Complex interactions at the materno-placental-foetal interface have a profound influence on the infants’ immune maturation and the likelihood of developing allergic sensitisation and disease. Gene/environment interactions and timing of exposures through pregnancy add further degrees of complexity. Understanding the early life origins of allergy will only be possible by embracing this complexity. Studies will now need to investigate combinations of dietary, pollutant, medication and microbial exposures during pregnancy in relation to genomics, epigenomics, metagenomics and metabolomics and their effect on infant/child outcomes. Controlled trials of a “healthy diet” during pregnancy are likely to yield better outcomes than focusing on single nutrients which hitherto have produced disappointing results. The manipulation of the neonates’ evolving microbiome is suggested as another focus for controlled prevention trials.
1. Introduction
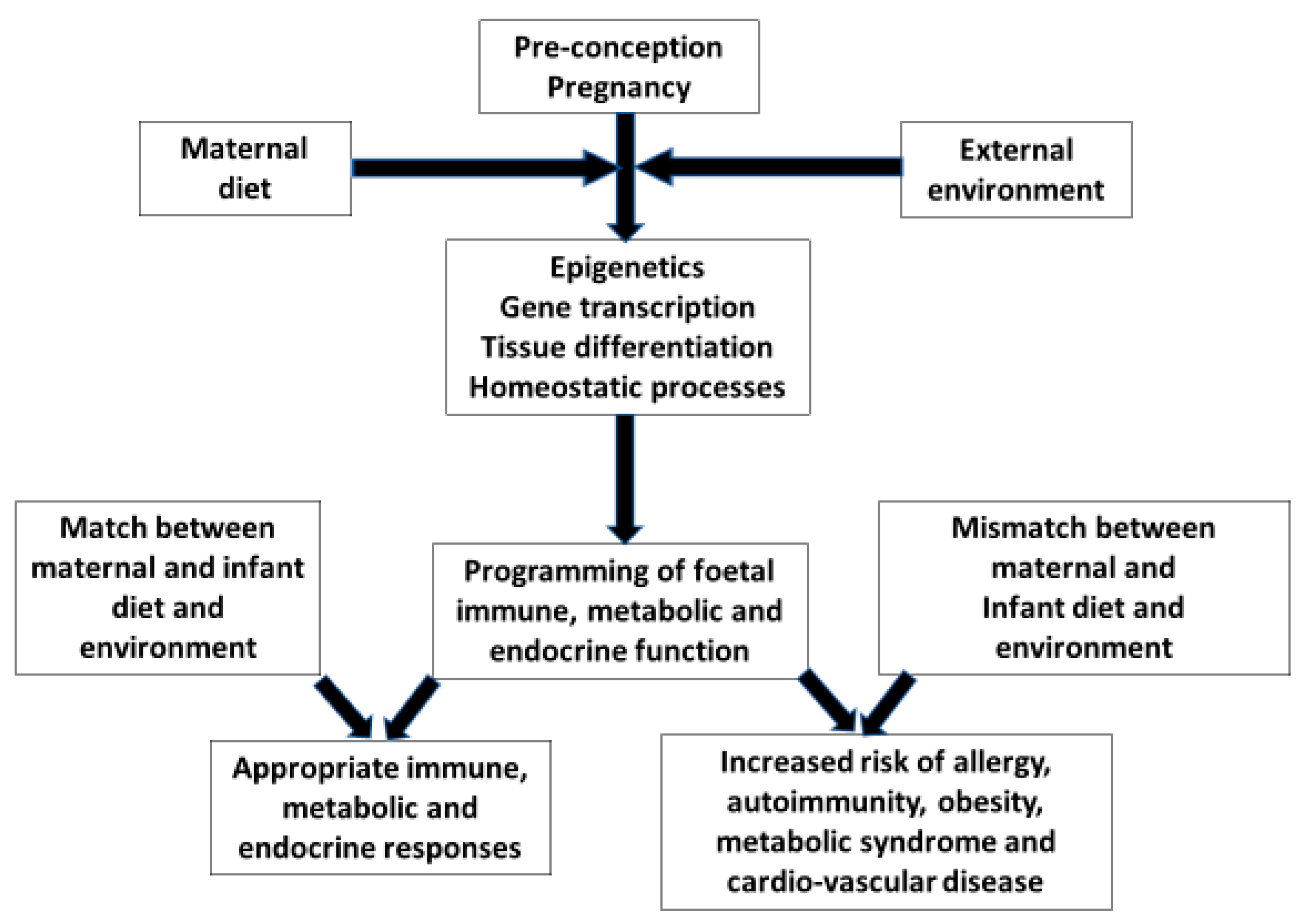
During delivery the neonate leaves the controlled and sterile environment of the uterus to be exposed to potentially overwhelming physical challenges. Development through pregnancy is in some respects a preparation for extra-uterine existence with maternal gene/environment interactions influencing organ development and the programming of foetal immune, metabolic and endocrine responses. This concept arises from the hypothesis that the origins of health or risks of most diseases are a consequence of minor perturbations during organogenesis and periods of rapid cell division. It has evolved into the science known as “The Developmental Origins of Health And Disease (DOHAD)” or in common parlance the first 1000 days from conception to the second birthday
[1]. A mismatch between the intra- and extra-uterine environment is more likely to result in adverse outcomes, because programming has not prepared the neonate for different exposures. Birth-cohort and migration studies have highlighted this phenomenon in relation to changing susceptibility to cardio-vascular disease, metabolic syndrome and non-communicable immune mediated diseases
[2] (
Figure 1). Studies have shown that first-generation people migrating from countries with a low prevalence of allergic disease had a lower prevalence of allergy than second generation immigrants. The younger the child on arrival in a new high allergy prevalence country and the longer duration resident in that country directly correlates with changes in the subsequent incidence of allergic disease. Many factors interact to affect outcomes, but all relate to changes in life style and environmental exposures
[3].
Figure 1. The evolutionary hypothesis based on Crespi BJ Front. Endocrinol. 2020
[2]. The pre-conception and pregnancy maternal gene/environment interactions are the mechanisms by which the foetus is prepared for extra-uterine life. If there is a mismatch between the maternal environment in her earlier life and that of her new-born, programming will be inappropriate for the infants’ environment. This will lead to endocrine, metabolic and immune responses which are ill-equipped to handle environmental exposures with increased risks of cardio-vascular disease, metabolic syndrome and non-communicable inflammatory diseases. Arrows indicate the direction of effect.
