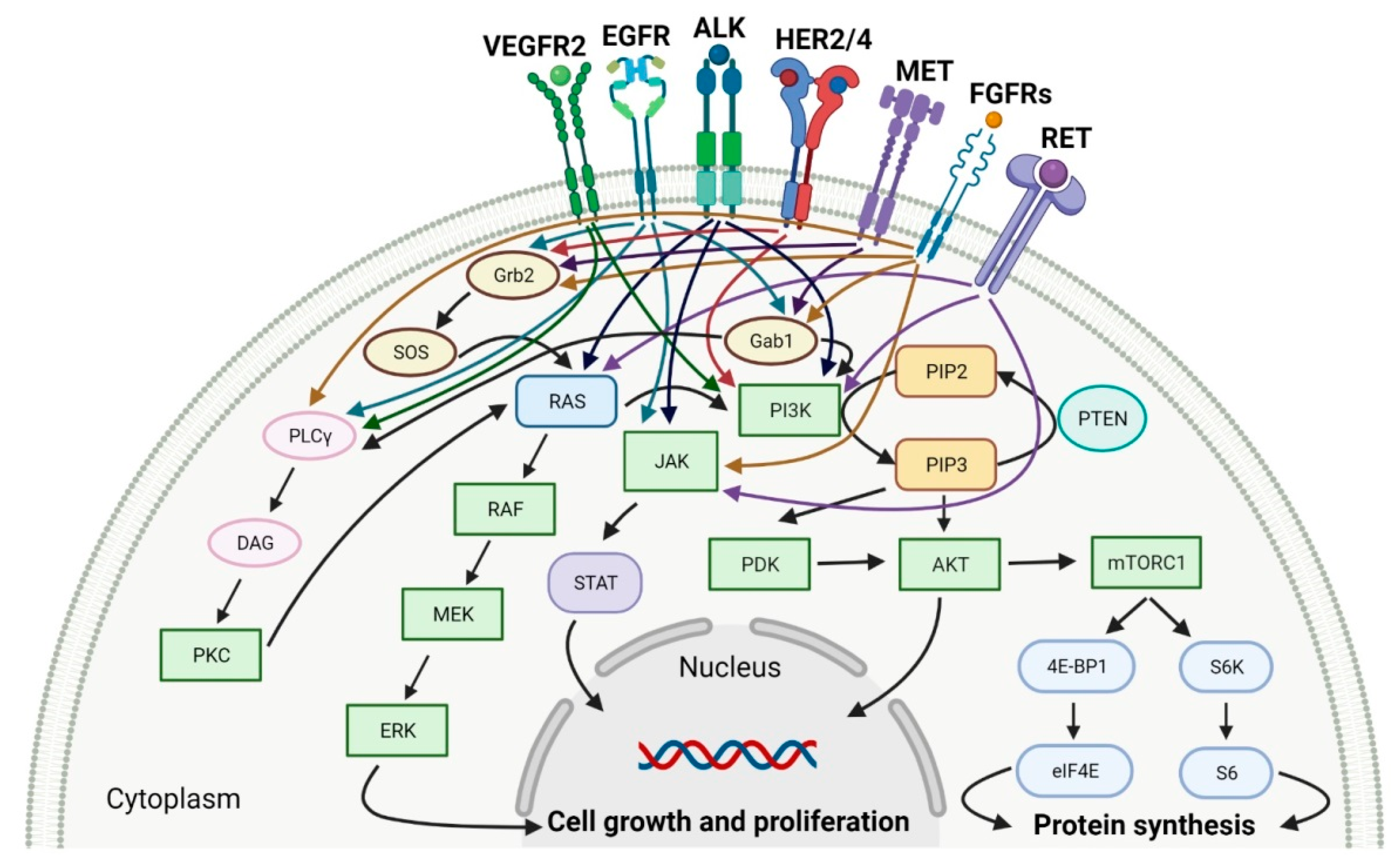
Protein kinases (PTKs) are enzymes that regulate the biological activity of proteins by phosphorylation of certain amino acid residues. This reaction causes a conformational change from an inactive to an active form of the protein, which is one of the most important regulatory mechanisms of the cell cycle and transduction of external signals. Dysregulation of protein kinases activity is implicated in the processes of carcinogenesis and the progression of various solid cancers. Therefore, protein kinases are prime targets for the development of selective anticancer drugs.
- Protein kinases
- Anticancer agents
- Small molecule inhibitors
1. Tyrosine Kinase (TK) Inhibitors
| No | Generic Name of Drug | Brand Name and Company |
First FDA/EMA Approval Date | Structure | Molecular Target | Route of Administration | Indication | Adverse Effects | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mobocertinib | EXKIVITY Takeda Pharmaceuticals America, Inc., Deerfield, IL, USA |
FDA: 15 September 2021 EMA: Not approved |
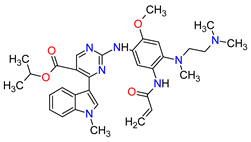 |  |
EGFR | 1 | Oral | Non-Small Cell Lung Cancer | Diarrhea, rash, stomatitis, vomiting, decreased appetite, nausea, paronychia, musculoskeletal pain, dry skin, fatigue, decreased hemoglobin, decreased lymphocytes, increased creatinine, amylase, and lipase, decreased potassium, and magnesium | [33] | ||||
| 2 | Infigratinib | TRUSELTIQ BridgeBio Pharma, Inc., Palo Alto, CA, USA | FDA: 28 May 2021 EMA: 21 August 2020 |
 |  |
FGFRs | 2 | Oral | Cholangiocarcinoma | Nail toxicity, stomatitis, dry eye, fatigue, increased creatinine, phosphate, alkaline phosphate, and alanine aminotransferase, decreased phosphate, and hemoglobin | [34][35] | ||||
| 3 | Tivozanib | FOTIVDA AVEO Oncology, Boston, MA, USA; Eusa Pharma (Netherlands) B.V., Schiphol-Rijk |
FDA: 10 March 2021 EMA: 24 August 2017 |
 |  |
VEGFRs | 3 | Oral | Renal Cell Carcinoma | Fatigue, hypertension, diarrhea, decreased appetite, nausea, dysphonia, hypothyroidism, cough, stomatitis, sodium decreased, lipase increased, and phosphate decreased | |||||
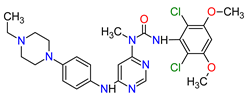
| 4 | Tepotinib | TEPMETKO EMD Serono, Inc., Darmstadt, Germany. | FDA: 3 February 2021 EMA: Not approved |
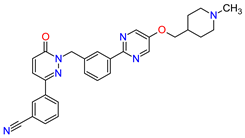 |  |
MET | 4 | Oral | Non-Small Cell Lung Cancer | Peripheral edema, diarrhea, fatigue, nausea, decreased appetite, increased blood creatinine levels, hypoalbuminemia, increased amylase levels | [38] | ||||
| 5 | Pralsetinib | GAVRETO Genentech, Inc., South San Francisco, CA, USA | FDA: 4 September 2020 EMA: 18 November 2021 |
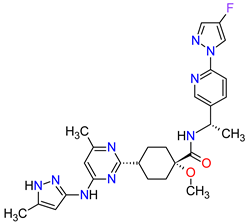 |  |
RET | 5 | Oral | Non-Small Cell Lung Cancer | Fatigue, constipation, musculoskeletal pain, hypertension | [39][40] | ||||
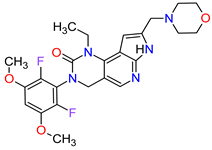
| 6 | Capmatinib | TABRECTA Novartis Pharmaceuticals Corporation, Basel, Switzerland | FDA: 6 May 2020 EMA: Not approved |
 |  |
MET | 4 | Oral | Non-Small Cell Lung Cancer | Peripheral edema, nausea, fatigue, vomiting, dyspnea, decreased appetite | [41] | ||||
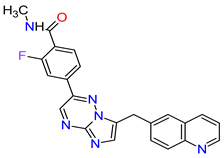
| 7 | Pemigatinib | PEMAZYRE Incyte Corporation, Wilmington, DE, USA | FDA: 17 April 2020 EMA: March 26, 2021 |
 |  |
FGFRs | 2 | Oral | Cholangiocarcinoma | Hyperphosphatasemia, alopecia, diarrhea, fatigue, dyspepsia | [42][43] | ||||
| 8 | Tucatinib | TUKYSA Seattle Genetics, Inc., Bothell, WA, USA | FDA: 17 April 2020 EMA: 11 February 2021 |
