later established during germ cell differentiation with distinct imprints in male and female germ cells. During reprogramming, the epigenome is highly susceptible to external and internal cues that can alter the reprogramming process and induce long-term disease risk in the future generation [16,17][16][17]. One of the most studied epigenetic modifications is DNA methylation [18], where a methyl group is added at the 5′ carbon position of the cytosine pyrimidine ring in the context of CG dinucleotide (CpG sites) [19]. Those epigenetic modifications are maintained by daughter cells throughout cell divisions by DNA methyltransferase 1 (DNMT1) [20]. Epigenetic modifications are crucial in regulating gene expression during embryo development, whereby any disruption to epigenetic states during this sensitive time window can lead to future consequences for development and disease [21,22][21][22]. Genomic imprinting is an epigenetic process resulting in monoallelic expression of either the maternally or the paternally inherited allele. This mechanism of parent-of-origin-specific expression is restricted to a limited number of ~200 imprinted genes described in humans [23,24][23][24]. Genomic imprinting has been mainly reported in eutherian mammals; however, similar phenomena were identified in flowering plants and in some insects indicating independent evolutionary origins [25]. Imprinted genes are regulated by cis-acting elements known as imprinting control regions (ICRs). For example, in the H19-Igf2 locus, the ICR located upstream of H19 along with enhancers controls the expression of H19 from the maternal allele and of the insulin-like growth factor (IGF2) gene from the paternal allele [26,27][26][27]. This exclusive monoallelic expression is controlled by specific epigenetic marks and regulatory elements such as DNA methylation, histone modifications, long non-coding RNA (lncRNA), and CCCTC binding factor (CTCF)-mediated boundaries [28]. The parental-specific imprints established in the germ line escape epigenetic reprogramming in preimplantation embryos, where imprinted genes play an important role in early development [29] and are essential for the regulation of energy balance between the mother and the developing fetus [30]. In humans, genetic mutations, copy number aberrations, and epigenetic alterations affecting imprinted genes have been linked to a number of disorders, e.g., Beckwith–Wiedemann syndrome (BWS), Angelman syndrome (AS), Silver–Russell syndrome (SRS), and Prader-Willi syndrome (PWS), Ref [31] characterized by clinical features affecting development, metabolism, and growth.
3. Epigenetic Alterations and Imprinting Disorders in ART
