The diagnosis of myocardial injury inflammatory lesions is a difficult and challenging task, and despite various imaging modalities, myocardial injury inflammatory lesions are still a diagnosis of exclusion. Currently, endomyocardial biopsy (EMB) combined with genomics and immunohistochemistry can be used to evaluate myocardial inflammatory lesions in clinical practice. However, due to its invasiveness, sampling variability, limited spatial information, and low clinical implementation, it is difficult to apply in clinical practice and has great limitations. For inflammatory lesions of myocardium injury, clinical treatment strategies are supportive treatment or treatment of the primary underlying diseases, which are focused on delaying the disease process, are unable to reverse and repair the myocardial injury, and have limited effects on reducing morbidity and mortality. Therefore, it is of clinical urgency to actively seek effective treatment methods for myocardial injury and inflammation.
1. Existing Methods of Diagnosis for Myocardial Injury Inflammatory Lesions
Most of the early stages of cardiomyopathy are subclinical, that is, without obvious symptoms
[14][1]. As the disease progresses, patients develop symptoms of cardiac insufficiency. The patient’s personal history, such as chemotherapy, history of diabetes, major trauma, etc., and clinical symptoms are the main basis of diagnosis of cardiomyopathy. Laboratory examination and radiographic analysis of myocardial injury caused by abnormal heart function or structure are essential. Evaluation methods include electrocardiogram, echocardiography, coronary computed tomographic angiography (CTA) and cardiac magnetic resonance imaging (MRI), etc. The patient’s medical history, symptoms and signs, and examination results are analyzed to exclude specific cardiomyopathy and endemic cardiomyopathy, and a diagnosis is finally reached
[12][2]. However, the above diagnostic methods have great limitations in accuracy and sensitivity for early myocardial injury
[15][3], which is one of the main causes of missed diagnosis and misdiagnosis in clinical practice. Therefore, timely and accurate assessment of the myocardial inflammatory microenvironment can help in the timely clinical diagnosis of myocardial injury.
2. Current Treatment Methods for Myocardial Injury and Inflammatory Lesions
Clinical treatment of various myocardial injuries and inflammatory lesions mainly follows two principles. One is to provide symptomatic support for the hemodynamic disorders of heart disease itself, including conventional anti-heart failure, anti-arrhythmia, anticoagulant thrombolytic, inhibition of cardiac remodeling, improvement of myocardial metabolism, etc., to prevent further damage to the cardiac cells and maintain cardiac function and hemodynamic stability. The other is targeted management for the primary underlying diseases that induce myocardial injury
[16[4][5][6],
17,18], such as septic cardiomyopathy. According to international guidelines for management of sepsis and septic shock, anti-infective therapy should be immediately given after diagnosis to treat primary sepsis
[19,20,21][7][8][9]. For diabetic cardiomyopathy, reasonable control of blood glucose, improvement of systemic and tissue insulin sensitivity, myocardial glucose uptake, and cardiac function are key factors to reduce the incidence and mortality of heart failure
[22,23,24][10][11][12].
When myocardial injury occurs, the homeostatic function of the cell population is unbalanced, and the stressed cells release pro-inflammatory factors, chemokines, exosomes, and senescence-associated secretory phenotypes (SASPs), leading to the formation of a pro-inflammatory microenvironment and myocardial tissue dysfunction, exacerbating the process of myocardial injury
[25,26,27,28,29,30,31][13][14][15][16][17][18][19]. With the development of cardiovascular precision medicine, targeted drugs, and the proposal of new therapeutic concepts to regulate the microenvironment of myocardial inflammation, it is possible to reverse and repair myocardial damage.
