Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Lindsay Dong and Version 2 by Lindsay Dong.
A bioink is a mixture of materials and biological molecules or cells to be used for bioprinting. Most bioinks are hydrogels, highly hydrated polymeric networks used to homogenously encapsulate cells by mimicking the natural extracellular matrix found in vivo. Hydrogels must meet certain characteristics to ensure they can support cell survival and function.
- 3D bioprinting
- biomaterials
- smart bioinks
1. Introduction
3D printing is a manufacturing technique based on a set of processes that creates physical objects by adding layers of material corresponding to successive sections of a computer-aided design (CAD) model [1]. Engineers and designers have been developing innovative applications using 3D printing as it is a rapid prototyping, mass-customizable process that enables the creation of complex geometries that are impossible to achieve by other manufacturing methods [2]. In recent years, 3D printing has become financially feasible at the small enterprise level, giving this type of process a chance to move from heavy industries to office environments. This progress allowed additive manufacturing to have new applications in the medical sector. Thus, advanced 3D printing no longer only involved making three-dimensional objects using plastics and metal alloys, but also human cells, leading to a breakthrough technology known as 3D bioprinting. 3D bioprinting has attracted significant attention in recent years due to its potential to enable the rapid production of tissue-engineered constructs [3].
3D bioprinting attempts to reproduce the three-dimensional organization of cells, replicating what the human body naturally does. This process can fabricate custom tissues or organs using patient-derived cells, thereby minimizing the risk of rejection after transplantation [4]. The final product usually consists of an assembly of specific cells based on a predefined digital design produced in a layer-by-layer fashion [5]. 3D bioprinting offers unprecedented adaptability in positioning cells and creating environments with precise control over their compositions, spatial distributions and architectural precision, allowing for a detailed reconstruction of printed tissues and organs [6]. This technology is already used for the production of several types of tissues, and there are examples in the literature for generating multi-layered skin [7], bones [8], vascular grafts [9], neural tissues [10], heart tissue [11] and cartilage structures [12]. Researchers also have employed 3D bioprinting to produce organs, such as mouse ovaries. For example, sterile mice implanted with artificial ovaries were able to ovulate, give birth and feed healthy baby mice in the normal way, demonstrating the potential to generate organs [13].
Compared to non-biological 3D printing, 3D bioprinting requires additional levels of consideration, such as the choice of cell-adequate nutrient medium, cell type and growth and differentiation factors [14]. Bioprinting requires a three-stage process to make a bioprinted structure. The first stage consists of (i) selecting materials, (ii) formulating a printable ink and (iii) generating sufficient quantities of bioink for printing.
Making a hydrogel-based bioink requires that the desired cells are obtained in sufficient quantities and then added to the ink. The bioprinted structure must then be cultured in a medium enriched with nutrients designed to promote the appropriate cell growth and function. The selection of materials has a great impact on the biocompatibility, cellular viability, and mechanical behaviour of a bioprinted structure and thus care must be taken when determining the most suitable bioink for a given tissue engineering application. Most bioinks rely on crosslinking to turn the liquid bioink into a gel-like substance containing cells. Extrusion-based bioprinting often builds the desired structure through the addition of layers of cell-laden bioinks crosslinked to achieve the expected structures and their associated mechanical properties. Finally, these bioprinted constructs are cultured in media often followed by cellular and mechanical tests to characterize their constructs [15]. Accordingly, formulating appropriate bioinks with the complete required properties for the building of engineered functional tissues and organs is one of the most significant challenges of 3D bioprinting for tissue engineering. As a result, certain bioinks must be functionalized or modified in order to generate the most suitable bioarchetypes [16].