2. Foetal Nutrition and Allergic Disease
The maternal diet will affect the mother’s gut microbiome and therefore the infant’s inoculum during delivery. However, the maternal microbiome and other confounders have hitherto not been comprehensively analyzed in relation to studies of the impact of pregnancy nutrition on allergy outcomes. This means that outcomes must be interpreted with caution.
Foetal growth and nutrition have an impact on the ontogeny of immune responses. There is an unexpected association between large head circumference at birth and levels of total IgE at birth, in childhood, and adulthood
[49][4]. It has been hypothesised that a large head circumference at birth is representative of an early rapid foetal growth trajectory because of good nutrient supply in early pregnancy. The foetus is subsequently programmed to maintain a rapid growth trajectory necessitating a high nutrient demand. If this is not met in the later stages of pregnancy there is continuing head growth at the expense of relatively poorer nutrient delivery to the body with consequent adverse effects on immune and lung development. Rapid immediate post-natal weight gain is often a consequence of late intra-uterine growth faltering and is associated with poorer infant lung function
[50][5]. The combination of compromised immune responses and lung function leads to a higher risk of infant wheezing and allergic asthma. High birth-weight has been associated with increased risks of subsequently positive allergy skin tests but not necessarily asthma
[51][6]. While allergy has been associated with high foetal abdominal girth growth velocity, infant wheeze was associated with low abdominal growth velocity
[52][7]. These observations are consistent with the concept that allergy is a consequence of affluence and good nutrition while airway disease follows gene/environment aberrations including the effects of impaired nutrition, which compromises airway development.
The key question is whether there are any specific nutrients of importance in promoting appropriate immune responsiveness. Reduced intake of fresh fruit and vegetables has been associated with a higher rate of allergic sensitisation in many studies
[53][8]. A high intake of fish in pregnancy has been associated with less subsequent allergy in the offspring
[54][9]. Conversely, a high maternal free sugar intake in pregnancy has been associated with a higher risk of allergy and asthma
[55][10]. A meta-analysis of studies investigating maternal pregnancy intake of vitamins and trace elements suggested a protective effect of vitamins D and E, and zinc against the development of wheezing illnesses in offspring, but inconclusive effects on the prevalence of asthma and other allergic conditions
[56][11]. This emphasizes the distinction between obstructive airway diseases resulting in wheeze and allergic asthma. Conflicting outcomes have been noted for pregnancy intake of numerous other trace elements, many associated with anti-oxidant activity, but heterogeneity of outcome pheno-typing precludes meaningful conclusions and association studies do not distinguish cause from consequence
[57][12]. Trials of supplementation in pregnancy are required to establish causal relationships.