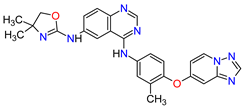 |  |
HER2 | 6 | Oral | Breast Cancer | Diarrhea, palmar–plantar erythrodysesthesia syndrome, decreased hemoglobin or phosphate, nausea | [44][45] | ||||
| 9 | Neratinib | NERLYNX Puma Biotechnology, Inc., Los Angeles, CA, USA |
FDA: 17 July 2017 EMA: 31 August 2018 |
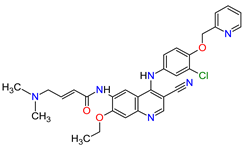 |  |
EGFR | 1 | , HER2 | 6 | , HER4 | 7 | Oral | Breast Cancer | Diarrhea | [46][47] |
| 10 | Osimertinib | TAGRISSO AstraZeneca, Cambridge, UK | FDA: 13 November 2015 EMA: 24 April 2017 |
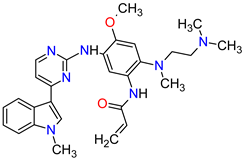 |  |
EGFR | 1 | Oral | Non-Small Cell Lung Cancer | Diarrhea, rash, dry skin, nail toxicity | [48][49] | ||||
| 11 | Ceritinib | ZYKADIA Novartis Pharmaceuticals Corporation, Basel, Switzerland | FDA: 29 April 2014 EMA: 6 May 2015 |
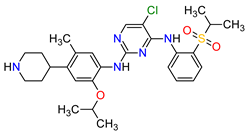 |  |
ALK | 8 | Oral | Non-Small Cell Lung Cancer | Diarrhea, nausea, vomiting, abdominal pain | [50][51] |
2. Cyclin-Dependent Kinase (CDK) Inhibitors
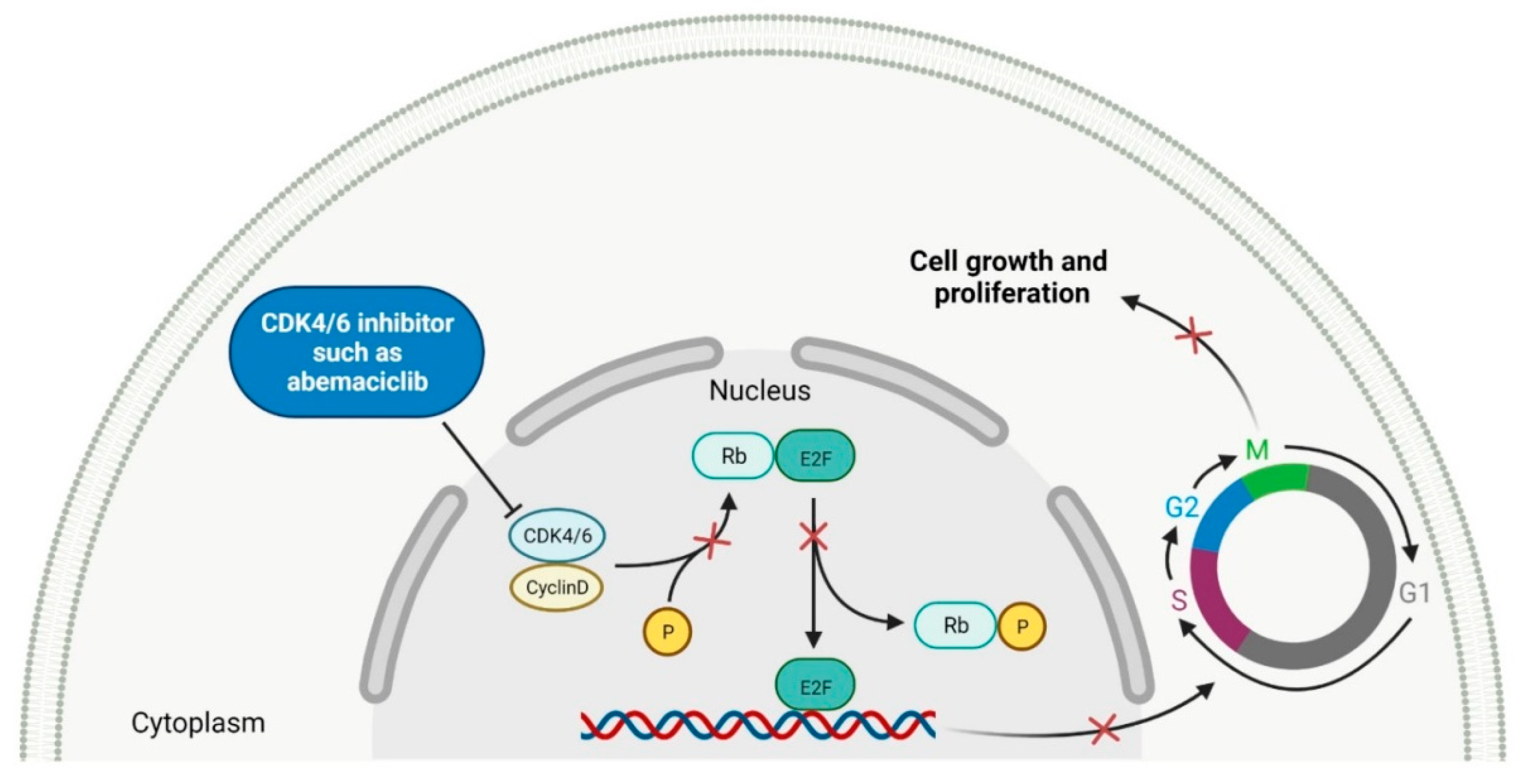
| No. | Generic Name of Drug | Brand Name and Company |
First FDA/EMA Approval Date | Structure | Molecular Target | Route of Administration | Indication | Adverse Effects | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Abemaciclib | VERZENIO Eli Lilly and Company, Indianapolis, IN, USA |
FDA: 28 September 2017 EMA: 27 September 2018 |
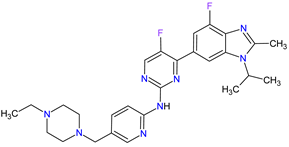 |  |
CDK4 | 1 | , CDK6 | 2 | Oral | Breast Cancer | Diarrhea, fatigue, nausea, decreased appetite, abdominal pain, neutropenia, vomiting, infections, anemia, headache, thrombocytopenia, leucopenia | [61][62] |
| 2 | Ribociclib | KISQALI Novartis Pharmaceuticals Corporation, Basel, Switzerland | FDA: 13 March 2017 EMA: 22 August 2017 |
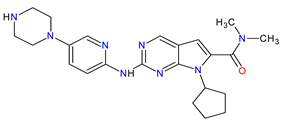 |  |
CDK4 | 1 | , CDK6 | 2 | Oral | Breast Cancer | Neutropenia, nausea, infections, fatigue, diarrhea | [63][64] |
| 3 | Palbociclib | IBRANCE Pfizer Inc., New York City, NY, USA |
FDA: 3 February 2015 EMA: 9 November 2016 |
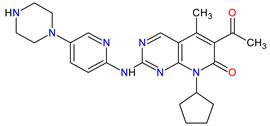 |  |
CDK4 | 1 | , CDK6 | 2 | Oral | Breast Cancer | Neutropenia, leukopenia, fatigue, anemia, nausea, arthralgia, alopecia, diarrhea, hot flush | [65][66] |
3. Multi-Kinase Inhibitors
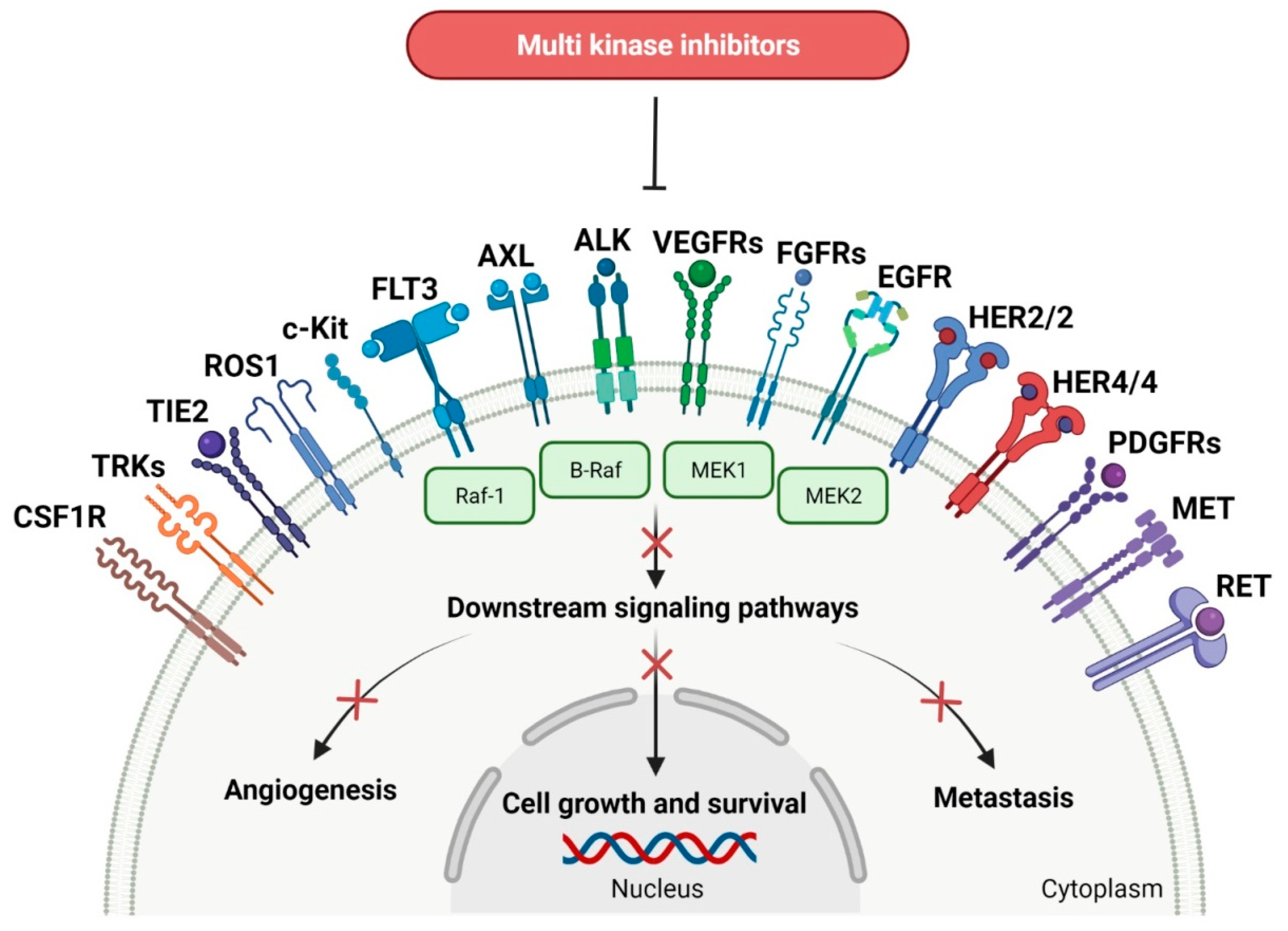
| No. | Generic Name of Drug | Brand Name and Company |
First FDA/EMA Approval Date | Structure | Molecular Target | Route of Administration | Indication | Adverse Effects | Ref. | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ripretinib | QINLOCK Deciphera Pharmaceuticals, Inc., Waltham, MA, USA | FDA: 15 May 2020 EMA: 18 November 2021 |
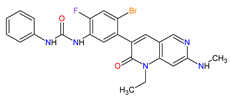 |  |
c-Kit | 1 | , PDGFRA | 2 | Oral | Gastrointestinal Stromal Tumor | Alopecia, fatigue, nausea, abdominal pain, constipation, myalgia, diarrhea, decreased appetite, palmar–plantar erythrodysesthesia syndrome, vomiting | [93][94] | ||||||||||||
| 2 | Selpercatinib | RETEVMO Eli Lilly and Company, Indianapolis, IN, USA |
FDA: 8 May 2020 EMA: 11 February 2021 |
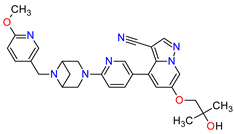 |  |
RET | 3 | Oral | Non-Small Cell Lung Cancer, Thyroid Cancer | Increased AST levels, increased glucose levels, decreased albumin levels, decreased leukocyte levels, decreased calcium levels, increased creatinine levels, dry mouth, diarrhea, increased alkaline phosphatase levels, hypertension, fatigue, decreased platelet levels, edema, increased total cholesterol levels, decreased sodium levels, rash, constipation, decreased magnesium levels, increased potassium levels, increased bilirubin levels, headache, decreased glucose levels, nausea, abdominal pain, cough, prolonged QT interval, dyspnea, vomiting, hemorrhage | [95][96] | ||||||||||||||