2. Types of Smart Bioinks
Various structures, including nanoparticles, nanofibers, microspheres, fillers and films, have been used for delivering drugs or therapeutic substances to tissues. These substances can promote cell growth, influence cell proliferation and differentiation, and control extracellular matrix (ECM) secretion [17]. For instance, statins [18], osteoprotegerin [19], v3 integrin antagonists [20], cathepsin K inhibitors [21], parathyroid hormone [22], transforming growth factor, and bone morphogenetic protein (BMP) [23] can stimulate bone growth. Angiogenic growth factors, vascular endothelial growth factors (VEGF), fibroblast growth factor, hepatocyte growth factor, and the platelet-derived growth factor have all been utilized to modulate blood vessel creation [17]. It is also critical to evaluate and optimize the chosen hydrogel’s biophysical and biochemical properties when 3D bioprinting functional tissues as they have a big impact on tissue behaviour and functionality [24]. The choice of bioink has a significant impact on the overall qualities of the printed constructs. For example, hydrogel-based bioinks often work not only as a structural substrate for printed tissue but also as a microenvironment for encapsulated cells, allowing them to direct their activities [25].2.1. Nanoparticles
Nanotechnology describes research applications focused on the principles and properties existing at the nanometric scale, at the level of atoms and molecules [26]. The objective of nanotechnology is to produce objects or materials smaller than 100 nanometers [27]. These nanomaterials can be composed of nanoparticles, as seen in Figure 1, which are produced intentionally unlike very fine particles of natural origin. Individual molecules and interacting groups of molecules in relation to the bulk macroscopic properties of the material or device become important at the nanoscales because they have control over the fundamental molecular structure, allowing control over the macroscopic chemical and physical properties [28].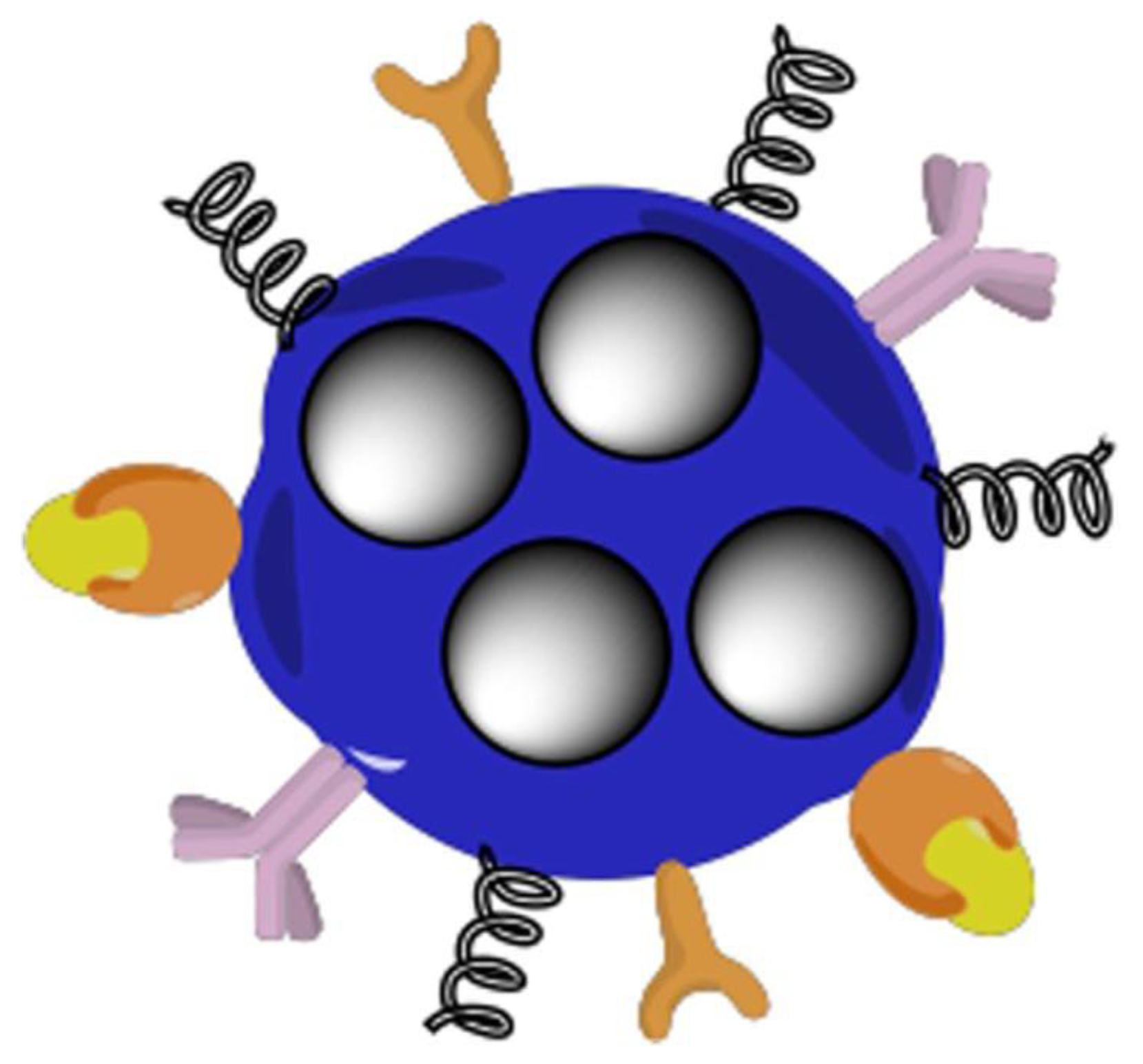
Figure 1. Representation of a smart multifunctional drug-loaded nanoparticle, decorated with various moieties for targeting, imaging and stealth properties. This image is reprinted under a Creative Commons CC BY 4.0 license from [29].