Following fertilization, the zygote develops into a structure called the “blastocyst” (Figure 2). At this stage, the embryo encloses about 150 or 200 cells differentiated into two types: the trophectoderm (TE), an epithelial sheet surrounding the fluid filled cavity (i.e., the blastocoele) and the inner cell mass (ICM), a group of cells attached to the inside of the trophectoderm that eventually give rise to the fetus. TE cells facilitate implantation into the uterine lining and form extraembryonic tissues including the placenta. During early development, embryonic cells are guided toward their future lineages through epigenetic reprogramming and subsequent re-establishment of cell-type-specific epigenetic signatures. This corresponds to the period when gametes and embryos are being in vitro manipulated and cultured inside the embryology laboratory. Therefore, such artificial intrusions during this critical time window might lead to epigenetic aberrations in the resultant offspring (Figure 1 and Figure 3). Several studies reported imprinted loci to be vulnerable to external environmental cues during in vitro embryo culture. For example, KvDMR1 has been observed to be abnormally methylated in ART-related BWS in humans [32,33][32][33] and hypomethylated in ART-produced bovine conceptuses with LOS [34]. Several studies have also shown that ART-related procedures including COS, ICSI, and embryo manipulation might induce epigenetic abnormalities [29,31,35][29][31][35]. A systematic review published by Lazaraviciute et al. compared the incidence of imprinting disorders and DNA methylation alterations at key imprinted genes in children conceived via ART versus those conceived naturally. A total of 18 papers were included in this review, and the combined odds ratio (95% confidence intervals) for the incidence of imprinting disorders in children conceived through ART was 3.67 in comparison to spontaneously conceived children. The authors concluded that an increased risk of imprinting disorders occurs in babies born via IVF and ICSI; nevertheless, there was limited evidence for a link between epigenetic alterations at imprinted genes and ART [36]. Another review summarizing data from eight studies on BWS and ART reported a significant positive association between IVF and ICSI procedures and BWS with increased relative risk of about 5.2 times (95% CI 1.6–7.4) [37]. However, the authors did not observe an association for either AS or PWS with IVF and ICSI, but rather a positive association with fertility problems. Regarding SRS, the number of children born following ART was small (n = 13); therefore, probable significance for SRS incidences could not be inferred. A more recent epidemiological study investigated the risk of imprinting disorders in IVF children born in Denmark and Finland, where