Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 3 by Camila Xu.
Blood–brain barrier (BBB) is one of the most specialized biological barriers in the human body. The pathogenesis of Alzheimer’s disease (AD) includes BBB dysfunction, which leads to the failure of Aβ transport from the brain to the peripheral circulatory system, causing neuroinflammation and oxidative stress. Moreover, the BBB is the first protective defense line to prevent foreign substances from traversing from the blood to the brain.
- blood–brain barrier
- liposomes
- dendrimers
- Alzheimer's Disease
- Nanoparticles
1. Blood–Brain Barrier (BBB) Physiology and Structure
The blood–brain barrier (BBB) is a formidable doorkeeper that protects the brain from foreign substances, including pathogens. However, it is a major obstacle in the management of neurological disorders, including Alzheimer’s disease (AD) and Parkinson’s disease (PD), because it prohibits many potential therapeutics from penetrating into the brain [1][2]. BBB dysfunction is directly correlated with amyloid and tau pathology in AD [3]. Recently, drug-loaded NPs have been actively investigated as precision medicines that enter the brain by binding to target proteins associated with the BBB [4]. Because AD is a neurodegenerative disease of the CNS, drugs used to treat AD must pass through the BBB, otherwise they will fail to achieve the expected efficacy, regardless of their action mechanism. Thus, permeability through the BBB is an indispensable prerequisite for therapeutic agents to treat AD.
The BBB is composed of multiple cell types, including endothelial cells (ECs), astrocytes, microglial cells, and pericytes. Transportation across the BBB can be achieved via both transcellular and paracellular pathways (Figure 1). Transcellular pathways play a pivotal role in transporting lipophilic agents, while paracellular pathways transport hydrophilic molecules [5]. Transcellular pathways involve endothelial carrier-mediated transport (CMT), receptor-mediated transcytosis (RMT), adsorptive-mediated transcytosis (AMT), and active efflux. CMT is responsible for the transportation of vitamins, hormones, organic anions/cations, and nucleotides across the BBB. CMT involves spontaneous passive transport via transmembrane integral proteins along a concentration gradient. The brain always needs glucose, so transport will occur even if blood glucose levels are low via glucose transporters such as GLUT-1 and GLUT-3 (Table 1). Different amino acids can also cross the BBB through the CMT pathway; large neutral amino acids such as levodopa, histidine, tryptophan, and valine are transported via LAT1 and excitatory amino acids, such as glutamate and aspartate, are transported via excitatory amino-acid transporters EAATs (Table 1). Large molecules such as antibodies, transferrin, insulin, and low-density lipoprotein (LDL) are transported through RMT. RMT is the primary pathway for the transport of macromolecules that are critical for brain function across the BBB. It involves transporters such as insulin receptor (IR), insulin-like growth factor receptor (IGFR), type 1 transferrin receptor (TfR1), low-density lipoprotein receptor (LDLR), leptin receptor (LEPR), and neonatal Fc receptor (FcRN) (Table 1). AMT involves electrostatic interactions between cationic and anionic molecules on the endothelial cell membrane, which aids in the transport of cationic proteins and basic oligopeptides, such as cell-penetrating peptides. Under these circumstances, efflux pumps such as glycoprotein-P (PgP), ATP-binding cassette transporters (ABC), and multidrug resistance proteins create a resistant barrier that prevents drugs from passing through the BBB. However, aging or pathological conditions can increase the influx of certain drugs into the brain. Paracellular pathways allow the transmission of constituents by penetrating the intercellular space across the epithelium. However, transcellular transport allows constituents to travel through a cell by crossing both the apical and basolateral membranes. There are three major types of transporters responsible for BBB penetration, which can be utilized as targets to deliver therapeutics into the brain, as shown in Table 1.