Drug nanoparticles possess increased solubility and hence better bioavailability due to their very small size and wide surface area [29] as well as the capacity to penetrate the blood–brain barrier (BBB), enter the pulmonary system, and be absorbed through the tight connections of skin endothelial cells [30]. Nanoparticles made from natural and synthetic polymers have been of considerable interest because they can be customized for targeted drug delivery, improved bioavailability, and controlled release of medication from a single dose; the system can also prevent endogenous enzymes from degrading the drug through adaptation [31].
2.2. Microparticles and Microspheres
Microparticles, ranging in size from 1 to 1000 µm, can also serve as an effective tool for delivery, especially in the context of 3D bioprinting applications [32]. These particles can both be used as a tool for drug delivery or alone as an additive to modify the properties of the bioink. For example, Kim et al. synthesized dECM microparticles by decellularizing and freeze-milling a pig liver. This novel bioink, dECM powder-based bioink (dECM pBio-ink), was made by dissolving dECM micro-particles in a gelatin solution, and it outperformed the traditional bioink in terms of layer stacking for 3D bioprinting, while the conventional bioink could not keep its shape. Finally, in vitro studies with endothelial cells and primary mouse hepatocytes showed that the dECM pBio-ink had comparable cytocompatibility to the regular dECM bio-ink [33]. Neufurth et al. created a morphogenetically active bioink prepared of amorphous microparticles made of calcium ions (Ca2+) and polyphosphate (polyP), reinforced with poly–caprolactone (PCL). The resulting granular PCL/Ca-polyP-MP hybrid material was used to 3D bioprint tissue-like scaffolds with open pores for cell migration using a layered architecture. The printed composite scaffold had biomechanical properties similar to cortical and trabecular bone. Staining for cell viability, cell density, and scanning electron microscopy (SEM) analyses revealed that this scaffold could attract and foster the growth of human bone-related osteosarcoma (SaOS-2) cells. Based on the findings, it was determined that granular PCL/Ca-polyP-MP hybrid material is ideal for the production of bioprintable scaffolds with morphogenetic potential as well as biomechanical stability [34]. Finally, Sun et al. 3D-bioprinted a protein-releasing cell-laden Hydrogel-PCL composite scaffold to create an integrated live meniscus construct. Transforming growth factor β3 (TGFβ3) or connective tissue growth factor (CTGF) were carried in distinct sections of the hydrogel, encasing PLGA microparticles to produce anisotropic phenotypes to be bioprinted into the microchannels between PCL fibres from different syringes. In vitro and in vivo, the regenerated meniscus construct had cell morphologies and matrix deposition that were similar to the native anisotropic meniscus. Furthermore, the 3D-bioprinted meniscus gave long-term chondroprotection when transplanted into goat knees [35]. Microspheres are engineered materials defined as spherical or round-shaped microparticles [36]. They are often used during the bioprinting and post-printing processes because they cushion the cells, preventing shear stress from occurring, thus allowing different types of cells to grow in a more ideal 3D environment [37]. Chen et al. seeded PC12 and Schwann cells on a new hydrogel they created using Gelatin methacryloyl (GelMA) and Chitosan Microspheres (GC-MSs). The 3D multiscale composite scaffolds were bioprinted using microspheres and hydrogel as the modular bioink to test neurite outgrowth and Schwann cell proliferation as a way to engineer neural tissue. The findings show that a multiscale composite scaffold provided an adequate 3D microenvironment to improve