| 3 | Selumetinib | KOSELUGO AstraZeneca, Cambridge, UK | FDA: 13 April 2020 EMA: 17 June 2021 |
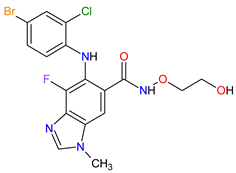 |  |
MEK1 | 4 | , MEK2 | 5 | Oral | Neurofibromatosis Type 1 | Vomiting, rash, abdominal pain, diarrhea, nausea, dry skin, musculoskeletal pain, fatigue, pyrexia, stomatitis, acneiform rash, headache, paronychia, pruritus, dermatitis, constipation, hair changes, epistaxis, hematuria, proteinuria, decreased appetite, decreased cardiac ejection fraction, edema, sinus tachycardia, skin infection | [97][98] | ||||||||||||
| 4 | Avapritinib | AYVAKIT Blueprint Medicines Corporation, Cambridge, MA, USA | FDA: 9 January 2020 EMA: 24 September 2020 |
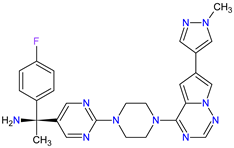 |  |
c-Kit | 1 | , PDGFRA | 2 | Oral | Gastrointestinal Stromal Tumor | Edema, nausea, fatigue/asthenia, cognitive impairment, vomiting, decreased appetite, diarrhea, increased lacrimation, abdominal pain | [99][100] | ||||||||||||
| 5 | Entrectinib | ROZLYTREK Genentech, Inc., South San Francisco, CA, USA | FDA: 15 August 2019 EMA: 31 July 2020 |
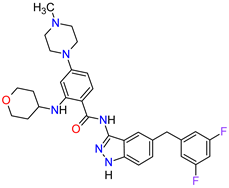 |  |
TRK | 6 | , ROS1 | 7 | , ALK | 8 | Oral | Solid Tumors, Non-Small Cell Lung Cancer | Dysgeusia, fatigue, dizziness, constipation, nausea, diarrhea, increased weight, paresthesia, increased blood creatinine, myalgia, peripheral edema, vomiting, anemia, arthralgia, increased aspartate aminotransferase (AST) | [101][102] | ||||||||||
| 6 | Pexidartinib | TURALIO Daiichi Sankyo, Tokyo, Japan |
FDA: 2 August 2019 EMA: Not approved |
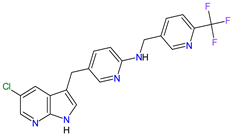 |  |
CSF1R | 9 | , c-Kit | 1 | , FLT3 | 10 | Oral | Tenosynovial Giant Cell Tumor | Hair color changes (depigmentation), fatigue, increased AST, increased alanine aminotransferase (ALT), dysgeusia, vomiting, periorbital edema, abdominal pain, decreased appetite, pruritus, hypertension, increased alkaline phosphatase | [103][104] | ||||||||||
| 7 | Erdafitinib | BALVERSA Janssen Pharmaceuticals, Inc., Raritan (HQ), NJ, USA | FDA: 12 April 2019 EMA: Not approved |
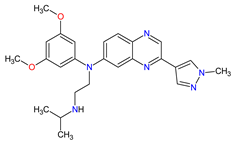 |  |
FGFRs | 11 | (1, 2, 3, 4) | Oral | Urothelial Carcinoma | Increased phosphate levels, stomatitis, fatigue, diarrhea, dry mouth, onycholysis, decreased appetite, dysgeusia, dry skin, dry eye, alopecia, palmar–plantar erythrodysaesthesia syndrome, constipation, abdominal pain, nausea, musculoskeletal pain | [72] | |||||||||||||
| 8 | Larotrectinib | VITRAKVI Loxo Oncology, Inc., Stamford, CT, USA |
FDA: 26 November 2018 EMA: 19 September 2019 |
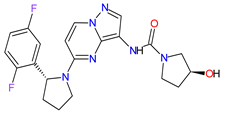 |  |
TRK | 6 | Oral | TRK Fusion Cancers | Fatigue, nausea, dizziness, vomiting, anemia, increased transaminase levels, cough, constipation, diarrhea | [105][106] | ||||||||||||||
| 9 | Lorlatinib | LORBRENA Pfizer Inc., New York City, NY, USA | FDA: 2 November 2018 EMA: 6 May 2019 |
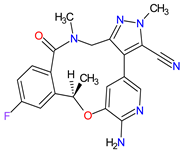 |  |
ALK | 8 | , ROS1 | 7 | Oral | Non-Small Cell Lung Cancer | Hypercholesterolemia, hypertriglyceridemia, edema, peripheral neuropathy | [107][108] | ||||||||||||
| 10 | Dacomitinib | VIZIMPRO Pfizer Inc., New York City, NY, USA |
FDA: 27 September 2018 EMA: 2 April 2019 |
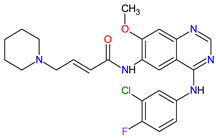 |  |