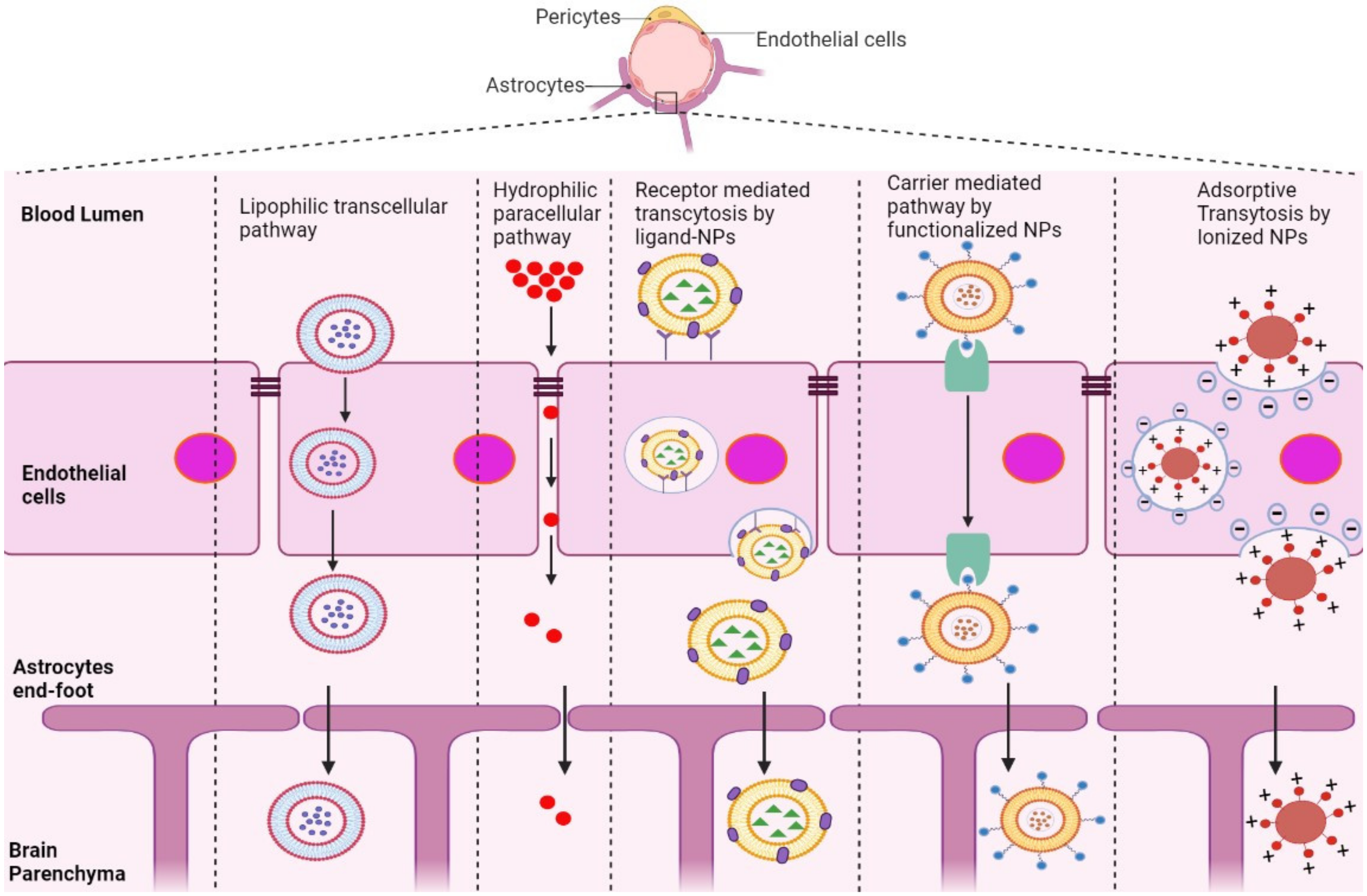
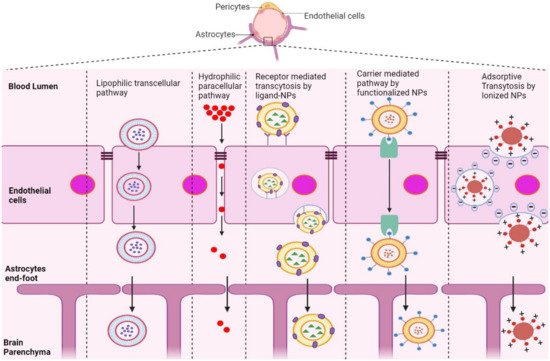
Figure 1. Schematic representation of potential pathways of NP-based drug delivery systems that penetrate the BBB for the treatment of AD.