neurite growth and that a 3D printed hydrogel network could offer a 3D macroenvironment that mimics the epineurium layer to proliferate Schwann cells and organize nerve cells, showing promise for neural tissue engineering applications [38]. To summarize, smart bioinks including microparticles and microspheres delivery systems are distinguished by certain characteristics such as (i) physical and chemical stability of the encapsulated active ingredient, which should be maintained throughout the process; (ii) simple, reproducible, and expandable manufacturing, ideally ending with optimal drug loading, maximum encapsulation, and maximum yield at the intended rate for an adequate time period; (iii) flowability and syringeabilitiy [39].2.3. Microswimmers
Magnetic helical microswimmers, also known as artificial bacterial flagella (ABFs), are microscale devices/robots that use rotating, oscillating magnetic fields, or magnetic field gradients to swim in liquid. They transform rotational motion into translational motion to perform 3D navigation in diverse liquids under low-strength rotating magnetic fields. ABFs microswimmers have been extensively researched as carriers for selective drugs and cells. Control of individual groups of swimmers within a swarm is required for numerous biological applications such as drug delivery and release or small-scale surgery in vivo and in vitro [40]. Wang et al. fabricated GelMA microswimmers with user-defined geometry and added Fe3O4 magnetic nanoparticles to their surface to render them magnetically responsive, then, human skin fibroblast cells were cultivated on arrays of the GelMA microstructures. Unlike prior rigid helical microrobots, the soft helical microswimmers were able to corkscrew over the step-out frequency while maintaining relatively high advancing velocity, indicating an unparalleled self-adaptive capability. GelMA microswimmers were also discovered to be highly cell compatible. They are also entirely degradable by collagenases, promote cell adhesion and development, and are gradually degraded throughout a culture by cell-released enzymes. These non-cytotoxic biodegradable hydrogel microswimmers are great prospects for several applications in medicine and tissue engineering as they reduce the worry of collecting microrobots after drug-release procedures [41].2.4. Nano/Micro/Macrogels
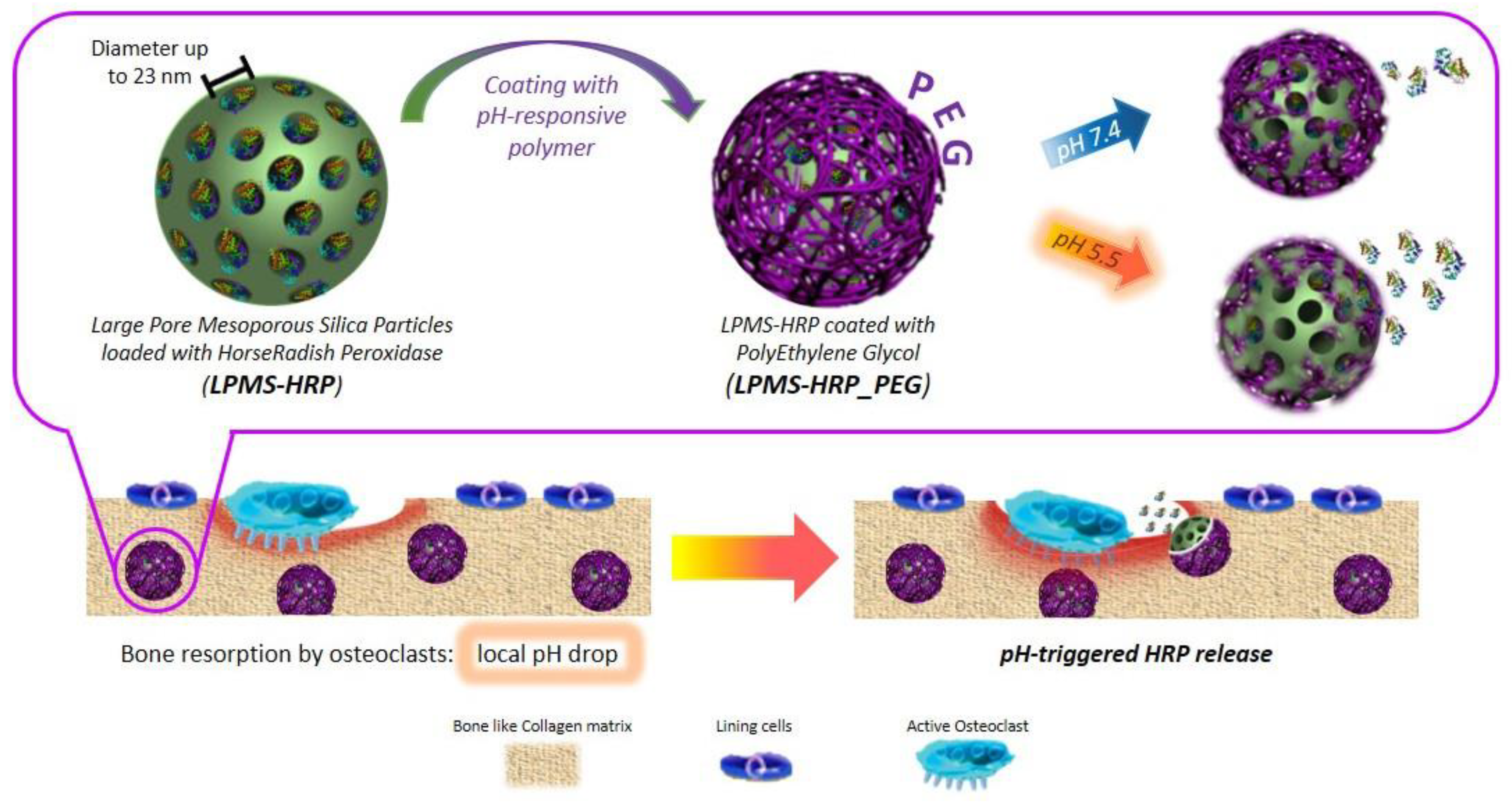
Hydrogels can regulate release performance by managing swelling or degradation thanks to their good compatibility and hydrophilicity [42]. Photo-polymerized hydrogels have, therefore, been employed for localized drug delivery depots for they can encapsulate cells, drugs, or nanoparticles and give physical support at the location of a printed tissue [17]. Banche-Niclot et al. developed large-pore mesoporous silicas (LPMSs) to transport large biomolecules and release them under a pH stimulation for use in bone regeneration [43]. The suggested pH-triggered approach intends to imitate the release of growth factors contained in the bone matrix because of bone resorption by osteoclasts (OCs) and the resulting pH drop in bone remodelling. To achieve this, large-pore mesoporous silicas were made using 1,3,5-trimethyl benzene (TMB) as a swelling agent, and the synthesis solution was hydrothermally treated to see how varied process temperatures and durations affected the final mesostructure. As summarized in Figure 2, LPMSs were coated with a pH-responsive polymer, poly(ethylene glycol) (PEG), to enable the transfer of the incorporated biomolecules in response to a pH decrease The results showed that in an acidic environment, PEG-coated carriers released horseradish peroxidase more quickly due to the protonation of poly(ethylene glycol) at low pH, which catalyzes the polymer hydrolysis reaction. Therefore, suggest that large-pore mesoporous silicas could be employed as carriers for large biomolecules and that poly(ethylene glycol) can be used as a pH-responsive coating [43]. Thus, this method of delivery might be adapted for 3D bioprinting.