3. Problems That Need to Be Solved
The inflammatory response is an important link in the pathogenesis and development of coronary heart disease, and the value of inflammatory signals in the prediction and prognosis evaluation of acute coronary syndrome of coronary heart disease, as well as the anti-inflammatory effects of drug therapy, is increasingly being recognized
[32][20]. There are several potential approaches for the diagnosis and treatment of cardiac inflammatory diseases, and targeted nanomedicine is one of the most promising approaches (
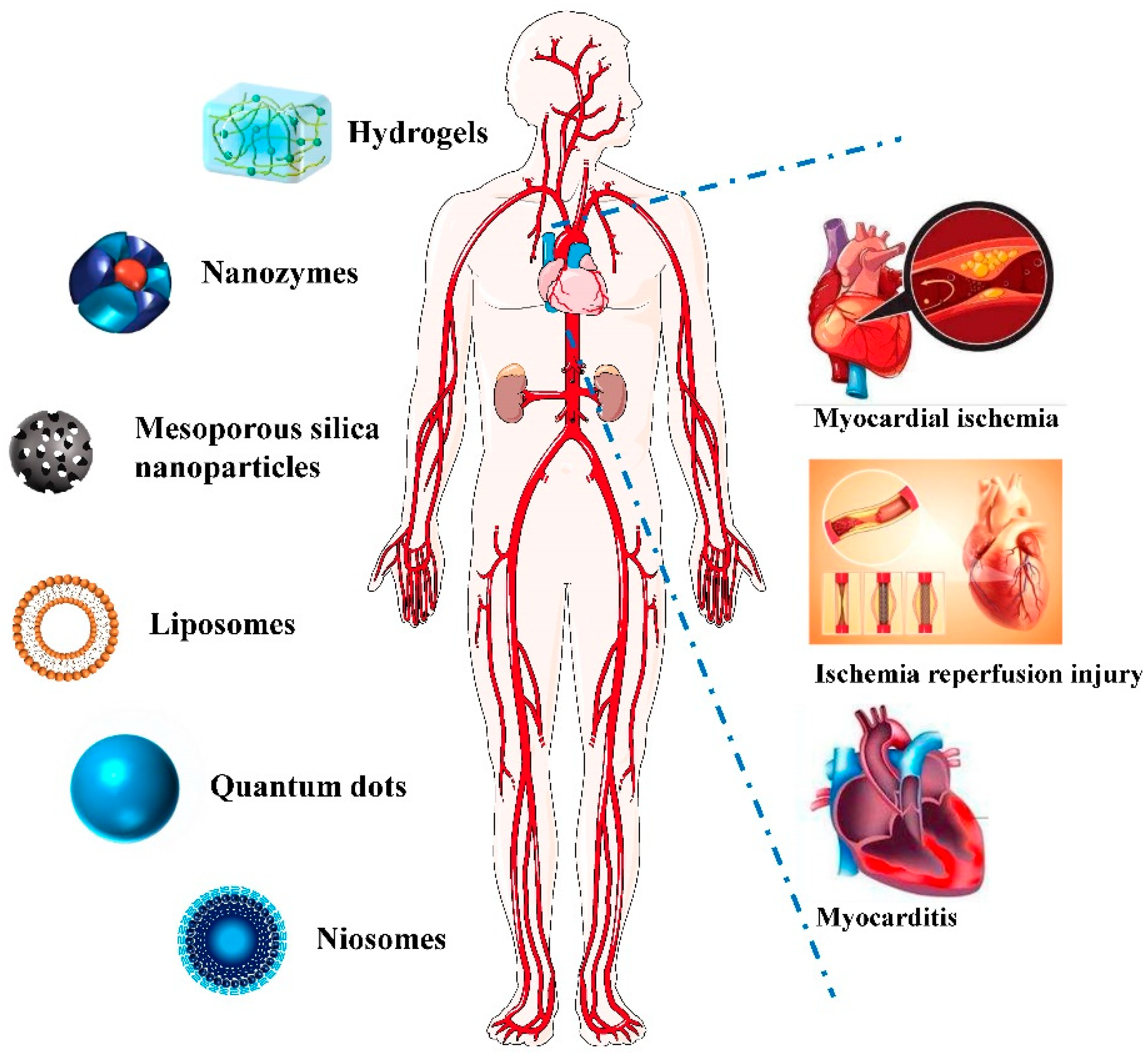
Figure 1).
Figure 1. Application of Nano-theranostics for myocardial injury.
3.1. Hydrogel
Hydrogels with particle sizes between 1–1000 nm can be used for drug delivery, medical diagnosis, as biosensors, and for biological separation
[33][21]. Hydrogels are similar to natural soft tissue and can provide a microenvironment for the extracellular matrix, showing great potential in tissue engineering applications. In particular, the development of biodegradable conductive hydrogels is of great significance for repairing electrically active tissues such as the myocardium, skeletal muscle and nerves
[33,34,35][21][22][23]. In recent years, hydrogels have been widely used in the diagnosis and treatment of myocardial injury and inflammatory lesions.
