You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by DIEGO PERONI and Version 2 by Peter Tang.
Long Coronavirus disease-19 (COVID-19) refers to the persistence of symptoms related to the infection with severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). This condition is described as persistent and can manifest in various combinations of signs and symptoms, such as fatigue, headache, dyspnea, depression, cognitive impairment, and altered perception of smells and tastes. Long COVID-19 may be due to long-term damage to different organs—such as lung, brain, kidney, and heart—caused by persisting viral-induced inflammation, immune dysregulation, autoimmunity, diffuse endothelial damage, and micro thrombosis.
- COVID-19
- SARS-CoV-2
- long-COVID
- vitamins
- antioxidant
- vitamin D
- magnesium
- selenium
- zinc
- oxidative stress
1. Introduction
Long Coronavirus disease-19 (COVID-19)—and the cognate term “long-haulers”—refers to the persistence of symptoms related to the infection with severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) [1]. This condition is described as persistent and can manifest in various combinations of signs and symptoms in 10% [2] to 87% [3] of adults, particularly females, following SARS-CoV-2 infection [4]. More than 50 long-term effects of SARS-Cov-2 infection have been identified, including fatigue, headache, dyspnea, cognitive impairment—numbness, depression, altered perception of smells and tastes, poor appetite, chronic cough, joint and chest pain, postural orthostatic tachycardia expression of autonomic dysregulation, thermoregulation abnormalities, skin eruptions, and gastrointestinal disorders [5]. Similar findings have also been reported in children and adolescents [6]. Recent evidence on vaccination against SARS-Cov-2 suggests that vaccines reduce the risk of long COVID by lowering the chances of contracting COVID-19 in the first place. However, for those who do experience a breakthrough infection, the vaccination might only halve the risk of long COVID—or have no effect on it at all [7]. Understanding the prevalence of long COVID among vaccinated people has urgent public-health implications as restrictions that limited viral spread are eased in some countries. It could also offer clues about what causes lingering COVID-19 symptoms long after the acute infection has cleared [7].
Long COVID-19 may be due to long-term damage to different organs—such as lung, brain, kidney, and heart—caused by persisting viral-induced inflammation, immune dysregulation, autoimmunity, diffuse endothelial damage, and micro thrombosis [8][15]. Intestinal dysbiosis could also play an important pathogenetic role given the close connections between the intestine immune system and central nervous system [9][16]. Furthermore, reactivation of the Epstein-Barr virus (EBV) has been documented in more than half of long haulers [10][17]. This is not a trivial effect because EBV reactivation has been associated with skin, cardiovascular, hematological, and neurological complications [11][18], which may also occur in long COVID-19.
2. The Possible Deep Roots of Long COVID-19 and Their Biological Plausibility