Figure 2. Schematic representation of the procedure of obtaining mPEG-silane on LPMS_HRP through PEGylation method. This image is reprinted under a Creative Commons CC BY 4.0 license from [43].
2.5. General Hydrogel-Colloid Composite Bioinks
A colloid is a dispersion of one or more substances suspended in a liquid, forming a system with two separate phases [44]. Colloids can be heterogeneous mixtures of nano- or microscopic particles of various shapes such as spheres, platelets, crystals, rods, and fibres [45]. Bhattacharyya et al. explained the parameter optimizations for semi-automated mixing of bioink components and 3D bioprinting with the twin-screw extruder head with alginate, alpha-tricalcium phosphate (α-TCP) micro/nanoparticles, and osteoblast cells [46]. The TSE-treated bioink samples outperformed the conventional ones in terms of bioprintability, mechanical properties, and biological properties. Even with continuous feeding and extrusion-based bioink printing, the micro/nanoparticles were uniformly dispersed in the bioink, and the live cell distribution in the printed structures was substantially better than conventional mixing. The control of consistent micro/nanomaterials and cell distribution throughout the directly mixed printed bioink was achieved with this novel extrusion head, with minimal cell damage. Due to their highly efficient variable screw pitch design, they also supplied increased batch uniformity in real-time mixing and bioink printing. Increased automation and reduced processing time resulted in higher repeatability than the traditional method of bioink component mixing and subsequent 3D bioprinting, showing a promising basis in tissue engineering applications through the controlled mixing of bioink components and subsequent 3D bioprinting without affecting the cells [46].3. Conclusions
Bioprinting is an additive manufacturing technology that uses bioinks in combination with cells to produce living structures [47]. These bioinks are made up of cytocompatible hydrogel precursor formulations that gel in a compatible way with various bioprinting techniques. The printability of bioink depends on its properties before, during, and after gelation, which includes structural resolution, form fidelity, and cell survival [48]. These properties are regulated by the number of cells in the construct, their proliferation, migration, and contact with the material during tissue growth. A well-calibrated computational framework can forecast tissue regeneration while also optimizing bioprinting input parameters including the beginning material, initial cell loading, and construct design [48]. Recent advances in bioprinting provide a valuable tool to fabricate biomimetic constructs, which can be applied in different stages of drug release research. This pMaper presented manny types of “smart” bioinks that can be used in 3D bioprinting. Furthermore, considering the major advances in engineering and healthcare that 3D bioprinting has enabled, bioprinting in four dimensions (4D) has become an area of increasing focus. 4D printing occurs when a printed 3D item is exposed to external energy inputs such as temperature, light, or other environmental stimuli to trigger a change [49]. 