the authors compared the incidence rate of PWS, SRS, BWS, and AS in ART-conceived babies in Denmark (n = 45,393 born 1994–2014) and Finland (n = 29,244 born 1990–2014). They observed an increased odds rate for BWS (OR 3.07, 95% CI: 1.49–6.31) in ART-conceived children; however, no significant difference was evident for PWS, SRS, and AS [38]. Similarly, a nation-wide study in Japan found a 4.46-fold increase in BWS and an 8.91-fold increase in SRS following ART including several with aberrant DNA methylation at imprinted genes [39]. The effect of altered epigenetics marks and epimutations on human health is just beginning to be understood. Further research in this area is needed help clarify whether ART-induced epigenetic changes affect growth, development, and health of future offspring. In the next sections, wresearchers discuss specific procedures applied during ART treatments to provide examples on how certain treatments may lead to epigenetic alterations.
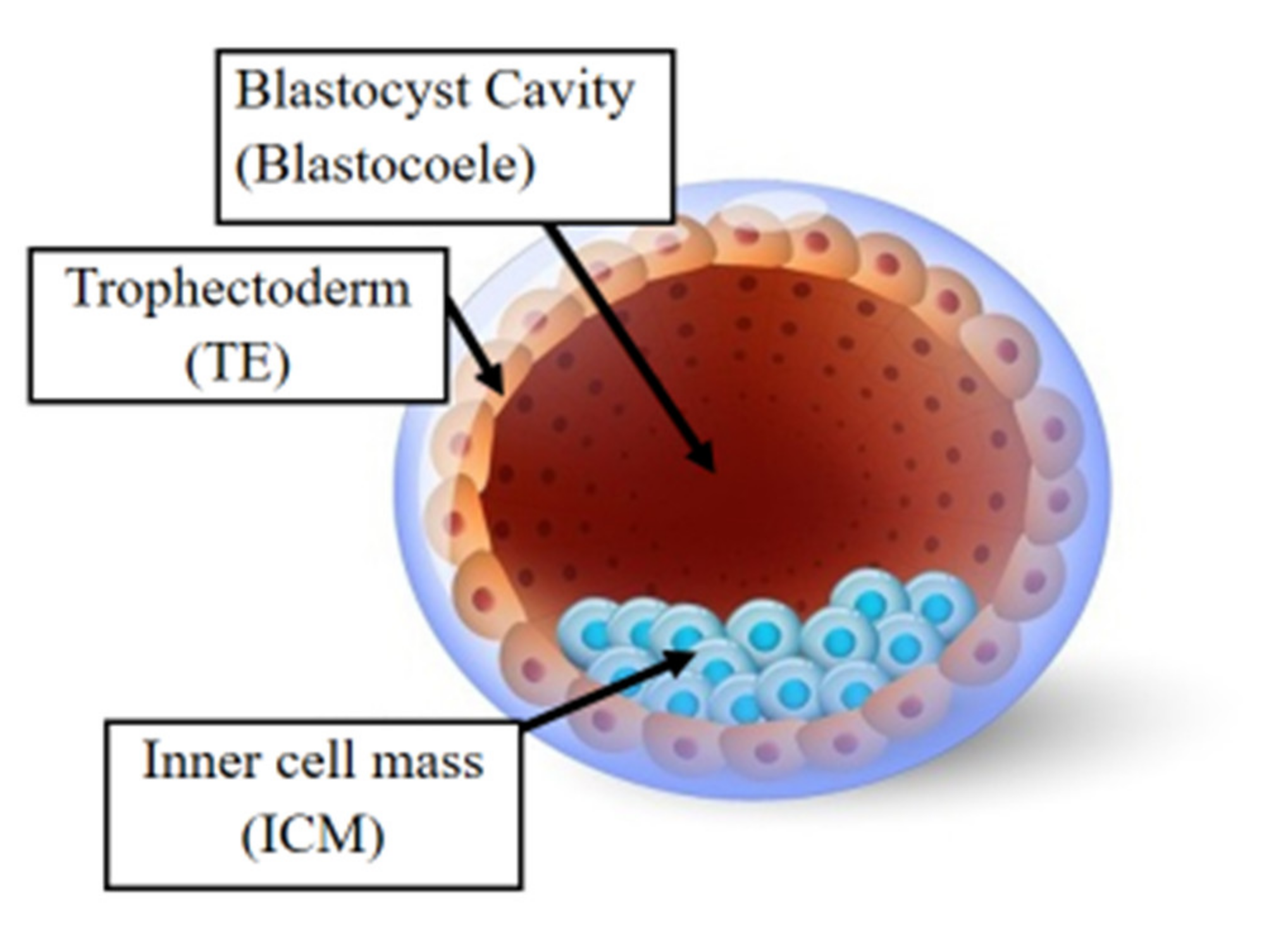
Figure 2. The human blastocyst. The structure comprises two differentiated cell types and a central cavity filled with fluid (blastocoel cavity). The inner cell mass (ICM) becomes the fetus and the trophectoderm (TE) cells later develop into the placenta.
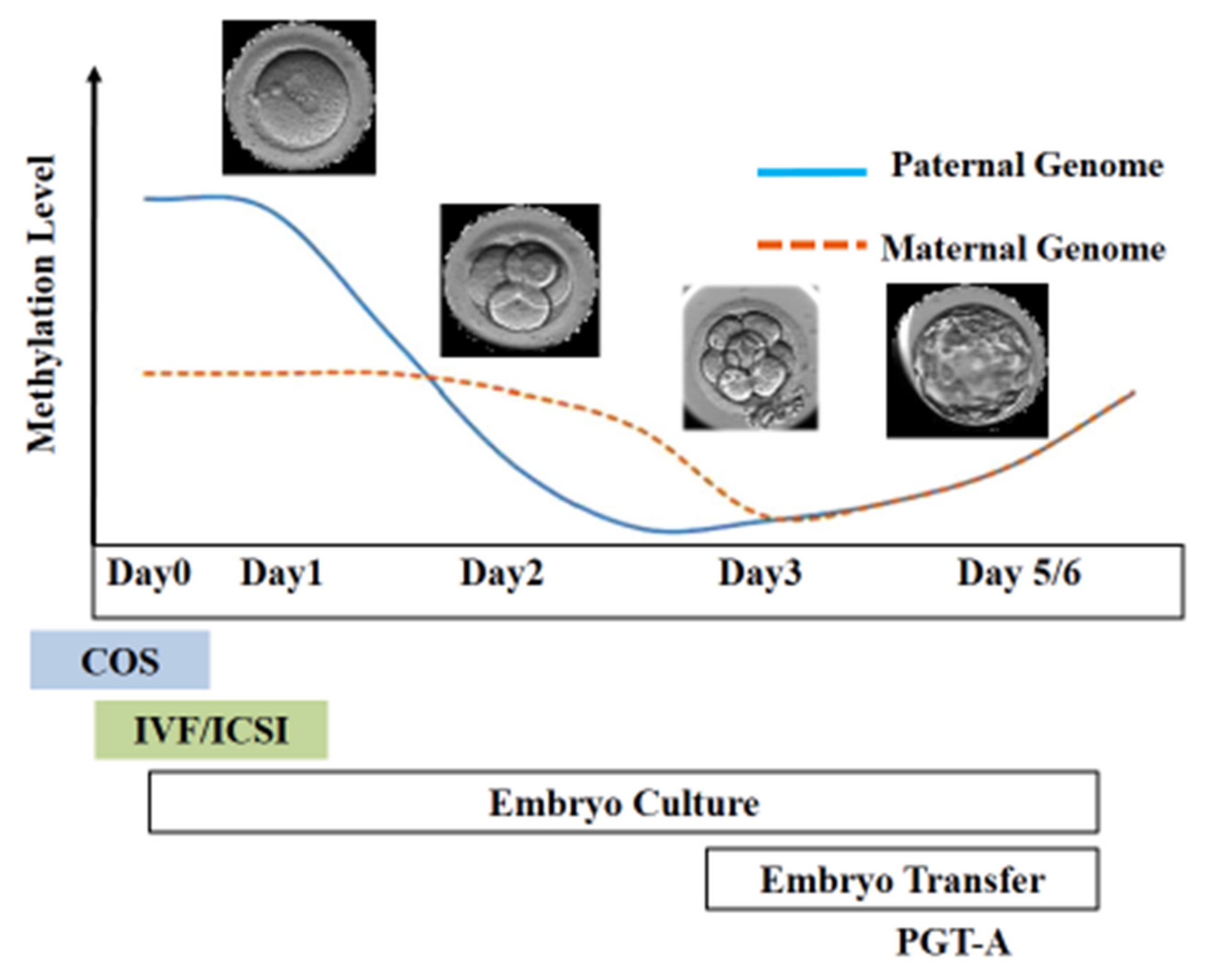
Figure 3. Epigenetic reprogramming during the early stage of embryo development. Post-fertilization, the paternal genome undergoes active demethylation, whereas the maternal genome is passively demethylated. The scheme illustrates the stage of development at which different ART techniques are employed.
4. Controlled Ovarian Stimulation in ART
Ovarian stimulation is one procedure likely responsible for epigenetic aberrations in the oocyte and embryo [40]. COS may lead to the selection of poor quality oocytes that are usually excluded in a natural cycle, and those oocytes might induce perturbed genomic imprinting during the early stage of embryo development and later in the placenta [41,42][41][42]. Medical records of women who gave birth to children with BWS following ART revealed ovarian stimulation medication as the only common factor among those patients [43]. Each month, the human ovaries typically produce a single dominant follicle which ovulates and releases a single oocyte. To increase the number of fertilized oocytes and improve IVF outcome, COS is applied using exogenous gonadotropins to stimulate the ovary and promote multifollicular development yielding multiple oocytes. Typically, a pharmacological dose of FSH is used to induce the growth of multiple follicles. As follicles grow and reach a specific width, LH is administered to produce the mid-cycle LH surge, which promotes oocyte maturation and later ovulation. Oocyte