Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Katarzyna Czarzasta and Version 2 by Rita Xu.
Obesity is a growing epidemiological problem, as two-thirds of the adult population are carrying excess weight. It is a risk factor for the development of cardiovascular diseases (hypertension, ischemic heart disease, myocardial infarct, and atrial fibrillation). It has also been shown that chronic obesity in people may be a cause for the development of heart failure with preserved ejection fraction (HFpEF), whose components include cellular hypertrophy, left ventricular diastolic dysfunction, and increased extracellular collagen deposition.
- cardiac fibrosis
- cardiac remodeling
- collagen
- extracellular matrix (ECM)
- metalloproteinase (MMPs)
1. Introduction
Obesity today has reached pandemic proportions, as excess weight may even be affecting up to two-thirds of the adult population in developed countries and is one of the main causes of disability worldwide [1][2][1,2]. It is a well-established risk factor for the development of metabolic disorders such as insulin resistance, diabetes, dyslipidemia, as well as cardiovascular diseases such as atherosclerosis, ischemic heart disease, myocardial infarction, hypertension, and atrial fibrillation [3][4][5][6][7][3,4,5,6,7]. It has been postulated that it may contribute to the development of heart failure with preserved ejection fraction (HFpEF) [8], as well as heart failure with reduced ejection fraction—HFrEF (usually by increasing the risk of myocardial infarction) [8]. Obesity cardiomyopathy is a term describing heart failure in the course of severe obesity of long duration, in which left ventricular hypertrophy (LVH) is the most common finding [8]. Importantly, obesity is associated with several hemodynamic changes and metabolic, inflammatory, and neurohormonal alterations such as increased activity of both the renin–angiotensin–aldosterone system (RAAs) and the sympathetic nervous system (SNS), hyperleptinemia, and hypoadiponectinemia, which may all have an impact on heart remodeling and heart function [2][9][2,9].
Cardiac remodeling is defined as a change in the size, shape, or structure of one or more of the cardiac chambers [9]. It results from changes in both the phenotype of the cardiomyocytes and the extracellular matrix (ECM) [10]. Excessive synthesis of ECM components, especially collagen, by residential fibroblasts in the myocardium, is known as fibrosis [11]. This process is inextricably associated with constant ECM turnover and may be related to an imbalance between ECM degradation by enzymes known as metalloproteinases (MMPs) and their specific inhibitors (tissue inhibitors of metalloproteinases; TIMPs) [12]. It also may lead to disruption of the cardiac architecture, enhancing its stiffness and, therefore, deteriorating the systolic or diastolic function and facilitating the occurrence of arrhythmia [11].
2. Obesity as a Heterogenous Disorder
The definition of obesity is based on the body mass index (BMI) (calculated as the ratio of body mass in kilograms (kg) divided by the height in meters squared (m2)) and is diagnosed when the BMI value exceeds 30 kg/m2, whereas a person is described as overweight when their BMI value is 25–29.9 kg/m2 [13][14][13,14]. In children, obesity is diagnosed when the BMI value is above the 95th percentile adjusting for both age and gender [15][16][15,16]. According to WHO recommendations, the severity of obesity may be assessed byits division into classes, categorized by BMI: I class,30–34.9 kg/m2; II class, 35–39.9 kg/m2; and III class, over 40 kg/m2 [17]. The third class is also referred to as severe, extreme, or massive obesity [17]. The etiology of obesity is multifactorial, involving a complex interaction between genetical, hormonal, environmental, dietary, and behavioral factors; obesity develops when the caloric intake is disproportionately higher than the energy expenditure [18][19][18,19]. It must be acknowledged that obesity is a heterogenous disorder and there are a few possible phenotypes of obese subjects: metabolically healthy obese (MHO); metabolically abnormal