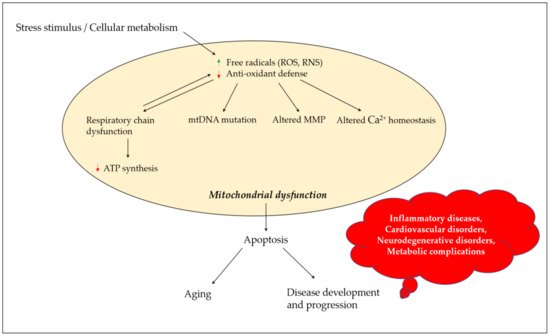
Mitochondrial dysfunction results in a series of defective cellular events, including decreased adenosine triphosphate (ATP) production, enhanced reactive oxygen species (ROS) output, and altered proteastasis and cellular quality control. An enhanced output of ROS may damage mitochondrial components, such as mitochondrial DNA and elements of the electron transport chain, resulting in the loss of proper electrochemical gradient across the mitochondrial inner membrane and an ensuing shutdown of mitochondrial energy production. Neurons have an increased demand for ATP and oxygen, and thus are more prone to damage induced by mitochondrial dysfunction. Mitochondrial dysfunction, damaged electron transport chains, altered membrane permeability and Ca2+ homeostasis, and impaired mitochondrial defense systems induced by oxidative stress, are pathological changes involved in neurodegenerative disorders. A growing body of evidence suggests that the use of antioxidants could stabilize mitochondria and thus may be suitable for preventing neuronal loss. Numerous natural products exhibit the potential to counter oxidative stress and mitochondrial dysfunction; however, science is still looking for a breakthrough in the treatment of neurodegenerative disorders. β-caryophyllene is a bicyclic sesquiterpene, and an active principle of essential oils derived from a large number of spices and food plants. As a selective cannabinoid receptor 2 (CB2) agonist, several studies have reported it as possessing numerous pharmacological activities such as antibacterial (e.g., Helicobacter pylori), antioxidant, anti-inflammatory, analgesic (e.g., neuropathic pain), anti-neurodegenerative and anticancer properties.
- oxidative stress
- mitochondrial dysfunction
- neurodegeneration
- β-caryophyllene
- neuroprotection
1. Introduction
2. Chemistry and Vegetable Sources of β-Caryophyllene
β-caryophyllene (Figure 2) is a bicyclic sesquiterpene, mainly occurring in the form of trans-caryophyllene in combination with small amounts of its isomers (iso-caryophyllene and α-caryophyllene or α-humulene) and its oxidative derivative β-caryophyllene oxide. β-caryophyllene and β-caryophyllene oxide are compounds with a strong wooden odor and are approved as flavorings by the Food and Drug Administration (FDA) and European Food Safety Authority (EFSA) [25]. β-caryophyllene exhibits low water solubility and thus aqueous media such as biological fluids decrease its absorption to the cell. However, the potential obstacles associated with its low water solubility can be overcome by liposomal formulation techniques, which could increase its bioavailability and ensure the desired biological cell effects [26]. β-caryophyllene is a major active principle of essential oils derived from a large number of spices and food plants (Table 1). As reported in the Essential Oil Database, in nature β-caryophyllene is commonly found in Ocimum basilicum L., Cinnamomum species, Piper nigrum L., Syzygium aromaticum (L.) Merr. and L.M. Perry, Cannabis sativa L., Lavandula angustifolia Mill., Origanum vulgare L., and Rosmarinus officinalis L. [27].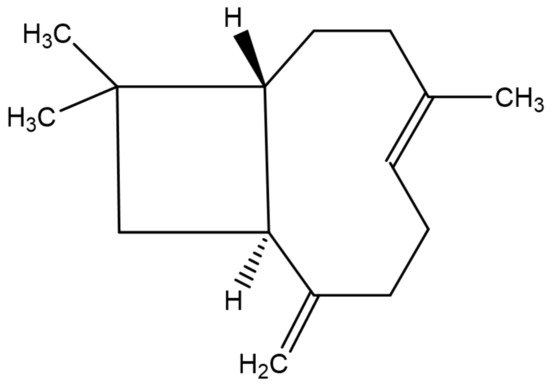
| Botanical Name | Family | Active Parts | Percentage 1 |
|---|---|---|---|
| Ocimum basilicum L. | Lamiaceae | Leaf | 0.3–3.1 |
| Cinnamomum species | Lauraceae | Leaf/bark a | 0.2–35.9 a |