Zhao Chao et al.
[36][24] invented a temperature-sensitive and biodegradable poly(lactic acid)-poly(ethylene glycol)-poly(N-iso-propylacrylamide) (PLLA-PEG-PNIPAM) block polymer, which could form nanofiber gel microspheres (NF-GMS) and be loaded with cardiocytes (CM) for cardiac regeneration and repair.
This research can be applied to cardiac myocyte transplantation, significantly improving cardiac function and opening up broad application prospects in the regeneration and repair of the heart. Ghanta et al.
[37][25] used a small molecule modified alginate to encapsulate rat mesenchymal stem cells (MSCs)in a core-shell hydrogel capsule, and constructed TMTD-alginate MSC capsules which improve ventricular function and remodeling in a post-MI rat model. Li Hekai et al.
[38][26] developed a mixed alloy nanoparticle (AuNP)-hyaluronic acid (HA) hydrogel matrix for encapsulating human induced pluripotent stem cell-derived cardiomyocytes(iPS-CM) to overcome th
ise limitation. MMP-2 reactive hydrogel was prepared by crosslinking with degradable peptides of matrix metalloproteinase-2 (MMP-2) using methacrylate-modified HA as the skeleton. RGD peptide was introduced as an adhesion point to enhance its biocompatibility. The addition of AuNPs regulates the mechanical and topological properties of the matrix by significantly increasing its stiffness and surface roughness, thus accelerating the formation of gap junctions in iPS-CM and coordinating calcium processing through the αnβ1 integrin-mediated ILK-1/P-Akt/GATA4 pathway. Transplanted AUNP-HA-hydrogel-encapsulated iPS-CM formed stronger gap junctions in infarcted mouse hearts and resynchronized ventricular electrical conduction after myocardial infarction. Hydrogel-delivered iPS-CMs play a strong role in angiogenesis, which also contributes to the recovery process.
3.2. Nano-Enzyme
As powerful biocatalysts, enzymes have high specificity and catalytic efficiency in in vivo and in vitro biochemical reactions under relatively mild conditions. However, due to their disadvantages, such as high preparation and purification costs, poor operation stability, sensitivity to the reaction environment, and difficulty in recycling, nano-enzyme technology has emerged as an alternative
[39][27]. As a kind of artificial enzyme, nano-enzymes have many unique advantages, such as low cost, high stability, easy modification, and adjustable catalytic activity. They can solve the limitations of natural enzymes and have been widely studied and applied
[40][28].
Nano-enzymes have the functions of protecting the myocardium and improving Alzheimer’s disease and ischemic stroke, and their application research has been extended from in vitro to in vivo, providing new ideas and methods for the diagnosis and treatment of myocardial injury and inflammatory lesions
[41][29]. Zhang Yue
[42][30] et al., with metal nanoparticles as the enzyme activity center, constructed a hybrid nanozyme which not only has SOD- and CAT-like activities, but can also overcome the biological barrier targeting cardiomyocyte mitochondria, providing a new treatment for alleviating myocardial injury.
Nitric oxide (NO) is an important signaling molecule in the cardiovascular system and is associated with the pathogenesis of ischemic cardiomyopathy, septic cardiomyopathy, and other cardiomyopathies. One of the effective treatment strategies for these cardiomyopathies is to provide a controlled and constant supply of nitric oxide (NO). Li Haiyun et al.
[43][31] constructed nitric oxide synthase (NOS-like NanoNOS), comprised of a noble metal nanoparticle core and mesoporous silica shell, and proved the catalytic capacity of NanoNOS for NO production. NanoNOS inhibits endothelial cell adhesion factor expression by releasing NO and protects endothelial cells from stimulus-induced damage and the resulting mono-endothelial cell adhesion, suggesting that NanoNOS therapy can help prevent myocardial injury.