retrieval is precisely timed following LH administration to retrieve mature oocytes prior to ovulation. LH exposure initiates meiosis and leads to oocyte maturation from the immature “metaphase I” (MI) stage to the mature “metaphase II” (MII) stage of development. During this time, the first polar body is extruded and the oocyte reaches the metaphase II stage, which indicates its competence to be fertilized [44]. Following ovulation, the rest of the follicle forms the corpus luteum, which produces high levels of progesterone to prepare the endometrium for the process of embryo implantation. Since the expected number of oocytes is low in patients with reduced ovarian reserve, several strategies mainly based on increased gonadotropin dose have been applied to collect more oocytes. In certain cases, it is only possible to retrieve immature oocytes after COS where in vitro maturation might be adopted to obtain matured MII oocytes. Culture systems for in vitro maturation of human oocytes holds great potential but is still considered experimental for clinical use in ART [45]. In the last decade, there has been a growing concern over an association between COS and epigenetic aberrations in oocytes and embryos, which further increases the risk of imprinting disorders in the offspring [46]. Indeed, DNA methylation analysis of imprinted genes revealed aberrations in PEG1, KCNQ1OT1, and ZAC in oocytes collected following COS when compared to oocytes obtained after natural ovulation [47,48][47][48]. Furthermore, reports described DNA methylation alterations and expression changes in the H19 imprinted control region in embryos obtained from superovulated oocytes [49]. Mature oocytes obtained following superovulation were shown to have conserved DNA methylation patterns at ICRs; however, methylation aberrations were detected in genes involved in glucose metabolism, nervous system development, mRNA processing, cell cycle, and cell proliferation [50]. This is in contrary to a genome-wide DNA methylation study in superovulated mouse oocytes, which showed minor methylation differences between superovulated versus naturally ovulated oocytes [51]. DNA methylation was also studied in embryos generated from superovulated oocytes, where superovulation was shown to interfere with the genome-wide DNA methylation reprogramming process that occurs during early embryogenesis [52]. Multiple superovulation cycles were also shown to have adverse effects on the structure and function of the ovaries, causing lower fertilization rate and decreased rate of early embryo development. In addition, repeated superovulation affected expression of pluripotency genes and led to aberrant histone modifications in early embryos and in the future