EGFR | 12 | , HER2 | 13 | , HER4 | 14 | Oral | Non-Small Cell Lung Cancer | Diarrhea, paronychia, dermatitis acneiform, stomatitis, decreased appetite | [109][110] | ||||||||||
| 11 | Encorafenib | BRAFTOVI Pfizer Inc., New York City, NY, USA |
FDA: 27 June 2018 EMA: 20 September 2018 |
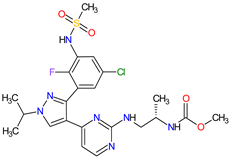 |  |
B-Raf | 15 | Oral | Melanoma Metastatic, Colorectal Cancer | Nausea, diarrhea, vomiting, fatigue, arthralgia | [111][112] | ||||||||||||||
| 12 | Binimetinib | MEKTOVI Array BioPharma Inc., Boulder, CO, USA |
FDA: 27 June 2018 EMA: 20 September 2018 |
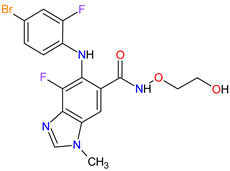 |  |
MEK1 | 4 | , MEK2 | 5 | Oral | Melanoma Metastatic | Nausea, diarrhea, vomiting, fatigue, arthralgia | [111][113] | ||||||||||||
| 13 | Brigatinib | ALUNBRIG Takeda Pharmaceuticals America, Inc., Deerfield, IL, USA |
FDA: 28 April 2017 EMA: 22 November 2018 |
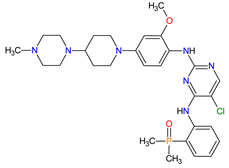 |  |
ALK | 8 | , EGFR | 12 | Oral | Non-Small Cell Lung Cancer | Nausea, diarrhea, fatigue, cough, headache, CPK elevation, pancreatic enzyme elevation, hyperglycemia | [114][115] | ||||||||||||
| 14 | Alectinib | ALECENSA Genentech, Inc., South San Francisco, CA, USA | FDA: 11 December 2015 EMA: 16 February 2017 |
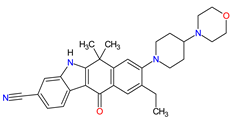 |  |
ALK | 8 | Oral | Non-Small Cell Lung Cancer | Constipation, nausea, diarrhea, vomiting, edema, increased levels of bilirubin, AST and ALT, myalgia, rash, anemia, increase in bodyweight | [116][117] | ||||||||||||||
| 15 | Cobimetinib | COTELLIC Genentech, Inc., South San Francisco, CA, USA | FDA: 10 November 2015: EMA: 20 November 2015. |
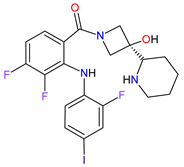 |  |
MEK1 | 4 | , MEK2 | 5 | Oral | Melanoma Metastatic | Diarrhea, nausea, rash, arthralgia, fatigue, increased creatine phosphokinase levels | [118][119] | ||||||||||||
| 16 | Lenvatinib | LENVIMA Eisai Inc., Tokyo, Japan, U.S. Corporate Headquarters in Nutley, NJ, USA |
FDA: 13 February 2015 EMA: 28 May 2015 |
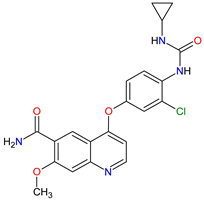 |  |
VEGFRs | 16 | (1, 2, 3), FGFR | 11 | (1, 2, 3, 4), PDGFRA | 2 | , RET | 3 | , c-Kit | 1 | Oral | Thyroid Cancer, Renal Cell Carcinoma, Hepatocellular Carcinoma, Endometrial Cancer | Hypertension, diarrhea, fatigue or asthenia, decreased appetite, bodyweight decreased, nausea, stomatitis, palmar–plantar erythrodysethaesia syndrome, proteinuria | [120][121] | ||||||
| 17 | Afatinib | GILOTRIF Boehringer Ingelheim Pharmaceuticals, Inc., Ingelheim, Germany | FDA: 12 July 2013 EMA: 25 September 2013 |
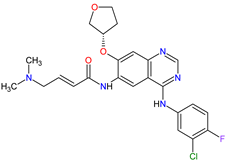 |  |
EGFR | 12 | , HER2 | 13 | , HER4 | 14 | Oral | Non-Small Cell Lung Cancer | Diarrhea, rash/acne, stomatitis/mucositis, paronychia, dry skin, decreased appetite, pruritus, nausea, fatigue, vomiting, epistaxis, cheilitis | [122][123] | ||||||||||
| 18 | Trametinib | MEKINIST GlaxoSmithKline, London, UK | FDA: 29 May 2013 EMA: 30 June 2014 |
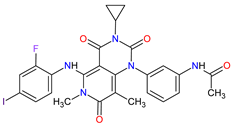 |  |
MEK1 | 4 | , MEK2 | 5 | Oral | Melanoma, Metastatic, Non-Small Cell Lung Cancer, Thyroid Cancer | Rash, diarrhea, fatigue, nausea/vomiting, peripheral edema | [124][125] | ||||||||||||
| 19 | Dabrafenib | TAFINLAR GlaxoSmithKline, London, UK | FDA: 29 May 2013 EMA: 26 August 2013 |
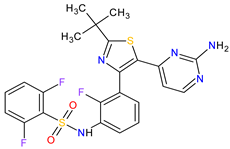 |  |
B-Raf | 15 | Oral | Melanoma, Metastatic, Non-Small Cell Lung Cancer, Thyroid Cancer | Alopecia, arthralgia, back pain, constipation, cough, erythrodysaesthesia, fever, headache, hyperkeratosis, muscle pain, nasopharyngitis, papilloma, squamous cell cancer | [126][127] | ||||||||||||||