Table 1. Various transporters that penetrate the BBB.
| Influx Transporters | Efflux Transporters | Receptor Transporters |
|---|---|---|
| Choline transporter (ChT) | Peptide transport system-6 (PTs-6) | Insulin receptors (IR) |
| Sodium-coupled glucose transporters (SGLTs) | Breast cancer resistant protein (BCRP) | Insulin-like growth factor receptor (IGFR) |
| Cationic amino acid transporter (CAT1) | P-glycoprotein (P-gp) | Transferrin receptors (TfR) |
| 1-type large amino-acid transporter (LAT1) Excitatory amino-acid transporters (EAATs) |
Leptin receptor (LepR) | |
| Glucose transporter (GLUT1) (GLUT3) | Low-density lipoprotein receptor (LDLR) | |
| Monocarboxylate lactate transporter (MCT1) Receptor for glycation end products (RAGE) |
Neonatal Fc receptor (FcRN) | |
| Lactoferrin receptor (LR) |
2. Strategies to Overcome the BBB
2.1. Crossing the BBB
NPs used to deliver drugs across the BBB are as small as 1–100 nm in size. NPs are adapted to overcome several hurdles faced by drugs in treating AD [6]. Many therapeutics for AD distribute throughout the body, with few reaching the brain, where it is primarily required due to the tightly organized BBB. Recently, various types of NPs have been shown to enhance the targeting efficiency to the brain and reduce the toxicity and adverse effects caused by the systemic circulation of a drug throughout the body [7].
RMT is the process by which NPs enter the brain microenvironment by taking advantage of overexpressed receptors in the BBB. This pathway induces endocytosis via clathrin-coated pits or caveolae, similar to AMT [8]. After NPs are internalized, they can comply with distinct cellular pathways, relying on their size, composition, charge, and ligand conjugation [9]. Most NPs with conjugated ligands, such as lactoferrin, transferrin, ApoE, and B6-peptide, are mediated by receptors, including LR, TfR, LDLR, IR, and IGFR, as shown in Table 1.
Positively charged NPs in contact with the negatively charged plasma membrane of the BBB undergo AMT [10][11]. This mechanism is stimulated by electrostatic interactions between the cationic groups of ligands conjugated on the surface of NPs and negative moieties present on the luminal surface of endothelial cells [11]. Positively charged ligands, such as lectin, cardiolipin, heparin, and CPPs, are functionalized on the surface of NPs, inducing BBB adsorption and transcytosis of the nanocarrier into the brain [12].
NPs conjugated with molecules including glucose, mannose, glutathione, and amino acids, which are recognized by transporters overexpressed in endothelial cells of the BBB, are mediated by CMT [13]. This involves transporters such as GLUT1, GLUT3, LAT1, and EAAT (Table 1), which covalently bind to glucose and mannose and ease penetration of nanocarriers into the BBB [14][15][16]. However, the small and stereospecific pores involved in CMT interfere with the uptake of large molecular drugs [17].
2.2. Avoiding the BBB
AD therapeutics can reach the CNS via local delivery routes without physically interacting with the BBB. Specifically, this pathway includes administrative routes that avoid passage through the BBB, such as injection either through surgical exposure to the brain by means of a catheter or pump (intracerebroventricular, intraparenchymal/intracerebral administration) or through spinal puncture (intrathecal administration). Intracerebral administration is a direct method of drug delivery, although drug diffusion is only possible if the target sites are adjacent to the parenchymal surface or ventricles. This route has been shown to be inefficient and clinically restricted [18][19]. However, Zhu et al., recently formulated angiopep-2-modified lipid-coated mesoporous silica NPs loaded with paclitaxel (ANG-LP-MSN-PTX) to target glioma. When blood–brain microdialysis was conducted to study their pharmacokinetic behavior, the half-life and AUCblood of ANG-LP-MSN-PTX displayed sustained systemic delivery via the intracerebral route of administration [20]. Likewise, Yurek et al., prepared DNA NPs that were injected intracerebrally into the denervated striatum of a rat model and showed significantly higher transgene expression in the brain [21].