offspring [53,54][53][54]. However, the effect of the ovarian superovulation on various epigenetic mechanisms are still to be fully elucidated. In animal models, reports have largely described that COS might alter the correct activities of DNA methyltransferases [53,54][53][54]. One of the first studies to determine that superovulation modifies expression levels of the DNMT proteins was published by Uysal et al. [55]. In tThis study, the authors compared DNMT protein levels in three groups (control, high dose, and normal dose of gonadotropins) and found that DNMT1, DNMT3A, and DNMT3B protein expression in the oocytes and developed embryos differed significantly when compared with controls. Similar data have been published by other groups confirming those results [53,54,56,57][53][54][56][57].
References
- De Geyter, C.; Wyns, C.; Calhaz-Jorge, C.; De Mouzon, J.; Ferraretti, A.P.; Kupka, M.; Andersen, A.N.; Nygren, K.G.; Goossens, V. 20 years of the European IVF-monitoring Consortium registry: What have we learned? A comparison with registries from two other regions. Hum. Reprod. 2020, 35, 2832–2849.
- Steptoe, P.; Edwards, R. Birth after the reimplantation of a human embryo. Lancet 1978, 312, 366.
- Thoma, M.E.; McLain, A.; Louis, J.F.; King, R.B.; Trumble, A.C.; Sundaram, R.; Louis, G.B. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil. Steril. 2013, 99, 1324–1331.
- Ventura-Juncá, P.; Irarrázaval, I.; Rolle, A.J.; Gutiérrez, J.I.; Moreno, R.D.; Santos, M.J. In vitro fertilization (IVF) in mammals: Epigenetic and developmental alterations. Scientific and bioethical implications for IVF in humans. Biol. Res. 2015, 48, 68.
- Young, L.E.; Sinclair, K.D.; Wilmut, I. Large offspring syndrome in cattle and sheep. Rev. Reprod. 1998, 3, 155–163.
- Young, L.E.; Fernandes, K.; McEvoy, T.G.; Butterwith, S.C.; Gutierrez, C.G.; Carolan, C.; Broadbent, P.J.; Robinson, J.J.; Wilmut, I.; Sinclair, K. Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nat. Genet. 2001, 27, 153–154.
- Doherty, A.S.; Mann, M.R.; Tremblay, K.D.; Bartolomei, M.S.; Schultz, R.M. Differential Effects of Culture on Imprinted H19 Expression in the Preimplantation Mouse Embryo1. Biol. Reprod. 2000, 62, 1526–1535.
- Barberet, J.; Binquet, C.; Guilleman, M.; Doukani, A.; Choux, C.; Bruno, C.; Bourredjem, A.; Chapusot, C.; Bourc’his, D.; Duffourd, Y.; et al. Do assisted reproductive technologies and in vitro embryo culture influence the epigenetic control of imprinted genes and transposable elements in children? Hum. Reprod. 2021, 36, 479–492.
- Schieve, L.A.; Meikle, S.F.; Ferre, C.; Peterson, H.B.; Jeng, G.; Wilcox, L.S. Low and Very Low Birth Weight in Infants Conceived with Use of Assisted Reproductive Technology. N. Engl. J. Med. 2002, 346, 731–737.
