Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jason Zhu and Version 1 by Ankit Srivastava.
Various disease-associated forms or strains of α-synuclein (αSynD) can spread and accumulate in a prion-like fashion during synucleinopathies such as Parkinson’s disease (PD), Lewy body dementia (DLB), and multiple system atrophy (MSA). This capacity for self-propagation has enabled the development of seed amplification assays (SAAs) that can detect αSynD in clinical samples. Notably, α-synuclein real-time quaking-induced conversion (RT-QuIC) and protein misfolding cyclic amplification (PMCA) assays have evolved as ultrasensitive, specific, and relatively practical methods for detecting αSynD in a variety of biospecimens including brain tissue, CSF, skin, and olfactory mucosa from synucleinopathy patients.
- α-synuclein
- prion
- seed amplification assays
- Parkinson’s disease
- synucleinopathies
- Lewy body dementia
- RT-QuIC
- PMCA
- multiple system atrophy
1. Introduction
Multiple neurodegenerative diseases (NDDs) are associated with accumulation of pathological aggregates of the protein α synuclein (αSyn). In Parkinson’s disease (PD) and dementia with Lewy bodies (DLB), disease-associated forms of αSyn (αSynD) are major components of neuronal Lewy bodies (LB) and Lewy neurites, and in multiple system atrophy (MSA), αSynD accumulates in oligodendrocytes as glial cytoplasmic inclusions (GCIs) [1].
αSyn is normally a presynaptic neuronal protein that exists primarily as an intrinsically disordered monomer within the cytoplasm. However, in synucleinopathies, αSyn can be converted to β-sheet-rich, protease-resistant αSynD aggregates that grow by refolding and incorporating additional monomers [2,3,4][2][3][4]. Multiple studies have shown that αSynD can replicate and spread in a prion-like fashion within and between cells [5,6][5][6] and is considered the major culprit in the molecular pathology of synucleinopathies. The ‘dual-hit’ hypothesis postulates that an unknown trigger (e.g., an exogenous pathogen) is responsible for initiating the misfolding of native αSyn to yield assemblies that may then propagate via cellular communication mechanisms including passive diffusion, endocytosis, or exosomes [7]. Braak and colleagues described a pathological staging system in PD based on αSynD immunopositivity in the brain and other anatomical regions [8]. They proposed two plausible routes, i.e., nasal and gastric, for the spreading of αSynD and progression of PD.
This scenario may be analogous to the propagation mechanism(s) in prion diseases in which PrPSc (scrapie isoform of the prion protein) spreads between the tissues of infected hosts. Early evidence for prion-like spreading of αSyn aggregates was observed in the grafted dopaminergic neurons of patients who had undergone neuronal replacement therapy [9]. The presence of LB pathology within newly grafted neurons upon postmortem analysis supported the concept that αSynD can propagate between cells in the human brain. Also, cell-to-cell transfer of αSynD was observed in grafted neurons in a rat model [10].
The initiation and spread of LB pathology have also been observed using synthetic preformed αSyn fibrils (PFFs) in both cell and animal models [11,12][11][12]. Interestingly, PFFs convert into a GCI-like strain inside oligodendrocytes that maintains high seeding activity, even when propagated in neurons [13]. Furthermore, PFFs and other recombinant α-synuclein forms injected into the gastrointestinal tract of mice and rats induce LB-like aggregates in the brainstem via the vagus nerve [14,15,16][14][15][16]. Recently, Challis et al. observed that inoculating the duodenal wall of mice with PFFs led to changes in the enteric nervous system and gastrointestinal deficits [17]. Ultimately, inoculation of αSyn PFFs resulted in progression of αSyn histopathology to the midbrain in aged mice. Related, but not identical, pathological spreading has been seen in MSA, with αSynD having properties of infectious prions in cell cultures and animal models [18,19,20,21][18][19][20][21].
αSyn fibrils obtained from brain tissue or formed in vitro from recombinant protein [22,23,24][22][23][24] have been found to be structurally heterogenous (reviewed in [25])[25]. Such αSyn fibril conformational polymorphs appear to be analogous to different prion strains and may be determinants of phenotypic diversity in synucleinopathies. Together, these observations support the one disease, one strain hypothesis for phenotypically distinct synucleinopathies [23,26][23][26]. To aid in synucleinopathy research and diagnostics, ultrasensitive seed amplification assays (SAAs) [27], including αSyn RT-QuIC [28,29][28][29] and the similar αSyn PMCA [30], have been developed to detect αSynD seeds in brain tissue, CSF, and other biospecimens.