| Piper nigrum L. | Piperaceae | Berries/Leaf/stem b | 3.3–46 b |
| Syzygium aromaticum (L.) Merr. and L.M. Perry | Myrtaceae | Floral bud | 3.2 |
| Cannabis sativa L. | Cannabaceae | Whole plant (fresh material) | 3–16.2 |
| Lavandula angustifolia Mill. | Lamiaceae/labiatae | Flower and stem | 1.08 |
| Lavandula angustifolia Mill. | Lamiaceae/labiatae | Whole plant | 0.3 |
| Origanum vulgare L. | Lamiaceae/labiatae | Leaf/Stem/Flower/Whole plantc | 0.4–24.5 c |
| Rosmarinus officinalis L. | Lamiaceae | Aerial parts | 0.5–13.6 |
3. β-Caryophyllene: Alteration of Oxidative Stress and Mitochondrial Dysfunction
A number of studies have suggested the alteration of oxidative stress and mitochondrial dysfunction by β-caryophyllene and β-caryophyllene-containing vegetable extracts, as one of the potential mechanisms in protecting neurons from degeneration [28]. Chávez-Hurtado et al. (2020) observed a reduction in DNA oxidation and overexpression of glial fibrillary acidic proteins with β-caryophyllene (10 mg/kg, p.o. for 4 weeks) in the prefrontal cortex and hippocampus of BALB/c mice withd-galactose induced aging [29]. In an in vivo model of PD, β-caryophyllene (50 mg/kg, i.p. for 4 weeks) ameliorated oxidative stress (restored antioxidant enzymes, increased GSH, and inhibited lipid peroxidation), neuroinflammation (decreased levels of IL-1β, IL-6, and TNF-α, and downregulated COX-2 and iNOS expression), and glial activation as well as rescuing dopaminergic neurons [30]. Javed et al. investigated the CB2 receptor mediated neuroprotective effects of β-caryophyllene in a rotenone induced animal model of PD [31]. Rotenone (2.5 mg/kg) induced a significant loss in dopaminergic neurons in the substantia nigra pars compacta and dopaminergic striatal fibers, following the activation of astrocytes and microglia when injected peritoneally once daily for 4 weeks. Moreover, rotenone downregulated antioxidant enzymes, increased nitrite levels and induced proinflammatory cytokines (IL-1β, IL-6 and TNF-α) and inflammatory mediators (NF-κB, COX-2, and iNOS). Supplementation with β-caryophyllene (50 mg/kg once daily for 4 weeks, 30 min prior to rotenone administration) attenuated the induction of pro-inflammatory cytokines and inflammatory mediators, prevented the depletion of glutathione, reduced lipid peroxidation, and augmented antioxidant enzymes (SOD and CAT). Tyrosine hydroxylase immunohistochemistry showed the rescue of dopaminergic neurons and fibers following decreased activation of glial cells. β-caryophyllene protected C6 glioma cells from glutamate induced cytotoxicity through alteration of antioxidant responses, mainly by inhibition of ROS production and restoration of MMP via CB2 receptor dependent nuclear factor erythroid 2–related factor 2 (Nrf2) activation [32]. In a neurovascular unit model of oxygen-glucose deprivation and re-oxygenation—induced injury, β-caryophyllene significantly decreased blood–brain barrier (BBB) permeability, reduced neuronal apoptosis, relieved oxidative stress damage, decreased secretion of inflammatory cytokines, downregulated metalloproteinase-9 expression/activity and Bcl-2-associated X protein (Bax) expression, and upregulated expression of claudin-5, occludin, zonula occludens-1 (ZO-1), growth-associated protein-43 (GAP-43) and B-cell lymphoma 2 (Bcl-2) [33]. Conversely, β-caryophyllene relieved seizures in mice induced by pentylenetetrazole, but anti-convulsant doses (0, 10, 30, and 100 mg/kg i.p.) showed no benefits over pentylenetetrazole related oxidative stress i.e., thiobarbituric acid-reactive substances and nonprotein thiol content [34]. Lou et al. (2016) found an attenuation of focal cerebral ischemia-reperfusion injury in rats by treatment with β-caryophyllene through enhanced expression of Nrf2 and HO-1, and restored activity and expression of antioxidant enzymes, i.e., superoxide dismutase (SOD) and catalase (CAT) [35]. In C57BL/6 mice, β-caryophyllene ameliorated the development of experimental autoimmune encephalomyelitis through inhibiting the production of hydrogen peroxide (H2O2), IFN-γ, TNF-α, IL-17 and NO, and decreasing the number of inflammatory infiltrates and neurological damage [36]. An in vitro study demonstrated the alleviation of 1-methyl-4-phenylpyridinium induced neurotoxicity by β-caryophyllene through restoring MMP and increasing intracellular activity of GSH and glutathione peroxidase (GPx), where antioxidant effects were found to be CB2 receptor dependent [37]. Apoptosis was prevented in same study by inhibition of the up-regulation of caspase-3 and Bax, restoring Bcl-2 expression, and suppressing heme oxygenase-1 (HO-1) activation and c-Jun N-terminal kinase (JNK) phosphorylation. Pinus halepensis Mill. essential oil attenuated Alzheimer’s toxic Aβ (1-42)-induced memory impairment and oxidative stress in rat hippocampus [38]. Inhalation of P. halepensis essential oil (1 and 3%) for 21 days resulted in the inhibition of hippocampal AChE activity, elevation of hippocampal antioxidant markers (SOD, CAT, GPx and GSH), and attenuation of Aβ-induced elevation of malondialdehyde (MDA) levels. Phytochemical screening of essential oils revealed the presence of 45 different compounds, with 33 of those compounds having been identified and quantified. Sesquiterpenes (β-caryophyllene) and monoterpenes (α-pinene, myrcene, terpinolene, and 2-phenylethylisovalerate) were the most abundant compounds present in the essential oil, while diterpenes (mainly cembrene) represent only 2.50% of the phytochemicals present. More importantly, β-caryophyllene was found to be the most abundant compound present with the highest percentage of 29.45%. Essential oils extracted from the dried leaves of Aloysia citrodora Palau displayed significant antioxidant and protective effects against both H2O2 and Aβ-induced neurotoxicity in CAD neuroblastoma cell lines [39]. H2O2 (250 μM) and Aβ (10 μM) failed to elicit neurotoxic responses in the presence of A. citrodora essential oil (0.01 and 0.001 mg/mL). The in vitro antioxidant effects of A. citrodora essential oil was confirmed by its Fe2+ chelating capacity. The major chemical components detected in this essential oil were limonene, geranial, neral, 1, 8-cineole, curcumene, spathulenol and caryophyllene oxide. The clove oil obtained from Syzygium aromaticum (L.) Merr. and L.M. Perry is known to contain eugenol as its most abundant compound (87.34%), with eugenol acetate (5.18%) and β-caryophyllene (2.01%) being present in smaller amounts [40]. Kumar et al. reported the neuroprotective potential of clove oil in intra-cerebroventricular (ICV) colchicine-induced memory impairment in rats [41]. Treatment of colchicine challenged rats with S. aromaticum (0.05 mL/kg and 0.1 mL/kg, i.p.) significantly improved cognitive dysfunction, with a marked reduction of AChE activity, lipid peroxidation