4D bioprinting can construct dynamic 3D patterned biological entities that change shape or behaviour in response to external inputs [50]. Multi-material prints with the potential to reshape over time, or a customized material system that can shift from one form to another, immediately off the print bed, are examples of 4D bioprinting [51]. This technique benefits from the development of smart materials, which can be designed to have a high degree of shape-changing potential. Recent efforts integrating naturally accessible polymers or hybrid smart materials have improved the ability to create volumetrically defined, cell-rich constructions with stimuli-responsive capabilities, shape memory properties, or dynamic motion in time [52]. For example, biocompatible stimuli-responsive shape memory hydrogels have been identified as interesting systems to use with this technology [53]. These materials are commonly used to assist cellular processes, as well as being able to be modified and mixed with other materials to obtain optimal properties for specific applications, making them extremely adaptable. Due to their inherent biocompatibility and biodegradability, intrinsic resemblance to natural tissues, ability to tune their properties through chemical modifications, and responsiveness to stimuli compatible with biological implementation, polymers of natural origin are being extensively investigated for “smart” bioink formulation. As a result, the 4D bioprinting approach has enabled the addition of several useful new ways to build engineered tissues. Vascularization, the capacity to execute a range of biological activities, and the integration of biophysical and biochemical signals to control cell fate and behaviour across time are all examples in the literature. These significant advancements make it straightforward to conclude that 4D bioprinting promotes enhanced integration with host tissues and functional regeneration [54]. Overall, 3D and 4D bioprinting strategies have the potential to revolutionize the field of tissue engineering.References
- Noorani, R. 3D Printing: Technology, Applications, and Selection, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-4987-8376-7.
- Conner, B.P.; Manogharan, G.P.; Martof, A.N.; Rodomsky, L.M.; Rodomsky, C.M.; Jordan, D.C.; Limperos, J.W. Making sense of 3-D printing: Creating a map of additive manufacturing products and services. Addit. Manuf. 2014, 1–4, 64–76.
- Xie, Z.; Gao, M.; Lobo, A.O.; Webster, T.J. 3D Bioprinting in Tissue Engineering for Medical Applications: The Classic and the Hybrid. Polymers 2020, 12, 1717.
- Vermeulen, N.; Haddow, G.; Seymour, T.; Faulkner-Jones, A.; Shu, W. 3D bioprint me: A socioethical view of bioprinting human organs and tissues. J. Med. Ethics 2017, 43, 618–624.
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434.
- Cui, H.; Nowicki, M.; Fisher, J.P.; Zhang, L.G. 3D Bioprinting for Organ Regeneration. Adv. Healthc. Mater. 2017, 6, 1601118.
- Vijayavenkataraman, S.; Lu, W.F.; Fuh, J.Y.H. 3D bioprinting of skin: A state-of-the-art review on modelling, materials, and processes. Biofabrication 2016, 8, 032001.
- Freeman, F.E.; Burdis, R.; Kelly, D.J. Printing New Bones: From Print-and-Implant Devices to Bioprinted Bone Organ Precursors. Trends Mol. Med. 2021, 27, 700–711.
- Hoch, E.; Tovar, G.E.M.; Borchers, K. Bioprinting of artificial blood vessels: Current approaches towards a demanding goal. Eur. J. Cardiothorac. Surg. 2014, 46, 767–778.
- de la Vega, L.; Lee, C.; Sharma, R.; Amereh, M.; Willerth, S.M. 3D bioprinting models of neural tissues: The current state of the field and future directions. Brain Res. Bull. 2019, 150, 240–249.
- Roche, C.D.; Brereton, R.J.L.; Ashton, A.W.; Jackson, C.; Gentile, C. Current challenges in three-dimensional bioprinting heart tissues for cardiac surgery. Eur. J. Cardiothorac. Surg. 2020, 58, 500–510.
- Aljohani, W.; Ullah, M.W.; Zhang, X.; Yang, G. Bioprinting and its applications in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2018, 107, 261–275.
- Zheng, J.-H.; Zhang, J.-K.; Tian, Y.-P.; Song, Y.-B.; Yang, Z.-W.; Huang, X.-H. A Stereological Study of Mouse Ovary Tissues for 3D Bioprinting Application. Cell. Mol. Bioeng. 2021, 14, 259–265.
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785.