Maternal obesity has been associated with higher risks of wheezing illnesses and asthma but not other allergic disorders in children. There are credible mechanistic explanations because mediators, known as adipokines, that are released by adipocytes are pro-inflammatory. They will have effects at the materno-foetal interface leading to foetal immune and neuro-endocrine dysregulation. Obesity also likely has epigenetic effects with enhanced pro-inflammatory gene expression
[58][13]. There are, however, confounding factors affecting outcomes which have not been accounted for in association studies. Overall dietary patterns may be different in obese mothers which could affect constituents implicated as influencing allergy outcomes as discussed above. Maternal obesity increases the risk of caesarean section delivery which in turn results in higher risks of infant asthma and food allergy, probably due to gut dysbiosis
[59,60][14][15]. A
res
tudyearch of elective caesarean section without medical indication showed an association with both infant asthma and allergic rhinitis. Breast feeding, which provides pre-biotic oligosaccharides, abrogated the effect, suggesting that gut dysbiosis was indeed the cause
[61][16]. Pregnancy antibiotics also modify the neonatal microbiome, and infants born to mothers who received antibiotics during pregnancy had increased prevalences of eczema and food allergy
[62][17].
Several studies have focused on lipids as being important in immune ontogeny. Indeed, fatty acids have a crucial role as a source of energy, as the principle component of cell membranes, and as precursors for the synthesis of prostaglandins and leukotrienes. Minor variations in levels could have a profound effect on immune responses. Fish oils have a high level of omega-3 polyunsaturated fatty acids (n-3 PUFAs) and Western diets have a diminished intake of n-3 PUFAs with corresponding increases in n-6 PUFAs. This change has been associated with increasing rates of allergic disease and asthma
[63][18]. Low cord blood n-3:n-6 ratios have been correlated with increased subsequent infant eczema and are related to higher maternal meat eating rather than fish eating
[64][19]. Several randomised controlled studies have employed the administration of a fish oil dietary supplement to mothers through pregnancy and lactation with monitoring of outcomes in the offspring, particularly in high risk cohorts, with conflicting outcomes. Even recent systematic reviews have produced differing results. One found no effect on eczema, wheeze, food allergy or allergic rhinitis, but moderate level evidence of a reduction in egg and peanut allergy in high risk cohorts
[65][20]. This re
vise
warch also showed that probiotic supplementation during the last month of pregnancy and lactation to six months may reduce eczema
[65][20]. However, another systematic review suggested that high dose fish oil supplementation reduced asthma
[66][21]. As a proof of concept that the pregnancy fish oil supplementation and microbiome manipulation has some effects, further studies would be worthwhile
Vitamin D receptors (VDR) have been identified on many immune active cells and vitamin D has important immuno-regulatory functions. Most notable are effects on T-regs through TGF-beta expression and signaling. Polymorphisms in the VDR have been linked to an increased risk of asthma
[67][22]. While some studies have associated low vitamin D levels with enhanced inflammation in patients with asthma, others have shown no or negative effects. A systematic review suggests that there is currently no evidence to support supplementation trials in pregnancy
[68][23]. The complexity of VDR function in relation to varying levels of vitamin D exposure and their effects on inflammatory processes requires considerably more elaboration before any clinical implications can be addressed. It is possible that there is a U-shaped curve of vitamin D levels and susceptibility to inflammation with both very high and low levels, increasing risks. Indeed, one study has shown this in relation to IgE levels
[69][24].
WThe researche
rs have yet to establish optimal levels for immunological health, which may be different from those for bone health.
Most studies of maternal dietary factors affecting allergic disease outcomes in off-spring have attempted to identify beneficial influences. There are a few recent studies suggesting adverse effects of some nutrients such as refined sugar and pro-inflammatory factors
[55,57][10][12]. A high intake of red meat increases levels of circulating advanced-glycation end products (AGEs), which are similar to molecules expressed on bacteria. They are recognized by a pattern recognition receptor known as (RAGE) which is particularly expressed in the airway epithelium and activates innate pro-inflammatory responses which are appropriate to handle infection, but a “false alarm” if related to dietary AGEs. Current child high dietary AGE intake has been associated with a high prevalence of asthma
[70,71][25][26]. However, effects of high AGE intake by pregnant mothers in the one published study to date did not affect child outcomes
[72][27]. A ten-year trans-generational cohort study utilised an early pregnancy dietary inflammatory index (predominantly a high fat intake) and healthy eating index (similar to a Mediterranean diet) to assess the effects on asthma development in children over a nine-year follow-up. A low quality pro-inflammatory diet increased asthma risk, while the health diet was protective
[71][26]. What is unclear is whether the impact on outcomes is exclusively due to either a healthy or unhealthy diet or a balance between each. However, the
res
tudyearch of total diet rather than individual nutrients may be more likely to show worthwhile effects.