- Sunkara, S.K.; Antonisamy, B.; Redla, A.C.; Kamath, M.S. Female causes of infertility are associated with higher risk of preterm birth and low birth weight: Analysis of 117 401 singleton live births following IVF. Hum. Reprod. 2020, 36, 676–682.
- Terho, A.M.; Pelkonen, S.; Opdahl, S.; Romundstad, L.B.; Bergh, C.; Wennerholm, U.B.; Henningsen, A.A.; Pinborg, A.; Gissler, M.; Tiitinen, A. High birth weight and large-for-gestational-age in singletons born after frozen compared to fresh embryo transfer, by gestational week: A Nordic register study from the CoNARTaS group. Hum. Reprod. 2021, 36, 1083–1092.
- Cox, G.F.; Bürger, J.; Lip, V.; Mau, U.A.; Sperling, K.; Wu, B.-L.; Horsthemke, B. Intracytoplasmic Sperm Injection May Increase the Risk of Imprinting Defects. Am. J. Hum. Genet. 2002, 71, 162–164.
- DeBaun, M.R.; Niemitz, E.L.; Feinberg, A.P. Association of In Vitro Fertilization with Beckwith-Wiedemann Syndrome and Epigenetic Alterations of LIT1 and H19. Am. J. Hum. Genet. 2003, 72, 156–160.
- Waddington, C.H. The epigenotype. Int. J. Epidemiol. 2012, 411, 10–13.
- Russo, V.E.A.; Martienssen, R.A.; Riggs, A.D. Epigenetic Mechanisms of Gene Regulation; Cold Spring Harbor Laboratory Press: Plainview, NY, USA, 1996.
- Skinner, M.K. Environmental epigenomics and disease susceptibility. EMBO Rep. 2011, 12, 620–622.
- Santos, F.; Hyslop, L.; Stojkovic, P.; Leary, C.; Murdoch, A.; Reik, W.; Stojkovic, M.; Herbert, M.; Dean, W. Evaluation of epigenetic marks in human embryos derived from IVF and ICSI. Hum. Reprod. 2010, 25, 2387–2395.
- Klose, R.J.; Bird, A.P. Genomic DNA methylation: The mark and its mediators. Trends Biochem. Sci. 2006, 31, 89–97.
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395.
- Rivera, C.M.; Ren, B. Mapping Human Epigenomes. Cell 2013, 155, 39–55.
- Hirst, M.; Marra, M.A. Epigenetics and human disease. Int. J. Biochem. Cell Biol. 2009, 41, 136–146.
- Weber, W. Cancer epigenetics. Prog. Mol. Biol. Transl. Sci. 2010, 95, 299–349.
- Skaar, D.A.; Li, Y.; Bernal, A.J.; Hoyo, C.; Murphy, S.; Jirtle, R.L. The Human Imprintome: Regulatory Mechanisms, Methods of Ascertainment, and Roles in Disease Susceptibility. ILAR J. 2012, 53, 341–358.
- Allegrucci, C.; Thurston, A.; Lucas, E.; Young, L. Epigenetics and the germline. Reproduction 2005, 129, 137–149.
- Glaser, R.L.; Ramsay, J.P.; Morison, I.M. The imprinted gene and parent-of-origin effect database now includes parental origin of de novo mutations. Nucleic Acids Res. 2006, 34, D29–D31.
- Tremblay, K.D.; Duran, K.L.; Bartolomei, M.S. A 5’ 2-kilobase-pair region of the imprinted mouse H19 gene exhibits exclusive paternal methylation throughout development. Mol. Cell. Biol. 1997, 17, 4322–4329.
- Thorvaldsen, J.L.; Duran, K.L.; Bartolomei, M.S. Deletion of the H19 differentially methylated domain results in loss of im-printed expression of H19 and Igf2. Genes Dev. 1998, 12, 3693–3702.
- Koerner, M.V.; Pauler, F.M.; Huang, R.; Barlow, D.P. The function of non-coding RNAs in genomic imprinting. Development 2009, 136, 1771–1783.