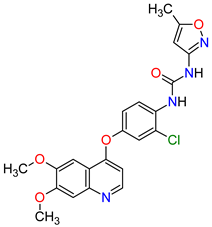
| 20 | Cabozantinib | CABOMETYX Exelixis, Inc., Alameda, CA, USA | FDA: 25 April 2016 EMA: 9 September 2016 |
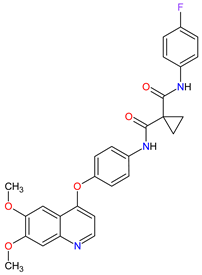 |  |
MET | 17 | , RET | 3 | , VEGFRs | 16 | (1, 2, 3), c-Kit | 1 | , FLT-3 | 10 | , TIE2 | 18 | , TRKB | 19 | , AXL | 20 | Oral | Renal Cell Carcinoma, Hepatocellular Carcinoma | Diarrhea, fatigue, nausea, vomiting, decreased appetite, hypertension, palmar–plantar erythrodysesthesia syndrome | [128][129][130] |
| 21 | Cabozantinib | COMETRIQ Exelixis, Inc., Alameda, CA, USA |
FDA: 29 November 2012 EMA: 21 March 2014 |
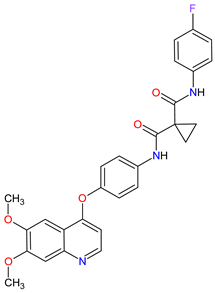 |  |
MET | 17 | , RET | 3 | , VEGFRs | 16 | (1, 2, 3), c-Kit | 1 | , FLT-3 | 10 | , TIE2 | 18 | , TRKB | 19 | , AXL | 20 | Oral | Thyroid Cancer | Diarrhea, stomatitis, palmar–plantar erythrodysesthesia syndrome, decreased weight, decreased appetite, nausea, fatigue, oral pain, hair color changes, dysgeusia, hypertension, abdominal pain, constipation, increased AST, increased ALT, lymphopenia, increased alkaline phosphatase, hypocalcemia, neutropenia, thrombocytopenia, hypophosphatemia, and hyperbilirubinemia | [131][132][133] |
| 22 | Regorafenib | STIVARGA Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ, USA |
FDA: 27 September 2012 EMA: 26 August 2013 |
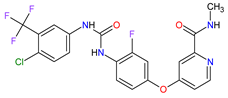 |  |
VEGFRs | 16 | (1, 2, 3), RET | 3 | , c-Kit | 1 | , PDGFRs | 21 | (A, B), FGFRs | 11 | (1, 2), TIE2 | 18 | , B-Raf | 15 | , RAF-1 | 22 | Oral | Colorectal Cancer, Gastrointestinal Stromal Tumor, Hepatocellular Carcinoma | Asthenia/fatigue, decreased appetite and food intake, hand-foot skin reaction, palmar–plantar erythrodysesthesia, diarrhea, mucositis, weight loss, infection, hypertension, dysphonia | [134][135][136] |
| 23 | Axitinib | INLYTA Pfizer Inc., New York City, NY, USA |
FDA: 27 January 2012 EMA: 3 September 2012 |
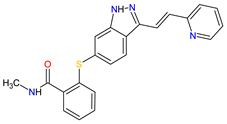 |  |
VEGFRs | 16 | (1, 2, 3), c-Kit | 1 | , PDGFRs | 21 | (A, B) | Oral | Renal Cell Carcinoma | Diarrhea, hypertension, fatigue, decreased appetite, nausea, dysphonia, palmar–plantar erythrodysesthesia (hand-foot) syndrome, weight decreased, vomiting, asthenia, constipation | [137][138][139] | |||||||||
| 24 | Crizotinib | XALKORI Pfizer Inc., New York City, NY, USA |
FDA: 26 August 2011 EMA: 23 October 2012 |
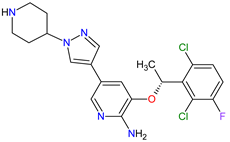 |  |
ALK | 8 | , MET | 17 | , ROS1 | 7 | Oral | Non-Small Cell Lung Cancer | Vision disorders, nausea, diarrhea, vomiting, edema, constipation, elevated transaminases, fatigue, decreased appetite, upper respiratory infection, dizziness, neuropathy | [140][141][142] | ||||||||||
| 25 | Vemurafenib | ZELBORAF Genentech, Inc., South San Francisco, CA, USA | FDA: 17 August 2011 EMA: 17 February 2012 |
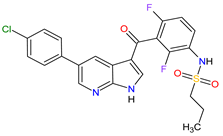 |  |
B-Raf | 15 | Oral | Melanoma Metastatic | Arthralgia, rash, alopecia, fatigue, photosensitivity reaction, nausea, | [143][144][145] | ||||||||||||||
| 26 | Vandetanib | CAPRELSA AstraZeneca, Cambridge, UK | FDA: 6 April 2011 EMA: 17 February 2012 |
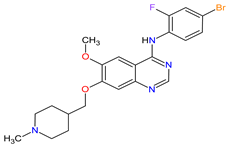 |  |
VEGFR-2 | 23 | , EGFR | 12 | , RET | 3 | Oral | Thyroid Cancer | Diarrhea, rash, nausea, hypertension, fatigue, headache, decreased appetite, acne, dermatitis acneiform, dry skin, photosensitivity reaction, erythema | [146][147] |
1
c-Kit: mast/stem cell growth factor receptor.