levels, and nitrite concentrations, and restoration of GSH and mitochondrial respiratory enzyme complex (I–IV) activities. Authors linked the attenuation of cognitive dysfunction with antioxidant and mitochondrial restoring mechanisms. Hyptis fruticosa Salzm. ex Benth (also known as Eplingiella fruticosa) leaf essential oil (containing β-caryophyllene, bicyclogermacrene and 1,8-cineole), complexed with β-cyclodextrin, showed neuroprotective effects in a mouse model of PD by decreasing membrane lipid peroxide levels in the striatum and preserving dopaminergic depletion in the striatum and substantia nigra pars compacta, when administered at a dose of 5 mg/kg, p.o. for 40 days [42]. Ocimum basilicum L. essential oil attenuated ethidium bromide-induced cognitive deficits as well as neuroinflammation, astrogliosis and mitochondrial dysfunction in the prefrontal cortex of rats, with induced MS like manifestations [43]. O. basilicum (100 and 200 μL/kg) significantly mitigated ethidium bromide-induced neuroinflammation by increasing the levels of proinflammatory cytokines (TNF-α and IL-6) and astrogliosis by increasing (Glial fibrillary acidic protein (GFAP) and Ionized calcium binding adaptor molecule-1 (Iba-1) levels. In addition, mitochondrial function, integrity, respiratory control rate, ATP production, and mitochondria-dependent apoptosis were positively regulated in the prefrontal cortex of rats by treatment with O. basilicum. Chemical analysis of the essential oil derived from O. basilicum L. demonstrated the presences of several phytoconstituents, with methyl chavicol, geranial, neral and caryophyllene oxide being major components [44]. Salvia rosmarinus Spenn. essential oil (comprised chemically of 1,8-cineole, α-pinene, camphor, and trans-caryophyllene) exhibited strong antioxidant effects evaluated by DPPH, ABTS, FRAP and β-carotene bleaching tests and confirmed by the relative antioxidant capacity index and significant acetylcholinesterase (AChE) inhibitory activities, suggesting neuroprotective potential in patients with AD [45].References
- Nunnari, J.; Suomalainen, A. Mitochondria: In Sickness and in Health. Cell 2012, 148, 1145–1159.
- Islam, M.T. Oxidative Stress and Mitochondrial Dysfunction-Linked Neurodegenerative Disorders. Neurol. Res. 2017, 39, 73–82.
- Facecchia, K.; Fochesato, L.A.; Ray, S.D.; Stohs, S.J.; Pandey, S. Oxidative Toxicity in Neurodegenerative Diseases: Role of Mitochondrial Dysfunction and Therapeutic Strategies. J. Toxicol. 2011, 2011, 683728.
- Kumar, A. Mitochondrial Dysfunction & Neurological Disorders. Curr. Neuropharmacol. 2016, 14, 565.
- Indo, H.P.; Davidson, M.; Yen, H.C.; Suenaga, S.; Tomita, K.; Nishii, T.; Higuchi, M.; Koga, Y.; Ozawa, T.; Majima, H.J. Evidence of ROS Generation by Mitochondria in Cells with Impaired Electron Transport Chain and Mitochondrial DNA Damage. Mitochondrion 2007, 7, 106–118.
- Chen, J.Q.; Yager, J.D.; Russo, J. Regulation of Mitochondrial Respiratory Chain Structure and Function by Estrogens/Estrogen Receptors and Potential Physiological/Pathophysiological Implications. Biochim. Biophys. Acta Mol. Cell Res. 2005, 1746, 1–17.
- Ghezzi, D.; Zeviani, M. Assembly Factors of Human Mitochondrial Respiratory Chain Complexes: Physiology and Pathophysiology. Adv. Exp. Med. Biol. 2012, 748, 65–106.
- Shoubridge, E.A.; Wai, T. Mitochondrial DNA and the Mammalian Oocyte. Curr. Top. Dev. Biol. 2007, 77, 87–111.