obese (MAO); metabolically obese, normal weight (MONW), who are individuals characterized by a BMI < 25 kg/m2, but affected with complications such as hyperinsulinemia/insulin resistance, abdominal and visceral adiposity, unfavorable adipokine and lipid profile, and hypertension; and sarcopenic obese (SO) (whose body composition is made up of a high fat content and low muscle mass with an accompanying “normal” BMI) [20][21][20,21]. Obesity is reaching pandemic proportions and may trigger the development of serious metabolic and cardiovascular complications [22]. In fact, it is believed that the majority of the adverse effects of obesity on the cardiovascular system and mortality risk are not attributable to obesity itself but to the concomitant metabolic syndrome [3]. BMI is not always considered to be a good predictor of future health complications, as it is not a reliable measure to assess individual body fatness and adipose tissue distribution (visceral vs. subcutaneous adipose tissue) [23]. It must be remembered that excessive adipose tissue itself is also responsible for several hormonal and proinflammatory disturbances [24]. Due to several pathomechanisms, obesity may switch macrophage polarization towards the M1 phenotype, which is considered to be proinflammatory and profibrotic [8]. Furthermore, adipose tissue excess in obese individuals is associated with chronic subclinical inflammation and increased infiltrations of the immune cells [25]. Adipose tissue is also considered to be an active endocrine organ, as it secretes several hormones known as adipokines [26]. Recently, there has beena plethora of new emerging substances. RIn this researchersview, we include two of the most common hormones, leptin and adiponectin, whose defective signaling in obesity can contribute to myocardial fibrosis. Several experimental models were developed to study obesity-related complications. The most typically encountered models for obesity induction are animals with genetic alterations, for example, null for the leptin gene (ob/ob), with a mutation in the leptin receptor gene (db/db, fa/fa), or fed with laboratory chow with a high percentage of fat content (high-fat diet—HFD) [27]. In animal models, similarly to obese people, obesity is accompanied by several metabolic disturbances (for example, hyperlipidemia, hyperinsulinemia, and glucose intolerance) [27].3. Distinctive Characteristics of the Cardiovascular System in the Course of Obesity
Obesity may affect the cardiovascular system via hemodynamic (increased workload) and non-hemodynamic factors (increased activation of the sympathetic nervous system (SNS) and the renin–angiotensin–aldosterone system (RAAs), insulin resistance/hyperinsulinemia, leptin insensitivity/hyperleptinemia, reduced adiponectin concentration/hypoadiponectinemia, overexpression of the peroxisome-proliferator-activated receptor (PPAR), decreased natriuretic peptides, lipotoxicity, oxidative stress, and chronic inflammation/hypoxia) [5][28][29][5,28,29]. The heart of obese individuals are characterized by cardiomyocyte hypertrophy, infiltration of fat into the ECM (steatosis), and accumulation of triglycerides among the contractile elements [28][30][28,30]. All of those factors impact the left ventricle (LV) geometry [28][30][28,30]. Moreover, the myocardium in obese people is surrounded by excessive epicardial adipose tissue (EAT) [8]. It has been shown that EAT may secrete several cytokines such as tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), activin A, connective tissue growth factor (cTGF), MMPs, and angiopoietin-like 2 (ANGPTL2) [26][31][32][26,31,32]. Moreover, there aredata showing that increased EAT in the course of obesity may facilitate cardiac fibrosis and promote the incidence of arrythmia [32][33][34][35][32,33,34,35]. Another adverse impact of obesity on the myocardium is associated with lipotoxicity [36]. Physiologically, in the healthy myocardium, free fatty acids (FFA) constitute an elementary energetic substrate (approximately 70%) for the synthesis of adenosine triphosphate (ATP) [37]. In obesity, there is high availability of