- Marcho, C.; Cui, W.; Mager, J. Epigenetic dynamics during preimplantation development. Reproduction 2015, 150, R109–R120.
- Tunster, S.J.; Jensen, A.B.; John, R.M. Imprinted genes in mouse placental development and the regulation of fetal energy stores. Reproduction 2013, 145, R117–R137.
- Eggermann, T.; de Nanclares, G.P.; Maher, E.R.; Temple, I.K.; Tümer, Z.; Monk, D.; Mackay, D.J.; Grønskov, K.; Riccio, A.; Linglart, A.; et al. Imprinting disorders: A group of congenital disorders with overlapping patterns of molecular changes affecting imprinted loci. Clin. Epigenet. 2015, 7, 123.
- White, C.R.; Denomme, M.M.; Tekpetey, F.R.; Feyles, V.; Power, S.G.A.; Mann, M.R.W. High Frequency of Imprinted Methylation Errors in Human Preimplantation Embryos. Sci. Rep. 2015, 5, 17311.
- Huntriss, J.D.; Hemmings, K.E.; Hinkins, M.; Rutherford, A.J.; Sturmey, R.G.; Elder, K.; Picton, H.M. Variable imprinting of the MEST gene in human preimplantation embryos. Eur. J. Hum. Genet. 2012, 21, 40–47.
- Chen, Z.; Robbins, K.M.; Wells, K.D.; Rivera, R.M. Large offspring syndrome: A bovine model for the human loss-of-imprinting overgrowth syndrome Beckwith-Wiedemann. Epigenetics 2013, 8, 591–601.
- Hiura, H.; Okae, H.; Chiba, H.; Miyauchi, N.; Sato, F.; Sato, A.; Arima, T. Imprinting methylation errors in ART. Reprod. Med. Biol. 2014, 13, 193–202.
- Lazaraviciute, G.; Kauser, M.; Bhattacharya, S.; Haggarty, P.; Bhattacharya, S. A systematic review and meta-analysis of DNA methylation levels and imprinting disorders in children conceived by IVF/ICSI compared with children conceived spontane-ously. Hum. Reprod. Update 2014, 20, 840–852.
- Vermeiden, J.P.; Bernardus, R.E. Are imprinting disorders more prevalent after human in vitro fertilization or intracytoplasmic sperm injection? Fertil. Steril. 2013, 99, 642–651.
- Henningsen, A.A.; Gissler, M.; Rasmussen, S.; Opdahl, S.; Wennerholm, U.B.; Spangmose, A.L.; Tiitinen, A.; Bergh, C.; Romundstad, L.B.; Laivuori, H.; et al. Imprinting disorders in children born after ART: A Nordic study from the CoNARTaS group. Hum. Reprod. 2020, 35, 1178–1184.
- Hattori, H.; Hiura, H.; Kitamura, A.; Miyauchi, N.; Kobayashi, N.; Takahashi, S.; Okae, H.; Kyono, K.; Kagami, M.; Ogata, T.; et al. Association of four imprinting disorders and ART. Clin. Epigenet. 2019, 11, 21.
- Chen, Z.; Hagen, D.E.; Elsik, C.G.; Ji, T.; Morris, C.J.; Moon, L.E.; Rivera, R.M. Characterization of global loss of imprinting in fetal overgrowth syndrome induced by assisted reproduction. Proc. Natl. Acad. Sci. USA 2015, 112, 4618–4623.
- Van der Auwera, I.; D’Hooghe, T. Superovulation of female mice delays embryonic and fetal development. Hum. Reprod. 2001, 16, 1237–1243.
- Market-Velker, B.A.; Zhang, L.; Magri, L.S.; Bonvissuto, A.C.; Mann, M.R. Dual effects of superovulation: Loss of maternal and paternal imprinted methylation in a dose-dependent manner. Hum. Mol. Genet. 2009, 19, 36–51.