Besides genetic predisposition, a diet poor in anti-inflammatory/antioxidant substances with potential immune-modulating and anti-viral activity can be a predisposing but preventable factor for more severe SARS-Cov-2 and very likely also for the development of persistent long symptoms after the acute phase of the disease. This proposition emerged from epidemiologic studies demonstrating that populations with very low death rates were found to have an unusual common feature of eating large quantities of fermented vegetables, including members of the cruciferous and Brassicaceae family [12][25]. At the beginning of the pandemic, it was suggested that treatment with a wide range of existing host-directed therapies, including nutrient supplements, was possibly beneficial in the care of 2019-nCoV infection [13][26]. Vitamin D deficiency has been associated with an increased number of cases with severity, and with deaths [14][27]. In COVID-19 infection, zinc deficiency was found to be linked to higher odds of complications, including deaths [15][28], particularly when combined with selenium deficiency [16][29], and selenium supplementation has been associated with a better prognosis in those patients [17][30]. In addition, low levels of magnesium, which are usually present in all COVID-19 comorbidities [18][31], are associated with an increased inflammatory state [19][32], while an increased likelihood of survival is seen in severe patients with COVID-19 with higher magnesium blood level on admission to hospital (odds ratio for mortality of 0.032) [20][33]. Collectively, these studies suggest that nutritional support may effectively reduce inflammation and oxidative stress, thereby strengthening the immune system during the COVID-19 crisis, and ecological studies have lent support to this suggestion [12][21][22][25,34,35]. These supplementations may offer additional benefits, providing significant antiviral, anti-inflammatory, antithrombotic, and cytoprotective effects, thus preventing further tissue damage [23][36] and favorably modifying the gut microbiome which, in patients with COVID-19, has been found to be concordant with the disease severity and plasma concentrations of several inflammatory cytokines, chemokines, and blood markers of tissue damage, as well as the persistence of symptoms [24][37].3. What Could Be Done for the Prevention
3.1. Vitamins
3.1.1. Vitamin B Group
B group vitamins represent essential micronutrients for energy metabolism, DNA and protein synthesis, and immune cell regulation [25][38]. Vitamin B1 influences mitochondrial membrane potential, cytochrome C release, protein kinases, and p38-MAPK; suppresses oxidative stress induced by nuclear factor-kappa b (NF-κB); and has anti-inflammatory properties. Deficiency of vitamin B1 may cause dysfunction of the nervous system; neuroinflammation; T cell infiltration; chemokine CCL2 activation; overexpression of proinflammatory cytokines such as interleukin (IL)-1, tumor necrosis factor (TNF), IL-6, and arachidonic acid products; and induces expression of CD40 by the microglia and CD40L by astrocytes, which provoke the death of neurons [26][39]. The active form of vitamin B6, pyridoxal 5′-phosphate (PLP), has consistently been shown to be low in inflammatory conditions and inversely associated with numerous inflammatory markers in clinical and population-based studies, and its low concentration predicts the risk of chronic diseases [27][40]. Furthermore, PLP serves as a co-factor in neurotransmitter biosynthesis, as well as a scavenger of reactive oxygen species (ROS) [28][41]. Vitamin B12 appears to possess antioxidant properties by scavenging ROS, by the preservation of glutathione, modulation of cytokine and growth factor production and reduction of oxidative stress caused by advanced glycation end-products [29][42]. Furthermore, Vitamins B1 (thiamine), B6 (pyridoxine), B12 (cobalamin), and folate play an important role in the pathogenesis of neuropathy and neuropathic pain and on the inflammatory basis of depression [30][43]. Pyridoxal-5-phosphate and methylcobalamin are cofactors in peripheral nerve functions and cobalamin facilitates myelinogenesis and nerve regeneration [31][44]. Methyl-folate has been shown to improve endothelial function [32][45], and the vitamin B group is an essential nutrient for host gut microbiota [33][46]. In effect, supplementation of vitamin B12 together with vitamin D and magnesium was shown to prevent severe outcome progression in patients with SARS-Cov2 [34][47].3.1.2. Vitamin C