Intracerebroventricular (ICV) and intrathecal procedures are performed by direct injection into the skull and ventricles or puncturing the lumbar region. Generally, the ICV route is used for injecting chemical compounds and peptides, such as colchicine, okadaic acid, streptozotocin, scopolamine, Aβ1-42, and hyperphosphorylated tau (p-tau), directly into the cerebral lateral ventricles to induce AD in different experimental animal models [22][23][24]. The intrathecal route accesses the subarachnoid space (SAS), where the cerebrospinal and interstitial fluids of the parenchyma are exchanged, which results in delivery to the entire CNS. Householder et al., intrathecally administered PEG-NPs into healthy mice and showed rapid distribution through the SAS with a reproducible pattern of brain delivery [25]. Polyamidoamine (PAMAM) dendrimers administered through the intrathecal route were also observed to be localized in activated microglia and astrocytes responsible for neuroinflammation. This method ensures the targeted delivery of therapeutics to the sites of diseases such as AD, cerebral palsy, and multiple sclerosis [26]. Despite the fact that they can deliver an enormous number of drugs to the CNS, these procedures are very intrusive and convey a significant risk of CNS infection or neurotoxicity [27]. For these reasons, intranasal (IN) routes that bypass the BBB via the sensory organs are preferred.
IN drug delivery is a non-invasive brain-targeting alternative because the brain and nose compartments are associated through olfactory, trigeminal, and systemic circulation. Among these, systemic circulation is only partially involved, reducing the risk of systemic side effects. Advantages of the IN route include ease of administration, high patient compliance, and rapid delivery kinetics within minutes [28][29]. The actual mechanism of IN drug delivery is not yet clear, but there is robust evidence that supports the significance of olfactory and trigeminal nerve pathways because of their direct and immediate connection from the nose to the brain [30]. For example, astaxanthin-loaded solid lipid NPs (SLNs) demonstrated a compelling neuroprotective effect against oxidative stress in PC-12 cell lines. IN delivery of the 99mTc-labeled SLN in experimental rats showed enhanced brain uptake, as measured by gamma scintigraphy and radiometry [31]. Curcumin is a natural anti-inflammatory and antioxidant phytochemical compound with the potential to treat AD, however, it has poor water solubility and limited BBB penetration. Mesoporous silica NPs (MSNP) were adapted to evaluate their efficacy in the nose-to-brain delivery of curcumin. Desirable loading efficiency, uptake, and release of this phytochemical were reported for curcumin-loaded MSNPs in a neuroblastoma cell line [32]. Similarly, tarenflurbil failed in phase III trials due to its poor BBB penetration; however, when incorporated into poly(lactic-co-glycolic acid (PLGA) NPs and SLNs and delivered intranasally, its overall pharmacokinetics were improved and the nose-to-brain route was observed to be superior to IV and oral administration [33].
2.3. Disrupting the BBB
Another strategy for overcoming the BBB involves opening the BBB through force using non-invasive physical methods, such as focused ultrasound sonication (FUS), and the administration of biochemical reagents, including cell-penetrating peptides (CPP), hyperosmotic agents, and surfactants.
FUS combines systematically circulating microbubbles embedded with NPs to locally improve vascular permeability and treat AD [34]. Microbubbles (MBs) are small drug delivery vehicles filled with gas that are typically between 0.5 μm and 10 μm in diameter, which is generally too bulky to enter the BBB. FUS is used to disrupt tight junctions and create openings in a specific localized area, resulting in enhanced BBB permeability [35]. Liu et al., prepared quercetin-modified sulfur NPs embedded in microbubbles (Qc-SuNPs-MB), which were demolished immediately upon exposure to ultrasonic waves. The sonoporation effect allowed the opening of the BBB and the release of Qc-SuNPs from the outer shell of the MB, which accumulated in the brain microenvironment. The rapid accumulation of Qc-SuNPs in the brain parenchyma effectively reduced the inflammatory response, neuronal death, oxidative stress, and calcium homeostasis imbalance, thereby treating AD [36]. Kafoed et al., investigated the transgene distribution and immune response of recombinant adeno-associated virus (rAAV) incorporated into MBs. Intravenous MBs of rAAV, when combined with FUS, triggered an immune reaction, including MHC class II expression, T cell infiltration, and microglial activation [37].
CPP facilitates the rapid internalization of exogenous cargo, such as proteins, nucleic acids, liposomes, and other NPs. Cheng et al., designed a co-delivery strategy in which positively charged CPP (TAT) and negatively charged plasmids were linked by electrostatic interactions and then incubated with positively charged mesoporous silica NP (MSN) to deliver curcumin, promote neuronal growth, and reduce oxidative stress [38].