- Chang, A.S.; Moley, K.H.; Wangler, M.; Feinberg, A.; DeBaun, M.R. Association between Beckwith-Wiedemann syndrome and assisted reproductive technology: A case series of 19 patients. Fertil. Steril. 2005, 83, 349–354.
- Voronina, E.; Wessel, G.M. The Regulation of Oocyte Maturation. Curr. Top. Dev. Biol. 2003, 58, 53–110.
- Telfer, E. Progress and prospects for developing human immature oocytes in vitro. Reproduction 2019, 158, F45–F54.
- Mak, W.; Weaver, J.R.; Bartolomei, M.S. Is ART changing the epigenetic landscape of imprinting? Anim. Reprod. 2010, 7, 168–176.
- Sato, A.; Otsu, E.; Negishi, H.; Utsunomiya, T.; Arima, T. Aberrant DNA methylation of imprinted loci in superovulated oo-cytes. Hum. Reprod. 2007, 22, 26–35.
- Laprise, S.L. Implications of epigenetics and genomic imprinting in assisted reproductive technologies. Mol. Reprod. Dev. 2009, 76, 1006–1018.
- Fauque, P.; Jouannet, P.; Lesaffre, C.; Ripoche, M.-A.; Dandolo, L.; Vaiman, D.; Jammes, H. Assisted Reproductive Technology affects developmental kinetics, H19 Imprinting Control Region methylation and H19 gene expression in individual mouse embryos. BMC Dev. Biol. 2007, 7, 116.
- Huo, Y.; Yan, Z.Q.; Yuan, P.; Qin, M.; Kuo, Y.; Li, R.; Yan, L.Y.; Feng, H.L.; Qiao, J. Single-cell DNA methylation sequencing reveals epigenetic alterations in mouse oocytes superovulated with different dosages of gonadotropins. Clin. Epigenetics 2020, 12, 75.
- Saenz-De-Juano, M.D.; Ivanova, E.; Billooye, K.; Herța, A.-C.; Smitz, J.; Kelsey, G.; Anckaert, E. Correction to: Genome-wide assessment of DNA methylation in mouse oocytes reveals effects associated with in vitro growth, superovulation, and sexual maturity. Clin. Epigenet. 2020, 12, 18.
- Yu, B.; Smith, T.H.; Battle, S.L.; Ferrell, S.; Hawkins, R.D. Superovulation alters global DNA methylation in early mouse embryo development. Epigenetics 2019, 14, 780–790.
- Kalthur, G.; Salian, S.R.; Nair, R.; Mathew, J.; Adiga, S.K.; Kalthur, S.G.; Zeegers, D.; Hande, M.P. Distribution pattern of cytoplasmic organelles, spindle integrity, oxidative stress, octamer-binding transcription factor 4 (Oct4) expression and de-velopmental potential of oocytes following multiple superovulation. Reprod. Fertil. Dev. 2016, 28, 2027–2038.
- Tang, S.-B.; Yang, L.-L.; Zhang, T.-T.; Wang, Q.; Yin, S.; Luo, S.-M.; Shen, W.; Ge, Z.-J.; Sun, Q.-Y. Multiple superovulations alter histone modifications in mouse early embryos. Reproduction 2019, 157, 511–523.
- Uysal, F.; Ozturk, S.; Akkoyunlu, G. Superovulation alters DNA methyltransferase protein expression in mouse oocytes and early em-bryos. J. Assist. Reprod. Genet. 2018, 35, 503–513.
- Kindsfather, A.J.; Czekalski, M.A.; Pressimone, C.A.; Erisman, M.P.; Mann, M.R. Perturbations in imprinted methylation from assisted reproductive technologies but not advanced maternal age in mouse preimplantation embryos. Clin. Epigenet. 2019, 11, 162.
- Chen, X.; Huang, Y.; Huang, H.; Guan, Y.; Li, M.; Jiang, X.; Yu, M.; Yang, X. Effects of superovulation, in vitro fertilization, and oocyte in vitro maturation on imprinted gene Grb10 in mouse blastocysts. Arch. Gynecol. Obstet. 2018, 298, 1219–1227.
More