Associations between omega-3 PUFAs, vitamins D, E and zinc at best show only weak beneficial effects, while intervention trials have produced conflicting results with variable outcomes, pheno-typing and confounding being critical issues. As is apparent from all pregnancy gene/environment interactions, the relationships are complex, being influenced by timing, dose, and combinations of exposures which are not normally analysed in observational or intervention studies. Some studies of the so-called Mediterranean diet (Med-Diet) with its high intake of fish, olive oil, fresh vegetables and fruits with low intake of chicken and red-meat (i.e., higher n-3:n-6 ratios) have suggested reductions in some but not all allergic pheno-types. A systematic review indicated that maternal adherence to the Med-Diet reduced offspring wheeze/asthma in the first year of life but not thereafter, and had minor effects on allergy but none on eczema
[73][28]. A more recent birth cohort observational study suggested that the Med-Diet in pregnancy improved offspring small-airway function but not the prevalence of any allergic disease
[74][29]. This would explain the reduction in infant wheeze suggested by the previous review
[66][21]. As the Med-Diet in pregnancy has been shown to be safe and beneficial to both mothers’ and infants’ health, further research into mechanisms and controlled trials are indicated
[75][30]. Principle component analysis of dietary diversity from the UK contingent of the “Prevalence of Infant Food Allergy (PIFA)” study revealed that infants having a more diverse intake of fresh fruit, vegetables and home prepared food had a significantly lower prevalence of food allergy by two years of age, as confirmed by controlled challenge
[76][31]. While the focus of this
res
tudyearch was on the diet of infants, it is very likely that the pattern of eating would have been similar for mothers during pregnancy. Therefore, the critical timing for the introduction of a diverse healthy diet remains to be established (
Table 1).
Table 1. Tabulation of the most promising interventions to prevent allergic disease based on published observational and interventional studies focusing on the first 1000 days. Published evidence referenced in brackets. However, before recommending any interventions it will not only be necessary to demonstrate worthwhile benefit but also to be mindful of potential adverse effects. For instance, paracetamol is the only mild to moderate analgesic recommended for use in pregnancy, while antibiotics for bacterial infection in pregnancy and caesarean section are sometimes essential for medical reasons. Based on all the published evidence, a “healthy diet” would appear to be the best and most practicable option, pre-conception, during pregnancy and lactation.
| Timing |
Target |
Intervention |
| Pre-conception |
Maternal obesity [58] | Maternal obesity [13] |
Weight loss
No maternal or grand-mother smoking [46] | Weight loss
No maternal or grand-mother smoking [32] |
| Pre-conception |
Maternal nutrition |
Healthy balanced diet [76] | Healthy balanced diet [31] |
| Pregnancy |
Maternal nutrition |
More fish less meat
Fresh fruit and vegetables [75]
Optimal vitamins D, E and zinc [67,68,69,70]
No allergen avoidance [29,30,31,32] | More fish less meat
Fresh fruit and vegetables [30]
Optimal vitamins D, E and zinc [22][23][24][25]
No allergen avoidance [33][34][35][36] |
| Pregnancy |
Medications to avoid if possible |
Antibiotics [62]
Paracetamol [43] | Antibiotics [17]
Paracetamol [37] |
| Pregnancy |
Maternal microbiome [6] | Maternal microbiome [38] |
Pre-/pro-/syn-biotics [6] | Pre-/pro-/syn-biotics [38] |
| Delivery |
Avoid if possible |
Caesarean section [59,60,61]
Bottle feeding [15] | Caesarean section [14][15][16]
Bottle feeding [39] |
| Neonatal period |
Infant microbiome [6] | Infant microbiome [38] |
Breast feeding [15]
Pre-/pro-/syn-biotics [6] | Breast feeding [39]
Pre-/pro-/syn-biotics [38] |
 Encyclopedia
Encyclopedia