2. Diagnostic Potential of αSynD
The clinical diagnosis of synucleinopathies and other NDDs has long been complicated by variable and overlapping symptoms, especially early in the pathologic process. Assessments of parkinsonism in patients can be helpful, but not definitive, with phenotypic manifestations including bradykinesia, resting tremor, rigidity, and postural instability. Parkinsonian traits are largely associated with PD and DLB and are less apparent in MSA [31]. Interestingly, DLB patients with parkinsonian traits are reportedly less responsive to L-DOPA (Levodopa) or similar treatments as compared with PD patients [32]. Another major difference is that DLB pathology involves a cognitive decline that is not found in PD patients, at least in the early and middle phases of disease. Thus, clinical diagnosis often involves a ‘one-year rule’, i.e., if cognitive alterations appear concurrent with, or before, movement symptoms, then the diagnosis is DLB and not late-phase PD dementia (PDD) [33]. It is also difficult to clinically differentiate synucleinopathies from other NDDs such as AD, progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and Creutzfeldt-Jakob disease (CJD) [34,35,36,37,38][34][35][36][37][38]. Even combined analyses of imaging (MRI, PET, and EMG) and fluid biomarkers (CSF Aβ42, NfL, p-tau, t-tau, αSyn, and HVA) do not routinely improve diagnostic accuracy for parkinsonism [39]. Thus, given the inconsistency and variability associated with current imaging and fluid biomarkers, the identification of more definitive biomarkers may help navigate these diagnostic ambiguities. Increasing evidence indicates that the detection of αSynD using SAAs can improve the clinical diagnosis of synucleinopathies [28,29,40[28][29][40][41][42][43][44],41,42,43,44], even in prodromal phases [27,40,45][27][40][45].3. SAAs for Detecting Pathological Prion Aggregates
SAA platforms such as PMCA and RT-QuIC were initially developed to amplify, detect, and quantify pathological TSE (transmissible spongiform encephalopathy) prion aggregates in a variety of biospecimens. These assays were built on earlier observations that infectious prion protein aggregates, e.g., PrPSc, induce the conversion of a normal cellular prion protein (PrPC) into an abnormal protease-resistant form in cell-free reactions with striking species and strain specificities [46,47,48,49,50,51][46][47][48][49][50][51]. PMCA reactions showed that such seeded conformational conversion occurs continuously under suitable conditions and is accompanied by increases in prion infectivity [27,52][27][52]. The original PMCA reactions involved the cyclic sonication and incubation of