- Mancha Sánchez, E.; Gómez-Blanco, J.C.; López Nieto, E.; Casado, J.G.; Macías-García, A.; Díaz Díez, M.A.; Carrasco-Amador, J.P.; Torrejón Martín, D.; Sánchez-Margallo, F.M.; Pagador, J.B. Hydrogels for Bioprinting: A Systematic Review of Hydrogels Synthesis, Bioprinting Parameters, and Bioprinted Structures Behavior. Front. Bioeng. Biotechnol. 2020, 8, 776.
- Trampe, E.; Koren, K.; Akkineni, A.R.; Senwitz, C.; Krujatz, F.; Lode, A.; Gelinsky, M.; Kühl, M. Functionalized Bioink with Optical Sensor Nanoparticles for O2 Imaging in 3D-Bioprinted Constructs. Adv. Funct. Mater. 2018, 28, 1804411.
- Mei, Q.; Rao, J.; Bei, H.P.; Liu, Y.; Zhao, X. 3D Bioprinting Photo-Crosslinkable Hydrogels for Bone and Cartilage Repair. Int. J. Bioprint. 2021, 7, 367.
- Gao, G.; Schilling, A.F.; Hubbell, K.; Yonezawa, T.; Truong, D.; Hong, Y.; Dai, G.; Cui, X. Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol. Lett. 2015, 37, 2349–2355.
- Doschak, M.R.; Kucharski, C.M.; Wright, J.E.I.; Zernicke, R.F.; Uludaǧ, H. Improved Bone Delivery of Osteoprotegerin by Bisphosphonate Conjugation in a Rat Model of Osteoarthritis. Mol. Pharm. 2009, 6, 634–640.
- Park, D.; Park, C.-W.; Choi, Y.; Lin, J.; Seo, D.-H.; Kim, H.-S.; Lee, S.Y.; Kang, I.-C. A novel small-molecule PPI inhibitor targeting integrin αvβ3-osteopontin interface blocks bone resorption in vitro and prevents bone loss in mice. Biomaterials 2016, 98, 131–142.
- Teno, N.; Masuya, K.; Ehara, T.; Kosaka, T.; Miyake, T.; Irie, O.; Hitomi, Y.; Matsuura, N.; Umemura, I.; Iwasaki, G.; et al. Effect of cathepsin K inhibitors on bone resorption. J. Med. Chem. 2008, 51, 5459–5462.
- Arrighi, I.; Mark, S.; Alvisi, M.; von Rechenberg, B.; Hubbell, J.A.; Schense, J.C. Bone healing induced by local delivery of an engineered parathyroid hormone prodrug. Biomaterials 2009, 30, 1763–1771.
- Rodan, G.A.; Martin, T.J. Therapeutic Approaches to Bone Diseases. Science 2000, 289, 1508–1514.
- Pei, M.; Mao, J.; Xu, W.; Zhou, Y.; Xiao, P. Photocrosslinkable chitosan hydrogels and their biomedical applications. J. Polym. Sci. Part Polym. Chem. 2019, 57, 1862–1871.
- Yi, H.-G.; Kim, H.; Kwon, J.; Choi, Y.-J.; Jang, J.; Cho, D.-W. Application of 3D bioprinting in the prevention and the therapy for human diseases. Signal Transduct. Target. Ther. 2021, 6, 177.
- Narducci, D. An Introduction to Nanotechnologies: What’s in it for Us? Vet. Res. Commun. 2007, 31, 131–137.
- Weiss, J.; Takhistov, P.; McClements, D.J. Functional Materials in Food Nanotechnology. J. Food Sci. 2006, 71, R107–R116.
- Saini, R.; Saini, S.; Sharma, S. Nanotechnology: The future medicine. J. Cutan. Aesthetic Surg. 2010, 3, 32–33.
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70.
- Kohane, D.S. Microparticles and nanoparticles for drug delivery. Biotechnol. Bioeng. 2007, 96, 203–209.
- Zhang, J.; Saltzman, M. Engineering biodegradable nanoparticles for drug and gene delivery. Chem. Eng. Prog. 2013, 109, 25–30.
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43.
- Kim, M.K.; Jeong, W.; Lee, S.M.; Kim, J.B.; Jin, S.; Kang, H.-W. Decellularized extracellular matrix-based bio-ink with enhanced 3D printability and mechanical properties. Biofabrication 2020, 12, 025003.
- Neufurth, M.; Wang, X.; Wang, S.; Steffen, R.; Ackermann, M.; Haep, N.D.; Schröder, H.C.; Müller, W.E.G. 3D printing of hybrid biomaterials for bone tissue engineering: Calcium-polyphosphate microparticles encapsulated by polycaprolactone. Acta Biomater. 2017, 64, 377–388.