Vitamin C is one of the body’s most important antioxidants and is involved as a co-factor in the synthesis of carnitine; the formation of serotonin, dopamine, and nitric oxide; the synthesis of noradrenaline; the biosynthesis of amidated peptides; hypomethylation of DN; and the degradation of the transcription factor hypoxia-inducible factor 1 alpha implicated in energy metabolism [35][48]. Vitamin C contributes to immune defense by supporting various cellular functions of both the innate and adaptive immune system [35][48], with a possible preventive effect on autoimmune diseases [36][49]. Furthermore, fatigue, pain, cognitive disorders, and depression-like symptoms are known symptoms of vitamin C deficiency [37][50] and, although vitamin C plasma levels have not been evaluated in patients with long COVID-19, a deficit is most probable because infections are known to be coupled with high intakes of vitamin C, and insufficiencies in acute infections are frequent [35][48]. Indeed, a systematic review of studies evaluating the effect of this vitamin on low energy and weakness suggested that high dose intravenous vitamin C could be a beneficial treatment option in treating fatigue in patients with long COVID-19 [38][51].3.1.3. Vitamin D
Vitamin D has an immune-modulating effect and reduces the frequency of infections when taken in daily doses [39][52]. The discordant results related to its effect can be attributed to its erroneous use in cumulative monthly doses that are associated with a sudden increase in its plasma levels, followed within a few days by their drastic reduction due to the catabolic effect of the 24-hydroxylase that transforms the vitamin in inactive metabolites [40][53]. These continuous oscillations not only neutralize its effect but could also be dangerous in relation to its epigenetic action. Furthermore, this vitamin reduces cellular damage from oxidative stress and stimulates the Nrf2 pathway of signal transduction by promoting the synthesis of anti-inflammatory cytokines (Figure 1) [41][54].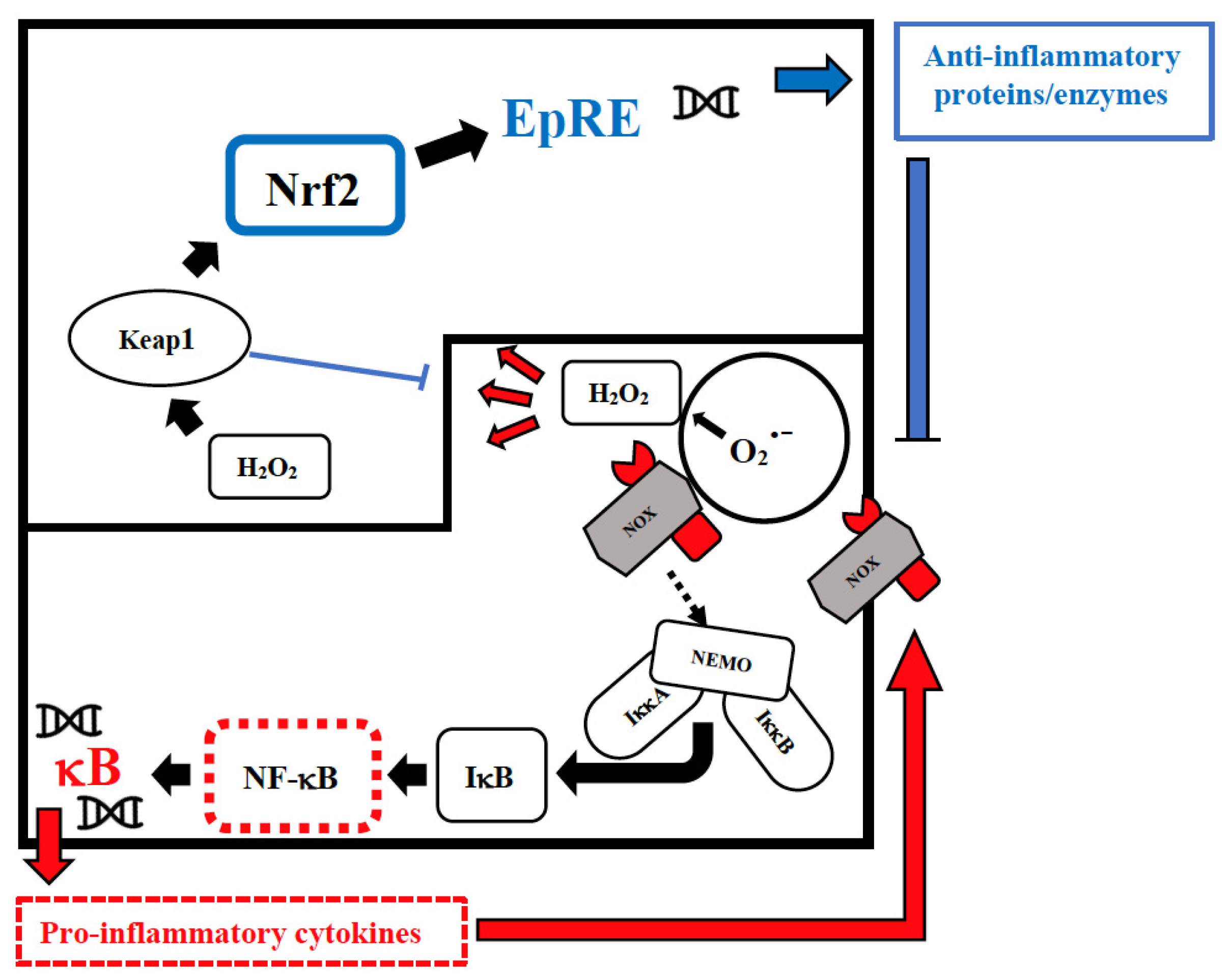
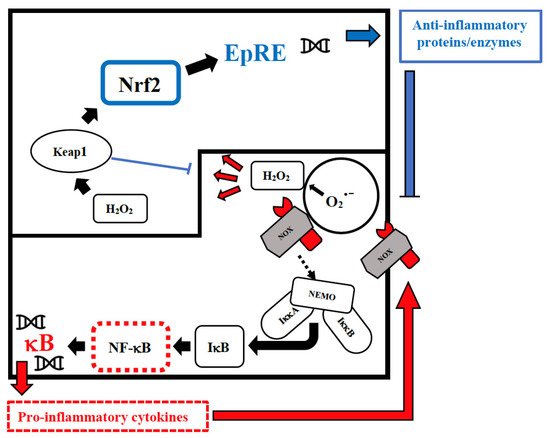
Figure 1. Intranuclear signal transductions can occur in two different pathways: while nuclear factor kappaB (NF-κB) tends to enhance and perpetuate the inflammatory response by triggering the expression of pro-inflammatory cytokines, nuclear factor erythroid 2–related factor 2 (Nrf2) activation through Kelch-like ECH-associated protein-1 (Keap1) oxidation dampens pro-inflammatory signaling by expression of peroxidases and other anti-inflammatory proteins. As E3-ligase, Keap1 also primes inhibitor of NF-κB kinase subunit beta (IKKβ) to degradation via ubiquitination, thereby directly interfering with NF-κB activation. For the sake of clarity, only the reactive oxygen species (ROS)-producing enzyme NADPH oxidase (NOX)-derived H2O2 is shown as an oxidant signal. Depending on the cellular system and the inflammatory stimulus, NOX-derived H2O2 may be supported or replaced by mitochondrial H2O2, lipoxygenase products, and S-alkylating electrophiles derived therefrom. NEMO, NF-κB essential modulator; IκB, Inhibitor of NF-κB.