2
PDGFRA: platelet-derived growth factor receptor α.
3
RET: receptor tyrosine kinase rearranged during transfection.
4
MEK1: mitogen-activated protein kinase kinase 1.
5
MEK2: mitogen-activated protein kinase kinase 2.
6
TRK: tropomyosin receptor tyrosine kinase.
7
ROS1: proto-oncogene tyrosine-protein kinase ROS.
8
ALK: anaplastic lymphoma kinase.
9
CSF1R: colony-stimulating factor 1 receptor.
10
FLT3: FMS-like tyrosine kinase-3.
11
FGFRs: fibroblast growth factor receptors.
12EGFR: epidermal growth factor receptor.
13
HER2: human epidermal growth factor receptor 2.
14
HER4: human epidermal growth factor receptor 4.
15
B-Raf: serine/threonine-protein kinase B-Raf.
16
VEGFRs: vascular endothelial growth factor receptors.
17
MET: mesenchymal-epithelial transition factor.
18
TIE2: tunica interna endothelial cell kinase 2.
19
TRKB: tropomyosin receptor kinase B.
20
AXL: AXL receptor tyrosine kinase.
21
PDGFRs: platelet-derived growth factor receptors.
22
RAF-1: RAF proto-oncogene serine/threonine-protein kinase.
23
VEGFR-2: vascular endothelial growth factor receptor-2.
References
- Manash Paul; Anup K. Mukhopadhyay; Tyrosine kinase – Role and significance in Cancer. International Journal of Medical Sciences 2004, 1, 101-115, 10.7150/ijms.1.101.
- Srinivasan Madhusudan; Trivadi S. Ganesan; Tyrosine kinase inhibitors in cancer therapy. Clinical Biochemistry 2004, 37, 618-635, 10.1016/j.clinbiochem.2004.05.006.
- Monique Nilsson; John V. Heymach; Vascular Endothelial Growth Factor (VEGF) Pathway. Journal of Thoracic Oncology 2006, 1, 768-770, 10.1016/s1556-0864(15)30404-4.
- Yixiao Feng; Mia Spezia; Shifeng Huang; Chengfu Yuan; Zongyue Zeng; Linghuan Zhang; Xiaojuan Ji; Wei Liu; Bo Huang; Wenping Luo; et al.Bo LiuYan LeiScott DuAkhila VuppalapatiHue H. LuuRex C. HaydonTong-Chuan HeGuosheng Ren Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes & Diseases 2018, 5, 77-106, 10.1016/j.gendis.2018.05.001.
- Lihua Huang; Liwu Fu; Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharmaceutica Sinica B 2015, 5, 390-401, 10.1016/j.apsb.2015.07.001.
- N. A. Seebacher; A. E. Stacy; G. M. Porter; A. M. Merlot; Clinical development of targeted and immune based anti-cancer therapies. Journal of Experimental & Clinical Cancer Research 2019, 38, 1-39, 10.1186/s13046-019-1094-2.
- Laura Pacini; Andrew Jenks; Nadia Lima; Paul Huang; Targeting the Fibroblast Growth Factor Receptor (FGFR) Family in Lung Cancer. Cells 2021, 10, 1154, 10.3390/cells10051154.
- Alberto Puccini; Nagore I. Marín-Ramos; Francesca Bergamo; Marta Schirripa; Sara Lonardi; Heinz-Josef Lenz; Fotios Loupakis; Francesca Battaglin; Safety and Tolerability of c-MET Inhibitors in Cancer. Drug Safety 2019, 42, 211-233, 10.1007/s40264-018-0780-x.
- Priscilla Cascetta; Vincenzo Sforza; Anna Manzo; Guido Carillio; Giuliano Palumbo; Giovanna Esposito; Agnese Montanino; Raffaele Costanzo; Claudia Sandomenico; Rossella De Cecio; et al.Maria Carmela PiccirilloCarmine La MannaGiuseppe TotaroPaolo MutoCarmine PiconeRoberto BiancoNicola NormannoAlessandro Morabito RET Inhibitors in Non-Small-Cell Lung Cancer. Cancers 2021, 13, 4415, 10.3390/cancers13174415.
- Julia Rotow; Trever G. Bivona; Understanding and targeting resistance mechanisms in NSCLC. Nature Reviews Cancer 2017, 17, 637-658, 10.1038/nrc.2017.84.
- Luc Friboulet; Nanxin Li; Ryohei Katayama; Christian C. Lee; Justin F. Gainor; Adam Crystal; Pierre-Yves Michellys; Mark M. Awad; Noriko Yanagitani; Sungjoon Kim; et al.Annemarie C. PferdekamperJie LiShailaja KasibhatlaFrank SunXiuying SunSu HuaPeter McNamaraSidra MahmoodElizabeth L. LockermanNaoya FujitaMakoto NishioJennifer L. HarrisAlice T. ShawJeffrey A. Engelman The ALK Inhibitor Ceritinib Overcomes Crizotinib Resistance in Non–Small Cell Lung Cancer. Cancer Discovery 2014, 4, 662-673, 10.1158/2159-8290.cd-13-0846.
- ZYKADIA (ceritinib). Prescribing information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- Elena Ardini; Maria Menichincheri; Patrizia Banfi; Roberta Bosotti; Cristina De Ponti; Romana Pulci; Dario Ballinari; Marina Ciomei; Gemma Texido; Anna Degrassi; et al.Nilla AvanziNadia AmboldiMaria Beatrice SaccardoDaniele CaseroPaolo OrsiniTiziano BandieraLuca MologniDavid AndersonGe WeiJason HarrisJean-Michel VernierGang LiEduard FelderDaniele DonatiAntonella IsacchiEnrico PesentiPaola MagnaghiArturo Galvani Entrectinib, a Pan–TRK, ROS1, and ALK Inhibitor with Activity in Multiple Molecularly Defined Cancer Indications. Molecular Cancer Therapeutics 2016, 15, 628-639, 10.1158/1535-7163.mct-15-0758.
- Suresh S. Ramalingam; Johan Vansteenkiste; David Planchard; Byoung Chul Cho; Jhanelle E. Gray; Yuichiro Ohe; Caicun Zhou; Thanyanan Reungwetwattana; Ying Cheng; Busyamas Chewaskulyong; et al.Riyaz ShahManuel CoboKi Hyeong LeeParneet CheemaMarcello TiseoThomas JohnMeng-Chih LinFumio ImamuraTakayasu KurataAlexander ToddRachel HodgeMatilde SaggeseYuri RukazenkovJean-Charles Soria Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. New England Journal of Medicine 2020, 382, 41-50, 10.1056/nejmoa1913662.
- Wenjun Zhou; Dalia Ercan; Liang Chen; Cai-Hong Yun; Danan Li; Marzia Capelletti; Alexis Cortot; Lucian Chirieac; Roxana E. Iacob; Robert Padera; et al.John EngenKwok Kin WongMichael J. EckNathanael S. GrayPasi A. Jänne Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature 2009, 462, 1070-1074, 10.1038/nature08622.