- Sas, K.; Robotka, H.; Toldi, J.; Vécsei, L. Mitochondria, Metabolic Disturbances, Oxidative Stress and the Kynurenine System, with Focus on Neurodegenerative Disorders. J. Neurol. Sci. 2007, 257, 221–239.
- Porter, R.K.; Brand, M.D. Mitochondrial Proton Conductance and H+/O Ratio Are Independent of Electron Transport Rate in Isolated Hepatocytes. Biochem. J. 1995, 310, 379–382.
- Zhang, Y.; Wang, M.; Li, H.; Zhang, H.; Shi, Y.; Wei, F.; Liu, D.; Liu, K.; Chen, D. Accumulation of Nuclear and Mitochondrial DNA Damage in the Frontal Cortex Cells of Patients with HIV-Associated Neurocognitive Disorders. Brain Res. 2012, 1458, 1–11.
- Wei, Y.H.; Lu, C.Y.; Wei, C.Y.; Ma, Y.S.; Lee, H.C. Oxidative Stress in Human Aging and Mitochondrial Disease-Consequences of Defective Mitochondrial Respiration and Impaired Antioxidant Enzyme System. Chin. J. Physiol. 2001, 44, 1–11.
- Hollensworth, S.B.; Shen, C.C.; Sim, J.E.; Spitz, D.R.; Wilson, G.L.; Ledoux, S.P. Glial Cell Type-Specific Responses to Menadione-Induced Oxidative Stress. Free Radic. Biol. Med. 2000, 28, 1161–1174.
- van Houten, B.; Woshner, V.; Santos, J.H. Role of Mitochondrial DNA in Toxic Responses to Oxidative Stress. DNA Repair 2006, 5, 145–152.
- Voets, A.M.; Huigsloot, M.; Lindsey, P.J.; Leenders, A.M.; Koopman, W.J.H.; Willems, P.H.G.M.; Rodenburg, R.J.; Smeitink, J.A.M.; Smeets, H.J.M. Transcriptional Changes in OXPHOS Complex I Deficiency Are Related to Anti-Oxidant Pathways and Could Explain the Disturbed Calcium Homeostasis. Biochim. Biophys. Acta Mol. Basis. Dis. 2012, 1822, 1161–1168.
- Castro, M.D.R.; Castro, M.D.R.; Suarez, E.; Kraiselburd, E.; Isidro, A.; Paz, J.; Ferder, L.; Ayala-Torres, S. Aging Increases Mitochondrial DNA Damage and Oxidative Stress in Liver of Rhesus Monkeys. Exp. Gerontol. 2012, 47, 29–37.
- Alexeyev, M.F. Is There More to Aging than Mitochondrial DNA and Reactive Oxygen Species? FEBS J. 2009, 276, 5768–5787.
- Andreazza, A.C.; Shoo, L.; Wang, J.F.; Trevor Young, L. Mitochondrial Complex I Activity and Oxidative Damage to Mitochondrial Proteins in the Prefrontal Cortex of Patients with Bipolar Disorder. Arch. Gen. Psychiatry 2010, 67, 360–368.
- Kirkinezos, I.G.; Bacman, S.R.; Hernandez, D.; Oca-Cossio, J.; Arias, L.J.; Perez-Pinzon, M.A.; Bradley, W.G.; Moraes, C.T. Cytochrome c Association with the Inner Mitochondrial Membrane Is Impaired in the CNS of G93A-SOD1 Mice. J. Neurosci. 2005, 25, 164–172.
- Stewart, V.C.; Heales, S.J.R. Nitric Oxide-Induced Mitochondrial Dysfunction: Implications for Neurodegeneration. Free Radic. Biol. Med. 2003, 34, 287–303.
- Mattson, M.R. Calcium and Neurodegeneration. Aging Cell 2007, 6, 337–350.