- Sun, Y.; You, Y.; Jiang, W.; Wu, Q.; Wang, B.; Dai, K. Generating ready-to-implant anisotropic menisci by 3D-bioprinting protein-releasing cell-laden hydrogel-polymer composite scaffold. Appl. Mater. Today 2020, 18, 100469.
- Wang, Y.-L.; Hu, J.-J. Sub-100-micron calcium-alginate microspheres: Preparation by nitrogen flow focusing, dependence of spherical shape on gas streams and a drug carrier using acetaminophen as a model drug. Carbohydr. Polym. 2021, 269, 118262.
- Tan, Y.J.; Tan, X.; Yeong, W.Y.; Tor, S.B. Hybrid microscaffold-based 3D bioprinting of multi-cellular constructs with high compressive strength: A new biofabrication strategy. Sci. Rep. 2016, 6, 39140.
- Chen, J.; Huang, D.; Wang, L.; Hou, J.; Zhang, H.; Li, Y.; Zhong, S.; Wang, Y.; Wu, Y.; Huang, W. 3D bioprinted multiscale composite scaffolds based on gelatin methacryloyl (GelMA)/chitosan microspheres as a modular bioink for enhancing 3D neurite outgrowth and elongation. J. Colloid Interface Sci. 2020, 574, 162–173.
- Vhora, I.; Khatri, N.; Misra, A. Applications of Polymers in Parenteral Drug Delivery. In Applications of Polymers in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2021; pp. 221–261. ISBN 978-0-12-819659-5.
- Wang, X.; Hu, C.; Schurz, L.; De Marco, C.; Chen, X.; Pané, S.; Nelson, B.J. Surface-Chemistry-Mediated Control of Individual Magnetic Helical Microswimmers in a Swarm. ACS Nano 2018, 12, 6210–6217.
- Wang, X.; Qin, X.-H.; Hu, C.; Terzopoulou, A.; Chen, X.-Z.; Huang, T.-Y.; Maniura-Weber, K.; Pané, S.; Nelson, B.J. 3D Printed Enzymatically Biodegradable Soft Helical Microswimmers. Adv. Funct. Mater. 2018, 28, 1804107.
- An, Y.; Hubbell, J.A. Intraarterial protein delivery via intimally-adherent bilayer hydrogels. J. Control. Release 2000, 64, 205–215.
- Banche-Niclot, F.; Montalbano, G.; Fiorilli, S.; Vitale-Brovarone, C. PEG-Coated Large Mesoporous Silicas as Smart Platform for Protein Delivery and Their Use in a Collagen-Based Formulation for 3D Printing. Int. J. Mol. Sci. 2021, 22, 1718.
- Petrucci, R.H.; Harwood, W.S.; Herring, F.G.; Madura, J.D. General Chemistry: Principles and Modern Applications, 9th ed.; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2007; ISBN 978-0-13-149330-8.
- Michel, R.; Auzély-Velty, R. Hydrogel-Colloid Composite Bioinks for Targeted Tissue-Printing. Biomacromolecules 2020, 21, 2949–2965.
- Bhattacharyya, A.; Janarthanan, G.; Tran, H.N.; Ham, H.J.; Yoon, J.; Noh, I. Bioink homogeneity control during 3D bioprinting of multicomponent micro/nanocomposite hydrogel for even tissue regeneration using novel twin screw extrusion system. Chem. Eng. J. 2021, 415, 128971.
- Jose, R.R.; Rodriguez, M.J.; Dixon, T.A.; Omenetto, F.; Kaplan, D.L. Evolution of Bioinks and Additive Manufacturing Technologies for 3D Bioprinting. ACS Biomater. Sci. Eng. 2016, 2, 1662–1678.
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002.
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Significant roles of 4D printing using smart materials in the field of manufacturing. Adv. Ind. Eng. Polym. Res. 2021, 4, 301–311.
- Li, Y.-C.; Zhang, Y.S.; Akpek, A.; Shin, S.R.; Khademhosseini, A. 4D bioprinting: The next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 2016, 9, 012001.
- Tibbits, S. 4D Printing: Multi-Material Shape Change. Archit. Des. 2014, 84, 116–121.
- Wan, Z.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2020, 101, 26–42.
- Subash, A.; Kandasubramanian, B. 4D printing of shape memory polymers. Eur. Polym. J. 2020, 134, 109771.
- Costa, P.D.C.; Costa, D.C.S.; Correia, T.R.; Gaspar, V.M.; Mano, J.F. Natural Origin Biomaterials for 4D Bioprinting Tissue-Like Constructs. Adv. Mater. Technol. 2021, 6, 2100168.
More