those substrates in circulatory form, which can directly interact with the PPAR receptors. Activation of PPAR receptors leads to increased uptake of FFA from the blood to the cardiomyocyte due to the CD36/fatty-acid transport protein (FAT) transportation to the cell membrane and stimulation of expression of enzymes necessary for FFA removal by β-oxidation in the mitochondria [38]. It was also shown that this process may be facilitated by leptin throughthe short-term observation [39]; such a prolonged condition may induce myocardial lipid accumulation [40]. The presence of triglycerides may not appear to be toxic;nevertheless, a lipid overload that exceeds the oxidative or storage capacity of the tissue may provoke the induction of other alternative biochemical pathways, for example, towards the production of ceramides, which may preserve proapoptotic properties [36][41][42][36,41,42]. Moreover, FFAs promote diacylglycerol (DAG) synthesis, which may activate protein kinase C (PKC), which in turn inhibits insulin signaling via the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt)-dependent pathway [36][38][36,38]. Lipotoxicity also contributes to the excessive production of reactive oxygen species (ROS) and impaired mitochondrial biogenesis, which favor both inflammation and cellular apoptosis [43]. In the next section, rwesearchers describe more comprehensively the hemodynamic factors, which are the main causative factor for cardiomyocyte hypertrophy in obese individuals [28]. Non-hemodynamic factors and their contribution to the changes in the cellular and ECM components will be presented in the paragraph entitled ‘The effect of obesity on myocardial ECM expression, heart fibrosis, and cellular hypertrophy’ in Section 7.3.1. Hemodynamic Changes Observed in Obesity
Obesity exerts several adaptive hemodynamic changes on the cardiac muscle (Figure 1) [44]. First, obesity is generally considered to be a hypercirculatory condition [45]. Elevated fat-free (lean) mass and the high activity of RAAs may be putative reasons [9][44][45][9,44,45]. In obese patients, increased cardiac output (CO) is observed, and as the heart rate (HR) remains normal or may be only slightly elevated, a major cause for this high CO is subjected to increased stroke volume (SV) [9]. Increased SV may also result in increased LV end-diastolic pressure, which was also detected in obese persons [9][44][9,44].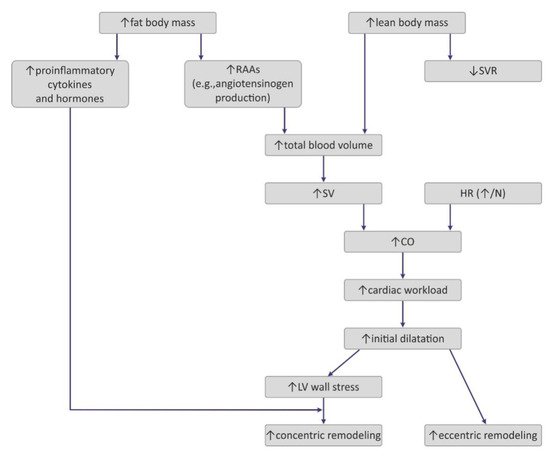
Figure 1. Pathogenesis of hemodynamic alterations in the course of obesity. RAAs—renin–angiotensin–aldosterone system; SVR—systemic vascular resistance; SV—stroke volume; HR—heart rate; CO—cardiac output; LV—left ventricle.
Interestingly, excess weight leads to decreased systemic vascular resistance (SVR), which may also lead to the augmentation of CO and LV dilatation [9]. Increased chamber volume exerts tension on the ventricular wall, according to the Law of Laplace, and such a chronic process may cause hypertrophy [29]. Such remodeling depicts the model of volume overload that contributes to eccentric hypertrophy, characterized by the lengthening of the myocytes [46].
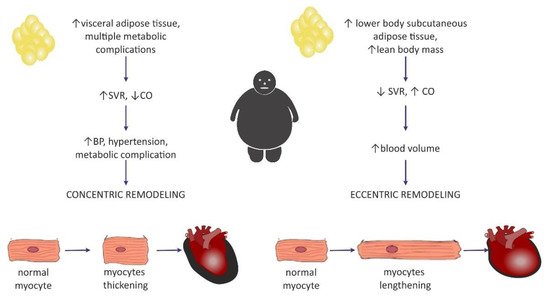
Clinical manifestation of hemodynamic changes in the course of obesity may differ entirely depending on the distribution of the adipose tissue in the body [47]. The Dallas Heart Study carried out on 2710 participants without organic heart disease suggested that hemodynamic features such as high CO and low SVR may pertain to individuals with an excess of subcutaneous adipose tissue (SAT), especially distributed within the gluteal-femoral region, whereas increased visceral adipose tissue (VAT) was rather related to lower CO and elevated SVR [47]. Patients with excessive VAT proportions developed a concentric hypertrophy of LV (i.e., increased myocyte thickness), and also had increased LV wall thickness, increased LV mass/volume ratio, and smaller LV end-diastolic volume [46][47][46,47]. Similarly, Liu et al. showed in their study that VAT in obese patients was a better predictor of cardiac remodeling and subclinical dysfunction of LV than elevated BMI [48].