- Douarre, C.; Sourbier, C.; Dalla Rosa, I.; Brata Das, B.; Redon, C.E.; Zhang, H.; Neckers, L.; Pommier, Y. Mitochondrial Topoisomerase I Is Critical for Mitochondrial Integrity and Cellular Energy Metabolism. PLoS ONE 2012, 7, e41094.
- Joshi, G.; Sultana, R.; Perluigi, M.; Butterfield, D.A. In Vivo Protection of Synaptosomes from Oxidative Stress Mediated by Fe2+/H2O2 or 2,2 Azobis-(2-Amidinopropane) Dihydrochloride by the Glutathione Mimetic Tricyclodecan-9-Yl Xanthogenate. Free Radic. Biol. Med. 2005, 38, 1023–1031.
- Ross, W.N. Understanding Calcium Waves and Sparks in Central Neurons. Nat. Rev. Neurosci. 2012, 13, 157–168.
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-Caryophyllene and β-Caryophyllene Oxide—Natural Compounds of Anticancer and Analgesic Properties. Cancer Med. 2016, 5, 3007–3017.
- Sarpietro, M.G.; di Sotto, A.; Accolla, M.L.; Castelli, F. Interaction of β-Caryophyllene and β-Caryophyllene Oxide with Phospholipid Bilayers: Differential Scanning Calorimetry Study. Thermochim. Acta 2015, 600, 28–34.
- EssOilDB. Available online: http://www.nipgr.ac.in/Essoildb/ (accessed on 7 February 2021).
- Machado, K.C.; Islam, M.T.; Ali, E.S.; Rouf, R.; Uddin, S.J.; Dev, S.; Shilpi, J.A.; Shill, M.C.; Reza, H.M.; Das, A.K.; et al. A Systematic Review on the Neuroprotective Perspectives of Beta-Caryophyllene. Phytother. Res. 2018, 32, 2376–2388.
- Chávez-Hurtado, P.; González-Castañeda, R.E.; Beas-Zarate, C.; Flores-Soto, M.E.; Viveros-Paredes, J.M. β-Caryophyllene Reduces DNA Oxidation and the Overexpression of Glial Fibrillary Acidic Protein in the Prefrontal Cortex and Hippocampus of d-Galactose-Induced Aged BALB/c Mice. J. Med. Food 2020, 23, 515–522.
- Ojha, S.; Javed, H.; Azimullah, S.; Haque, M.E. β-Caryophyllene, a Phytocannabinoid Attenuates Oxidative Stress, Neuroinflammation, Glial Activation, and Salvages Dopaminergic Neurons in a Rat Model of Parkinson Disease. Mol. Cell. Biochem. 2016, 418, 59–70.
- Javed, H.; Azimullah, S.; Haque, M.E.; Ojha, S.K. Cannabinoid Type 2 (CB2) Receptors Activation Protects against Oxidative Stress and Neuroinflammation Associated Dopaminergic Neurodegeneration in Rotenone Model of Parkinson’s Disease. Front. Neurosci. 2016, 10, 321.
- Assis, L.C.; Straliotto, M.R.; Engel, D.; Hort, M.A.; Dutra, R.C.; de Bem, A.F. β-Caryophyllene Protects the C6 Glioma Cells against Glutamate-Induced Excitotoxicity through the Nrf2 Pathway. Neuroscience 2014, 279, 220–231.
- Tian, X.; Peng, J.; Zhong, J.; Yang, M.; Pang, J.; Lou, J.; Li, M.; An, R.; Zhang, Q.; Xu, L.; et al. β-Caryophyllene Protects in Vitro Neurovascular Unit against Oxygen-Glucose Deprivation and Re-Oxygenation-Induced Injury. J. Neurochem. 2016, 39, 757–768.