Chemical compounds such as bradykinin induce vasodilation, resulting in enhanced BBB permeability [39]. Visible to near-infrared light (NIR) irradiation has likewise been exploited in several studies to further develop NP-based brain delivery, as NIR applications increase the BBB opening through the local heating effect and prompt a temporal disruption of the BBB [40]. A nanogel system made up of carbonyl-functionalized poly(N-vinyl pyrrolidone) produced by ionizing radiation was covalently bonded to insulin, serving as a nanocarrier that was efficiently transported across the BBB for the treatment of AD [41]. Zhou et al., designed a nanocomposite using hollow nano-ruthenium (Ru) as a carrier, which was loaded with nerve growth factor (NGF) and sealed with a phase change material (PCM). Under NIR radiation, the nanocomposite produced an excellent photothermal effect as it effectively penetrated the BBB and released NGF, which inhibited tau hyperphosphorylation and aggregation, reduced oxidative stress, and restored nerve damage, ultimately improving memory in an AD-induced mouse model [42]. Likewise, NIR application was used to propel the motion of the Janus nanomotor (JNM-I), which demonstrated an increased BBB permeability and promoted contact between the Aβ fibrils and the Aβ inhibitors [43]. Although these strategies provide new alternatives to overcome the BBB, it is not certain whether translational, repetitive, and prolonged BBB opening might also induce neurotoxicity [27][44].
References
- Palmer, A.M. The role of the blood brain barrier in neurodegenerative disorders and their treatment. J. Alzheimers Dis. 2011, 24, 643–656.
- Duwa, R.; Jeong, J.-H.; Yook, S. Development of immunotherapy and nanoparticles-based strategies for the treatment of Parkinson’s disease. J. Pharm. Investig. 2021, 51, 465–481.
- Cai, Z.; Qiao, P.F.; Wan, C.Q.; Cai, M.; Zhou, N.K.; Li, Q. Role of Blood-Brain Barrier in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 63, 1223–1234.
- Gonzalez-Carter, D.; Liu, X.; Tockary, T.A.; Dirisala, A.; Toh, K.; Anraku, Y.; Kataoka, K. Targeting nanoparticles to the brain by exploiting the blood-brain barrier impermeability to selectively label the brain endothelium. Proc. Natl. Acad. Sci. USA 2020, 117, 19141–19150.
- Lee, C.S.; Leong, K.W. Advances in microphysiological blood-brain barrier (BBB) models towards drug delivery. Curr. Opin. Biotechnol. 2020, 66, 78–87.
- Nguyen, T.T.; Nguyen, T.T.D.; Nguyen, T.K.O.; Vo, T.K.; Vo, V.G. Advances in developing therapeutic strategies for Alzheimer’s disease. Biomed. Pharmacother. 2021, 139, 111623.
- Nguyen, T.T.; Vo, T.K.; Vo, G.V. Therapeutic Strategies and Nano-Drug Delivery Applications in Management of Aging Alzheimer’s Disease. Adv. Exp. Med. Biol. 2021, 1286, 183–198.
- Béduneau, A.; Saulnier, P.; Benoit, J.P. Active targeting of brain tumors using nanocarriers. Biomaterials 2007, 28, 4947–4967.
- Han, L.; Jiang, C. Evolution of blood-brain barrier in brain diseases and related systemic nanoscale brain-targeting drug delivery strategies. Acta Pharm. Sin. B 2021, 11, 2306–2325.
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control. Release 2018, 270, 290–303.
- Kumagai, A.; Eisenberg, J.B.; Pardridge, W. Absorptive-mediated endocytosis of cationized albumin and a beta-endorphin-cationized albumin chimeric peptide by isolated brain capillaries. Model system of blood-brain barrier transport. J. Biol. Chem. 1987, 262, 15214–15219.
- Hervé, F.; Ghinea, N.; Scherrmann, J.M. CNS delivery via adsorptive transcytosis. Aaps J. 2008, 10, 455–472.
- Song, J.; Lu, C.; Leszek, J.; Zhang, J. Design and Development of Nanomaterial-Based Drug Carriers to Overcome the Blood-Brain Barrier by Using Different Transport Mechanisms. Int. J. Mol. Sci. 2021, 22, 10118.
- Zheng, P.P.; Romme, E.; van der Spek, P.J.; Dirven, C.M.; Willemsen, R.; Kros, J.M. Glut1/SLC2A1 is crucial for the development of the blood-brain barrier in vivo. Ann. Neurol. 2010, 68, 835–844.