infected samples with vast excesses of normal brain homogenates containing PrPC as the substrate for amplification [53]. PMCA products were then subjected to protease digestion and Western blotting to detect amplified conversion products. This protocol allowed the detection of prion aggregates with extraordinary sensitivity and selectivity [53,54][53][54]. However, limitations of this assay as a routine clinical test include the weeks-long reaction time for optimal sensitivity, the need for Western blotting, and the biohazardous infectivity of the amplified products. The development of the amyloid seeding assay [55] and prion RT-QuIC [56,57][56][57] improved the practicality of SAAs by providing formats based on multiwell plates; shaking instead of sonication; recombinant, rather than brain-derived, PrPC substrate; fluorescence (Thioflavin T (ThT)) instead of Western blot readout; much shorter assay times (e.g., 1–2 days); and noninfectious amplification products [58] (Figure 1A,B). Further development of prion RT-QuIC assays has improved their sensitivity and specificity and their applicability to most prion diseases (e.g., [59]); many specimen types including brain tissue [57[57][60][61],60,61], CSF [56[56][62][63][64][65],62,63,64,65], skin [66[66][67][68],67,68], eye [69], and olfactory mucosa [70,71,72][70][71][72]; and the cervid pregnancy microenvironment [73,74,75][73][74][75]. In some sample matrices, analytical sensitivities in the atto- or femtogram ranges have been documented [54,56,57,60,61,76,77,78,79][54][56][57][60][61][76][77][78][79].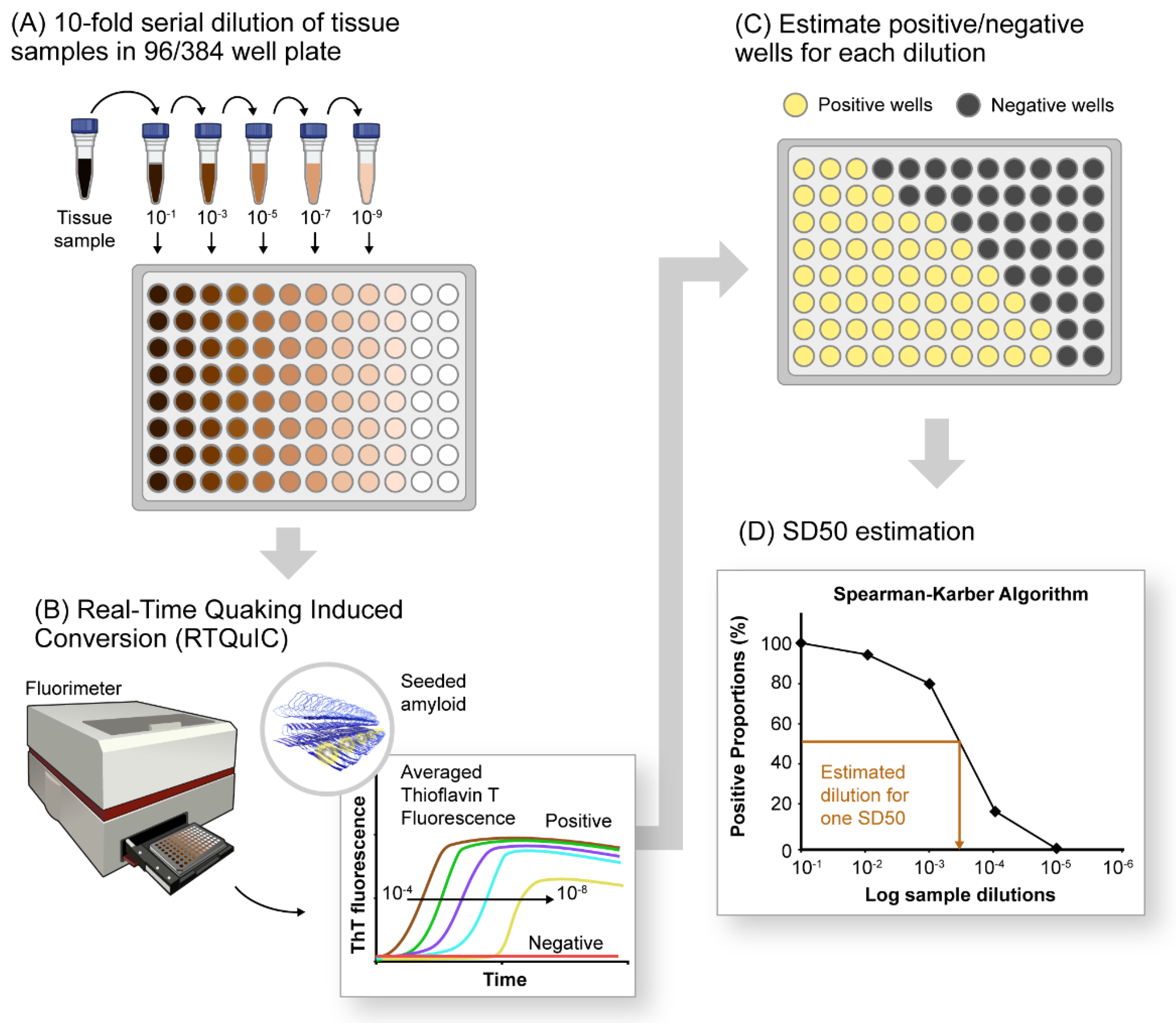
Figure 1. A schematic of αSyn RT-QuIC platform for detection and relative quantification of αSynD seeds in pathological tissues. (A) Serial dilutions (10−fold) of tissue sample (4 or 8 replicates) in a 96−well plate containing components of RT-QuIC reaction mixture including the recombinant, monomeric substrate protein and amyloid detection dye Thioflavin-T (ThT). (B) End-point estimation of serially diluted samples by the RT-QuIC assay. The resultant outcome is plotted as averaged ThT fluorescence versus time, showing declining fluorescence traces with increasing dilutions of positive samples. Negative samples do not exhibit any significant increase in ThT fluorescence under tested RT-QuIC conditions. (C) RT-QuIC outcomes for each dilution documented as number of positive and negative wells (for 4 or 8 replicates) per dilution tested. (D) Plot showing percentage of positive wells (shown as positive proportion percentages) for each sample dilution utilized for estimating seeding dose or sample dilutions in which 50% of the wells are ThT-positive (SD50).