- de Oliveira, C.C.; de Oliveira, C.V.; Grigoletto, J.; Ribeiro, L.R.; Funck, V.R.; Grauncke, A.C.B.; de Souza, T.L.; Souto, N.S.; Furian, A.F.; Menezes, I.R.A.; et al. Anticonvulsant Activity of β-Caryophyllene against Pentylenetetrazol-Induced Seizures. Epilepsy Behav. 2016, 56, 26–31.
- Lou, J.; Cao, G.; Li, R.; Liu, J.; Dong, Z.; Xu, L. β-Caryophyllene Attenuates Focal Cerebral Ischemia-Reperfusion Injury by Nrf2/HO-1 Pathway in Rats. Neurochem. Res. 2016, 41, 1291–1304.
- Fontes, L.B.A.; Dias, D.; dos, S.; Aarestrup, B.J.V.; Aarestrup, F.M.; Filho, A.A.D.S.; Corrêa, J.O.d.A. β-Caryophyllene Ameliorates the Development of Experimental Autoimmune Encephalomyelitis in C57BL/6 Mice. Biomed. Pharmacother. 2017, 91, 257–264.
- Wang, G.; Ma, W.; Du, J. β-Caryophyllene (BCP) Ameliorates MPP+ Induced Cytotoxicity. Biomed. Pharmacother. 2018, 103, 1086–1091.
- Postu, P.A.; Sadiki, F.Z.; el Idrissi, M.; Cioanca, O.; Trifan, A.; Hancianu, M.; Hritcu, L. Pinus Halepensis Essential Oil Attenuates the Toxic Alzheimer’s Amyloid Beta (1-42)-Induced Memory Impairment and Oxidative Stress in the Rat Hippocampus. Biomed. Pharmacother. 2019, 112, 108673.
- Abuhamdah, S.; Abuhamdah, R.; Howes, M.-J.R.; Al-Olimat, S.; Ennaceur, A.; Chazot, P.L. Pharmacological and Neuroprotective Profile of an Essential Oil Derived from Leaves of Aloysia Citrodora Palau. J. Pharm. Pharmacol. 2015, 67, 1306–1315.
- Mehta, A.K.; Halder, S.; Khanna, N.; Tandon, O.P.; Sharma, K.K. The Effect of the Essential Oil of Eugenia Caryophyllata in Animal Models of Depression and Locomotor Activity. Nutr. Neurosci. 2013, 16, 233–238.
- Kumar, A.; Aggrawal, A.; Pottabathini, R.; Singh, A. Possible Neuroprotective Mechanisms of Clove Oil against Icv-Colchicine Induced Cognitive Dysfunction. Pharmacol. Rep. 2016, 68, 764–772.
- Beserra-Filho, J.I.A.; de Macêdo, A.M.; Leão, A.H.F.F.; Bispo, J.M.M.; Santos, J.R.; de Oliveira-Melo, A.J.; Menezes, P.D.P.; Duarte, M.C.; de Souza Araújo, A.A.; Silva, R.H.; et al. Eplingiella Fruticosa Leaf Essential Oil Complexed with β-Cyclodextrin Produces a Superior Neuroprotective and Behavioral Profile in a Mice Model of Parkinson’s Disease. Food Chem. Toxicol. 2019, 124, 17–29.
- Garabadu, D.; Singh, D. Ocimum Basilicum Attenuates Ethidium Bromide-Induced Cognitive Deficits and Pre-Frontal Cortical Neuroinflammation, Astrogliosis and Mitochondrial Dysfunction in Rats. Metab. Brain Dis. 2020, 35, 483–495.
- Sajjadi, S.E. Analysis of the Essential Oils of Two Cultivated Basil (Ocimum basilicum L.) from Iran. DARU J. Pharm. Sci. 2006, 14, 128–130.
- Leporini, M.; Bonesi, M.; Loizzo, M.R.; Passalacqua, N.G.; Tundis, R. The Essential Oil of Salvia Rosmarinus Spenn. From Italy as a Source of Health-Promoting Compounds: Chemical Profile and Antioxidant and Cholinesterase Inhibitory Activity. Plants 2020, 9, 798.