This study broadens the biomedical application of nanomases and provides a new idea for the prevention and treatment of diseases related to myocardial injury.3.3. Extracellular Vesicles
Extracellular vesicles (EVs) are lipid bilayer particles without the ability to replicate that are secreted by cells into the extracellular microenvironment
[44][32]. Exosomes have a wide range of manifestations in terms of size, source, biochemical components, and biological functions, including intercellular communication, immune response regulation, and disease progression
[45][33], and can be divided into four subgroups: exosomes, microvesicles, apoptotic bodies, and cancer bodies, among which exosomes are the most well studied. Exosomes have been found to be secreted by endothelial cells, cardiac progenitor cells, cardiac fibroblasts, and cardiomyocytes, suggesting that exosomes play a potentially important role in cardiovascular diseases
[46][34]. Recent advances in extracellular vesicle research have not only highlighted their importance in cardiac physiology and pathology, but also attracted attention since these extracellular vesicles may have utility in the diagnosis and treatment of cardiac inflammatory diseases
[47][35], particularly those that are functionalized by nucleic acids
[48][36].
Anselmo Achille et al.
[49][37] identified CD172a+ EVs as cardiogenic EVs, and found that cardiomyocytes (CMs) shed more EVs under hypoxic stress, thus promoting the positive inotropic effect of unstressed CMs. Clinically, patients with aortic stenosis with higher levels of CD172a+ EVs in the circulatory center have a higher survival rate than those with lower levels of CD172a+ EVs during transcranial aortic valve replacement. These results suggest that CD172a+ EVs are a promising prognostic biomarker for myocardial diseases.
Cardiac progenitor cell-derived exosomes (CPCS-EXs) are of cardiac protection and repair value. Li Xin et al.
[50][38] found that exosomes secreted by cardiac progenitor cells can protect cardiac myocytes in viral myocarditis models. Its protective function is achieved by reducing virus proliferation and regulating the mTOR, Bcl-2, and Caspase signaling pathways.
This study identified the anti-apoptotic effect of CPCS-EX in Coxsackie virus B3-infected cells and rats, suggesting that CPCS-EX may be an effective tool for the treatment of viral myocarditis, and this work provides a potential cell therapy for diseases such as viral myocarditis.3.4. Other Nanomaterials
Nanotechnology, due to its unique physical and chemical properties, presents many attractive prospects in the field of cardiovascular diseases
[51][39]. The potential of drug delivery technology based on lipid nanoparticles has been fully demonstrated. For example, nano-probes are mainly used for targeted imaging of biochemical changes in life systems
[52][40]. The combination of drug delivery integrating with diagnosis and therapeutic monitoring is an interesting evolution of this concept, which will contribute to the development of general targeted drug delivery tools for the effective treatment of cardiovascular disease and some other diseases
[53][41]. Zhao Xueli
[54][42] assembled 17β-estradiol nano-probes with peptides that can specifically bind primary cardiomyocytes and used perfluorocarbons as the core to obtain a new nano-probe, PCM-E2/PFPs. Both in vivo and in vitro studies have demonstrated that these PCM-E2/PFPs can be used as contrast agents when exposed to low-intensity focused ultrasound (LIFU). The significantly accelerated release of 7β-estradiol enhances the efficacy of the drug without systemic side effects. PCM-E2/PFPs and low-intensity focused ultrasound therapy also significantly increased the myocardial targeting and circulation time. In-depth treatment evaluation showed that PCM-E2/PFPs and low-intensity focused ultrasound significantly inhibited myocardial hypertrophy, especially with lower expression levels of β-MHC, Collagen 1, and Collagen 3 among other treatment groups, revealing the high efficacy and protective effect of myocardium targeted delivery. This new integrated nano-platform can provide a diagnostic and treatment carrier for myocardial injury diseases and has very broad application prospects.
Creatine kinase is a commonly used marker of myocardial injury, and its activity is an important indicator of myocardial injury
[55][43]. Li Xingjun et al.
[56][44] synthesized monodisperse spherical EU-QPTCA rare earth MOF nanomaterials with a controllable particle size by the ligand exchange method, and used oleic acid and acetic acid as modulators. EU-QPTCA nanomaterials show strong red luminescence. Studies have found that both ATP and ADP can quench the red luminescence of EU-QPTCA nanomaterials, and ATP has a stronger quenching effect than ADP. Creatine kinase can catalyze the formation of ADP and phosphocreatine from ATP and creatine at pH 9.0, thus restoring the fluorescence of EU-QPTCA nanomaterials. Therefore, this nanomaterial can be used as a novel fluorescent probe for the highly sensitive and specific detection of creatine kinase activity, providing a new approach to the detection of creatine kinase activity in human blood, and has important significance for the early diagnosis of myocardial injury.