- Huttunen, J.; Peltokangas, S.; Gynther, M.; Natunen, T.; Hiltunen, M.; Auriola, S.; Ruponen, M.; Vellonen, K.S.; Huttunen, K.M. L-Type Amino Acid Transporter 1 (LAT1/Lat1)-Utilizing Prodrugs Can Improve the Delivery of Drugs into Neurons, Astrocytes and Microglia. Sci. Rep. 2019, 9, 12860.
- Akanuma, S.I. . Yakugaku Zasshi 2020, 140, 1235–1242.
- Jones, A.R.; Shusta, E.V. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm. Res. 2007, 24, 1759–1771.
- Brasnjevic, I.; Steinbusch, H.W.; Schmitz, C.; Martinez-Martinez, P. Delivery of peptide and protein drugs over the blood-brain barrier. Prog. Neurobiol. 2009, 87, 212–251.
- Scheld, W.M. Drug delivery to the central nervous system: General principles and relevance to therapy for infections of the central nervous system. Rev. Infect. Dis. 1989, 11 (Suppl. 7), S1669–S1690.
- Zhu, J.; Zhang, Y.; Chen, X.; Zhang, Y.; Zhang, K.; Zheng, H.; Wei, Y.; Zheng, H.; Zhu, J.; Wu, F.; et al. Angiopep-2 modified lipid-coated mesoporous silica nanoparticles for glioma targeting therapy overcoming BBB. Biochem. Biophys. Res. Commun. 2021, 534, 902–907.
- Yurek, D.M.; Hasselrot, U.; Cass, W.A.; Sesenoglu-Laird, O.; Padegimas, L.; Cooper, M.J. Age and lesion-induced increases of GDNF transgene expression in brain following intracerebral injections of DNA nanoparticles. Neuroscience 2015, 284, 500–512.
- Noor, N.A.; Hosny, E.N.; Khadrawy, Y.A.; Mourad, I.M.; Othman, A.I.; Aboul Ezz, H.S.; Mohammed, H.S. Effect of curcumin nanoparticles on streptozotocin-induced male Wistar rat model of Alzheimer’s disease. Metab. Brain Dis. 2022, 37, 343–357.
- Heydari, S.; Hedayati Ch, M.; Saadat, F.; Abedinzade, M.; Nikokar, I.; Aboutaleb, E.; Khafri, A.; Mokarram, A.R. Diphtheria toxoid nanoparticles improve learning and memory impairment in animal model of Alzheimer’s disease. Pharm. Rep. 2020, 72, 814–826.
- Mahdi, O.; Baharuldin, M.T.H.; Nor, N.H.M.; Chiroma, S.M.; Jagadeesan, S.; Moklas, M.A.M. Chemicals used for the induction of Alzheimer’s disease-like cognitive dysfunctions in rodents. Biomed. Res. Ther. 2019, 6, 3460–3484.
- Householder, K.T.; Dharmaraj, S.; Sandberg, D.I.; Wechsler-Reya, R.J.; Sirianni, R.W. Fate of nanoparticles in the central nervous system after intrathecal injection in healthy mice. Sci. Rep. 2019, 9, 12587.
- Dai, H.; Navath, R.S.; Balakrishnan, B.; Guru, B.R.; Mishra, M.K.; Romero, R.; Kannan, R.M.; Kannan, S. Intrinsic targeting of inflammatory cells in the brain by polyamidoamine dendrimers upon subarachnoid administration. Nanomedicine 2010, 5, 1317–1329.
- Formicola, B.; Cox, A.; Dal Magro, R.; Masserini, M.; Re, F. Nanomedicine for the Treatment of Alzheimer’s Disease. J. Biomed. Nanotechnol. 2019, 15, 1997–2024.
- Islam, S.U.; Shehzad, A.; Ahmed, M.B.; Lee, Y.S. Intranasal Delivery of Nanoformulations: A Potential Way of Treatment for Neurological Disorders. Molecules 2020, 25, 1929.
- Prabakaran, A.; Agrawal, M.; Dethe, M.R.; Ahmed, H.; Yadav, A.; Gupta, U.; Alexander, A. Nose-to-brain drug delivery for the treatment of Alzheimer’s disease: Current advancements and challenges. Expert. Opin. Drug Deliv. 2022, 19, 87–102.
- Fonseca, L.C.; Lopes, J.A.; Vieira, J.; Viegas, C.; Oliveira, C.S.; Hartmann, R.P.; Fonte, P. Intranasal drug delivery for treatment of Alzheimer’s disease. Drug Deliv. Transl. Res. 2021, 11, 411–425.