3.2. The Impact of Obesity on Myocardial Geometry and the Ejection Fraction
Left ventricular hypertrophy appears to be the most predominant observation in the hearts of obese individuals [44][49][50][51][44,49,50,51]. The results from the Framingham Heart Study, which was carried out on 3922 healthy participants, concluded that BMIwas strongly correlated with left ventricular mass, LV wall thickness, and LV internal dimensions [50]. In a different study, an increase in BMI by 1 kg/m2 and an increase in waist circumference by 1 cm increased the risk of LV hypertrophy by 5.1% and 2.6%, respectively [52]. It may be concluded that increased LV mass is commonly encountered among obese individuals and, in particular, it may be dependent on the central distribution of fat depots (abdominal fat) [53]. Nevertheless, in the literature, it is commonly discussed whether heart enlargement in the course of obesity results from concentric or rather eccentric hypertrophy [54]. A meta-analysis that included 4999 obese individuals and 6623 nonobese controls showed that eccentric hypertrophy was more frequent in obese subjects than the concentric phenotype [29][55][29,55]. This is in agreement with the initially formulated hypothesis of cardiomyopathy of obesity, based on increased cardiac output and blood volume, which in turn exerts tension on the LV wall, leading to its dilatation [56]. Today, investigators are more inclined to think that the concentric pattern may be more prevalent than initially expected [9][29][9,29] and is even independent of the occurrence of hypertension [54]. It is also believed that the distribution of fat tissue in the viscera was more correlated with concentric hypertrophy [29], as well as with higher blood pressure (BP) values in comparison with healthy, nonobese subjects, even without a diagnosis of hypertension, which may predispose to such remodeling [9]. Fat distribution and hemodynamic alterations also have an impact on cardiac remodeling, as mentioned above. Lower body SAT prompted eccentric cardiac remodeling, whereas abdominal subcutaneous adipose tissue did not have an effect on the hemodynamics. It was concluded that visceral abdominal adiposity (more often associated with the presence of metabolic syndrome) was a causative factor for concentric remodeling [47]. The existence of two different patterns of hemodynamic alterations in obese individuals may contribute to different LV remodeling (Figure 2).
Figure 2. Two distinctive patterns of cardiac remodeling in the course of obesity and a proposition for their pathogenesis based on adipose tissue distribution. SVR—systemic vascular resistance; CO—cardiac output.
While considering LV diastolic dysfunction, it was shown that it is present in all classes of isolated obesity [1][57][58][1,57,58]. Moreover, its degree correlated with BMI [57]. This supports the high prevalence of HFpEF in obese individuals [59].
Data about the systolic LV function are inconsistent, as various authors have reported that the LV ejection fraction (EF) was decreased, normal, or even supernormal in obese subjects [27]. An increased EF may be observed in the early stages of obesity due to increased volume overload [57].Some authors believe that long-lasting obesity without metabolic and cardiovascular comorbidities may not be conducive to the impairment of the systolic function [1][58][1,58]. Khan et al. concluded that there is not enough evidence in clinical studies to make the claim that obese patients have left ventricular ejection fraction (LVEF) < 35% [58]. Overt systolic dysfunction may suggest the presence of concomitant heart disease, especially coronary artery disease (CAD) [1][58][1,58].
Similarly, the right ventricle (RV) in obese individuals may by characterized by a mild increase in size and wall thickness [60]. In addition, a mild dysfunction of RV was reported [8]. This may be a potential reason for the high prevalence of sleep apnea among obese individuals [9]. Nevertheless, Wong et al. concluded that increased BMI was associated with the severity of RV dysfunction in overweight and obese subjects without overt heart disease, even independent of sleep apnea [60]. Moreover, obesity may also induce the enlargement of the left atrium (LAE) as a consequence of LV diastolic dysfunction [61][62][61,62]. The large MONICA/KORA study conducted on 1212 men and women showed that the presence of obesity was the strongest predictive factor for LAE development [63] Lavie et al. presumed that obesity may constitute a risk factor for atrial fibrillation [5].