- Francois Gonzalvez; Sylvie Vincent; Theresa E. Baker; Alexandra E. Gould; Shuai Li; Scott D. Wardwell; Sara Nadworny; Yaoyu Ning; Sen Zhang; Wei-Sheng Huang; et al.Yongbo HuFeng LiMatthew T. GreenfieldStephan G. ZechBiplab DasNarayana I. NarasimhanTim ClacksonDavid DalgarnoWilliam C. ShakespeareMichael FitzgeraldJohara ChouitarRobert J. GriffinShengwu LiuKwok-Kin WongXiaotian ZhuVictor M. Rivera Mobocertinib (TAK-788): A Targeted Inhibitor of EGFR Exon 20 Insertion Mutants in Non–Small Cell Lung Cancer. Cancer Discovery 2021, 11, 1672-1687, 10.1158/2159-8290.cd-20-1683.
- Johan Filip Vansteenkiste; Charlotte Van De Kerkhove; Els Wauters; Pierre Van Mol; Capmatinib for the treatment of non-small cell lung cancer. Expert Review of Anticancer Therapy 2019, 19, 659-671, 10.1080/14737140.2019.1643239.
- Paul K. Paik; Enriqueta Felip; Remi Veillon; Hiroshi Sakai; Alexis B. Cortot; Marina C. Garassino; Julien Mazieres; Santiago Viteri; Helene Senellart; Jan Van Meerbeeck; et al.Jo RaskinNiels ReinmuthPierfranco ConteDariusz KowalskiByoung Chul ChoJyoti D. PatelLeora HornFrank GriesingerJi-Youn HanYoung-Chul KimGee-Chen ChangChen-Liang TsaiJames C.-H. YangYuh-Min ChenEgbert F. SmitAnthonie J. van der WekkenTerufumi KatoDilafruz JuraevaChristopher StrohRolf BrunsJosef StraubAndreas JohneJürgen ScheeleJohn V. HeymachXiuning Le Tepotinib in Non–Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. New England Journal of Medicine 2020, 383, 931-943, 10.1056/nejmoa2004407.
- NERLYNX (neratinib). Prescribing Information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- TUKYSA (tucatinib). Prescribing Information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- Cristina Saura; Mafalda Oliveira; Yin-Hsun Feng; Ming-Shen Dai; Shang-Wen Chen; Sara A. Hurvitz; Sung-Bae Kim; Beverly Moy; Suzette Delaloge; William Gradishar; et al.Norikazu MasudaMarketa PalacovaMaureen E. TrudeauJohanna MattsonYoon Sim YapMing-Feng HouMichelino De LaurentiisYu-Min YehHong-Tai ChangThomas YauHans WildiersBarbara HaleyDaniele FagnaniYen-Shen LuJohn CrownJohnson LinMasato TakahashiToshimi TakanoMiki YamaguchiTakaaki FujiiBin YaoJudith BebchukKiana KeyvanjahRichard BryceAdam Brufskyfor the NALA Investigators Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. Journal of Clinical Oncology 2020, 38, 3138-3149, 10.1200/jco.20.00147.
- Rashmi K. Murthy; Sherene Loi; Alicia Okines; Elisavet Paplomata; Erika Hamilton; Sara A. Hurvitz; Nancy U. Lin; Virginia Borges; Vandana Abramson; Carey Anders; et al.Philippe L. BedardMafalda OliveiraErik JakobsenThomas BachelotShlomit S. ShacharVolkmar MüllerSofia BragaFrancois P. DuhouxRichard GreilDavid CameronLisa A. CareyGiuseppe CuriglianoKaren GelmonGabriel HortobagyiIan KropSibylle LoiblMark PegramDennis SlamonM. Corinna Palanca-WesselsLuke WalkerWentao FengEric P. Winer Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. New England Journal of Medicine 2020, 382, 597-609, 10.1056/nejmoa1914609.
- Anita Kulukian; Patrice Lee; Janelle Taylor; Robert Rosler; Peter De Vries; Daniel Watson; Andres Forero-Torres; Scott Peterson; Preclinical Activity of HER2-Selective Tyrosine Kinase Inhibitor Tucatinib as a Single Agent or in Combination with Trastuzumab or Docetaxel in Solid Tumor Models. Molecular Cancer Therapeutics 2020, 19, 976-987, 10.1158/1535-7163.mct-19-0873.
- Sridhar K. Rabindran; Carolyn M. Discafani; Edward Rosfjord; Michelle Baxter; M. Brawner Floyd; Jonathan Golas; William A. Hallett; Bernard D. Johnson; Ramaswamy Nilakantan; Elsebe Overbeek; et al.Marvin F. ReichRu ShenXiaoqing ShiHwei-Ru TsouYu-Fen WangAllan Wissner Antitumor Activity of HKI-272, an Orally Active, Irreversible Inhibitor of the HER-2 Tyrosine Kinase. Cancer Research 2004, 64, 3958-3965, 10.1158/0008-5472.can-03-2868.
- Ghassan K Abou-Alfa; Vaibhav Sahai; Antoine Hollebecque; Gina Vaccaro; Davide Melisi; Raed Al-Rajabi; Andrew S Paulson; Mitesh J Borad; David Gallinson; Adrian G Murphy; et al.Do-Youn OhEfrat DotanDaniel V CatenacciEric Van CutsemTao JiChristine F LihouHuiling ZhenLuis FélizArndt Vogel Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. The Lancet Oncology 2020, 21, 671-684, 10.1016/s1470-2045(20)30109-1.
- Milind Javle; Sameek Roychowdhury; Robin Kate Kelley; Saeed Sadeghi; Teresa Macarulla; Karl Heinz Weiss; Dirk-Thomas Waldschmidt; Lipika Goyal; Ivan Borbath; Anthony El-Khoueiry; et al.Mitesh J BoradWei Peng YongPhilip A PhilipMichael BitzerSurbpong TanasanvimonAi LiAmit PandeHarris S SoiferStacie Peacock ShepherdSusan MoranAndrew X ZhuTanios S Bekaii-SaabGhassan K Abou-Alfa Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. The Lancet Gastroenterology & Hepatology 2021, 6, 803-815, 10.1016/s2468-1253(21)00196-5.
- PEMAZYRE (pemigatinib). Prescribing information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- TRUSELTIQ (infigratinib). Prescribing information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- Saadia A Aziz; Joshua Sznol; Adebowale Adeniran; John W Colberg; Robert L Camp; Harriet M Kluger; Vascularity of primary and metastatic renal cell carcinoma specimens. Journal of Translational Medicine 2013, 11, 15-15, 10.1186/1479-5876-11-15.
- Muhammad Omer Jamil; Amanda Hathaway; AmitKumar Mehta; Tivozanib: Status of Development. Current Oncology Reports 2015, 17, 1-7, 10.1007/s11912-015-0451-3.
- Brian I Rini; Sumanta K Pal; Bernard J Escudier; Michael B Atkins; Thomas Hutson; Camillo Porta; Elena Verzoni; Michael N Needle; David F McDermott; Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. The Lancet Oncology 2019, 21, 95-104, 10.1016/s1470-2045(19)30735-1.
- FOTIVDA (tivozanib). Prescribing information. . Aveo Oncology. Retrieved 2022-4-21
- Anthony Markham; Mobocertinib: First Approval. Drugs 2021, 81, 2069-2074, 10.1007/s40265-021-01632-9.