- Bhatt, P.C.; Srivastava, P.; Pandey, P.; Khan, W.; Panda, B.P. Nose to brain delivery of astaxanthin-loaded solid lipid nanoparticles: Fabrication, radio labeling, optimization and biological studies. RSC Adv. 2016, 6, 10001–10010.
- Lungare, S.; Hallam, K.; Badhan, R.K.S. Phytochemical-loaded mesoporous silica nanoparticles for nose-to-brain olfactory drug delivery. Int. J. Pharm. 2016, 513, 280–293.
- Muntimadugu, E.; Dhommati, R.; Jain, A.; Challa, V.G.; Shaheen, M.; Khan, W. Intranasal delivery of nanoparticle encapsulated tarenflurbil: A potential brain targeting strategy for Alzheimer’s disease. Eur. J. Pharm. Sci. 2016, 92, 224–234.
- Wu, S.K.; Tsai, C.L.; Huang, Y.; Hynynen, K. Focused Ultrasound and Microbubbles-Mediated Drug Delivery to Brain Tumor. Pharmaceutics 2020, 13, 15.
- Burgess, A.; Shah, K.; Hough, O.; Hynynen, K. Focused ultrasound-mediated drug delivery through the blood-brain barrier. Expert. Rev. Neurother. 2015, 15, 477–491.
- Liu, Y.; Gong, Y.; Xie, W.; Huang, A.; Yuan, X.; Zhou, H.; Zhu, X.; Chen, X.; Liu, J.; Liu, J.; et al. Microbubbles in combination with focused ultrasound for the delivery of quercetin-modified sulfur nanoparticles through the blood brain barrier into the brain parenchyma and relief of endoplasmic reticulum stress to treat Alzheimer’s disease. Nanoscale 2020, 12, 6498–6511.
- Kofoed, R.H.; Heinen, S.; Silburt, J.; Dubey, S.; Dibia, C.L.; Maes, M.; Simpson, E.M.; Hynynen, K.; Aubert, I. Transgene distribution and immune response after ultrasound delivery of rAAV9 and PHP.B to the brain in a mouse model of amyloidosis. Mol. Methods Clin. Dev. 2021, 23, 390–405.
- Cheng, C.S.; Liu, T.P.; Chien, F.C.; Mou, C.Y.; Wu, S.H.; Chen, Y.P. Codelivery of Plasmid and Curcumin with Mesoporous Silica Nanoparticles for Promoting Neurite Outgrowth. ACS Appl. Mater. Interfaces 2019, 11, 15322–15331.
- Raymond, J.J.; Robertson, D.M.; Dinsdale, H.B. Pharmacological modification of bradykinin induced breakdown of the blood-brain barrier. Can. J. Neurol. Sci. 1986, 13, 214–220.
- Praça, C.; Rai, A.; Santos, T.; Cristovão, A.C.; Pinho, S.L.; Cecchelli, R.; Dehouck, M.P.; Bernardino, L.; Ferreira, L.S. A nanoformulation for the preferential accumulation in adult neurogenic niches. J. Control. Release 2018, 284, 57–72.
- Picone, P.; Ditta, L.A.; Sabatino, M.A.; Militello, V.; San Biagio, P.L.; Di Giacinto, M.L.; Cristaldi, L.; Nuzzo, D.; Dispenza, C.; Giacomazza, D.; et al. Ionizing radiation-engineered nanogels as insulin nanocarriers for the development of a new strategy for the treatment of Alzheimer’s disease. Biomaterials 2016, 80, 179–194.
- Zhou, H.; Gong, Y.; Liu, Y.; Huang, A.; Zhu, X.; Liu, J.; Yuan, G.; Zhang, L.; Wei, J.A.; Liu, J. Intelligently thermoresponsive flower-like hollow nano-ruthenium system for sustained release of nerve growth factor to inhibit hyperphosphorylation of tau and neuronal damage for the treatment of Alzheimer’s disease. Biomaterials 2020, 237, 119822.
- Liu, W.; Wang, W.; Dong, X.; Sun, Y. Near-Infrared Light-Powered Janus Nanomotor Significantly Facilitates Inhibition of Amyloid-β Fibrillogenesis. ACS Appl. Mater. Interfaces 2020, 12, 12618–12628.
- Yamazaki, Y.; Kanekiyo, T. Blood-Brain Barrier Dysfunction and the Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2017, 18, 1965.
More