- Connie Kang; Infigratinib: First Approval. Drugs 2021, 81, 1355-1360, 10.1007/s40265-021-01567-1.
- Infigratinib for the treatment of cholangiocarcinoma. . European Medicines Agency.. Retrieved 2022-4-21
- Esther S. Kim; Tivozanib: First Global Approval. Drugs 2017, 77, 1917-1923, 10.1007/s40265-017-0825-y.
- Fotivda: EPAR - Product Information. . European Medicines Agency. Retrieved 2022-4-21
- Anthony Markham; Tepotinib: First Approval. Drugs 2020, 80, 829-833, 10.1007/s40265-020-01317-9.
- Anthony Markham; Pralsetinib: First Approval. Drugs 2020, 80, 1865-1870, 10.1007/s40265-020-01427-4.
- Gavreto: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Sohita Dhillon; Capmatinib: First Approval. Drugs 2020, 80, 1125-1131, 10.1007/s40265-020-01347-3.
- Sheridan M. Hoy; Pemigatinib: First Approval. Drugs 2020, 80, 923-929, 10.1007/s40265-020-01330-y.
- Pemazyre: EPAR - Product Information. . European Medicines Agency. Retrieved 2022-4-21
- Arnold Lee; Tucatinib: First Approval. Drugs 2020, 80, 1033-1038, 10.1007/s40265-020-01340-w.
- Tukysa: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Emma D. Deeks; Neratinib: First Global Approval. Drugs 2017, 77, 1695-1704, 10.1007/s40265-017-0811-4.
- Nerlynx: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Sarah L. Greig; Osimertinib: First Global Approval. Drugs 2016, 76, 263-273, 10.1007/s40265-015-0533-4.
- Tagrisso: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Sohita Dhillon; Madeleine Clark; Ceritinib: First Global Approval. Drugs 2014, 74, 1285-1291, 10.1007/s40265-014-0251-3.
- Zykadia: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Robert Roskoski; Cyclin-dependent protein serine/threonine kinase inhibitors as anticancer drugs. Pharmacological Research 2018, 139, 471-488, 10.1016/j.phrs.2018.11.035.
- Concepción Sánchez-Martínez; María José Lallena; Sonia Gutiérrez Sanfeliciano; Alfonso de Dios; Cyclin dependent kinase (CDK) inhibitors as anticancer drugs: Recent advances (2015–2019). Bioorganic & Medicinal Chemistry Letters 2019, 29, 126637, 10.1016/j.bmcl.2019.126637.
- Francesco Schettini; Irene De Santo; Carmen G. Rea; Pietro De Placido; Luigi Formisano; Mario Giuliano; Grazia Arpino; Michelino De Laurentiis; Fabio Puglisi; Sabino De Placido; et al.Lucia Del Mastro CDK 4/6 Inhibitors as Single Agent in Advanced Solid Tumors. Frontiers in Oncology 2018, 8, 608, 10.3389/fonc.2018.00608.
- Julia A. Beaver; Laleh Amiri-Kordestani; Rosane Charlab; Wei Chen; Todd Palmby; Amy Tilley; Jeanne Fourie Zirkelbach; Jingyu Yu; Qi Liu; Liang Zhao; et al.Joyce CrichXiao Hong ChenMinerva HughesErik BloomquistShenghui TangRajeshwari SridharaPaul G. KluetzGeoffrey KimAmna IbrahimRichard PazdurPatricia Cortazar FDA Approval: Palbociclib for the Treatment of Postmenopausal Patients with Estrogen Receptor–Positive, HER2-Negative Metastatic Breast Cancer. Clinical Cancer Research 2015, 21, 4760-4766, 10.1158/1078-0432.ccr-15-1185.
- Amanda J. Walker; Suparna Wedam; Laleh Amiri-Kordestani; Erik Bloomquist; Shenghui Tang; Rajeshwari Sridhara; Wei Chen; Todd R. Palmby; Jeanne Fourie Zirkelbach; Wentao Fu; et al.Qi LiuAmy TilleyGeoffrey KimPaul G. KluetzAmy E. McKeeRichard Pazdur FDA Approval of Palbociclib in Combination with Fulvestrant for the Treatment of Hormone Receptor–Positive, HER2-Negative Metastatic Breast Cancer. Clinical Cancer Research 2016, 22, 4968-4972, 10.1158/1078-0432.ccr-16-0493.
- Joyce O’Shaughnessy; Katarina Petrakova; Gabe S. Sonke; Pierfranco Conte; Carlos L. Arteaga; David A. Cameron; Lowell L. Hart; Cristian Villanueva; Erik Jakobsen; Joseph T. Beck; et al.Deborah LindquistFarida SouamiShoubhik MondalCaroline GermaGabriel N. Hortobagyi Ribociclib plus letrozole versus letrozole alone in patients with de novo HR+, HER2− advanced breast cancer in the randomized MONALEESA-2 trial. Breast Cancer Research and Treatment 2017, 168, 127-134, 10.1007/s10549-017-4518-8.
- Amita Patnaik; Lee S. Rosen; Sara M. Tolaney; Anthony W. Tolcher; Jonathan W. Goldman; Leena Gandhi; Kyriakos P. Papadopoulos; Muralidhar Beeram; Drew W. Rasco; John F. Hilton; et al.Aejaz NasirRichard P. BeckmannAndrew E. SchadeAngie D. FulfordTuan S. NguyenRicardo MartinezPalaniappan KulanthaivelBeeram MuralidharMartin FrenzelDamien M. CronierEdward M. ChanKeith T. FlahertyPatrick Y. WenGeoffrey I. Shapiro Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients with Breast Cancer, Non–Small Cell Lung Cancer, and Other Solid Tumors. Cancer Discovery 2016, 6, 740-753, 10.1158/2159-8290.cd-16-0095.
- Thomas J. Raub; Graham N. Wishart; Palaniappan Kulanthaivel; Brian A. Staton; Rose T. Ajamie; Geri A. Sawada; Lawrence M. Gelbert; Harlan E. Shannon; Concepcion Sanchez-Martinez; Alfonso De Dios; et al. Brain Exposure of Two Selective Dual CDK4 and CDK6 Inhibitors and the Antitumor Activity of CDK4 and CDK6 Inhibition in Combination with Temozolomide in an Intracranial Glioblastoma Xenograft. Drug Metabolism and Disposition 2015, 43, 1360-1371, 10.1124/dmd.114.062745.
- Sara M. Tolaney; Solmaz Sahebjam; Emilie Le Rhun; Thomas Bachelot; Peter Kabos; Ahmad Awada; Denise Yardley; Arlene Chan; Pierfranco Conte; Véronique Diéras; et al.Nancy U. LinMelissa BearSonya C. ChapmanZhengyu YangYanyun ChenCarey K. Anders A Phase II Study of Abemaciclib in Patients with Brain Metastases Secondary to Hormone Receptor–Positive Breast Cancer. Clinical Cancer Research 2020, 26, 5310-5319, 10.1158/1078-0432.ccr-20-1764.
- Esther S. Kim; Abemaciclib: First Global Approval. Drugs 2017, 77, 2063-2070, 10.1007/s40265-017-0840-z.
- Verzenios: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Yahiya Y. Syed; Ribociclib: First Global Approval. Drugs 2017, 77, 799-807, 10.1007/s40265-017-0742-0.
- Kisqali: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Sohita Dhillon; Palbociclib: First Global Approval. Drugs 2015, 75, 543-551, 10.1007/s40265-015-0379-9.
- Ibrance: EPAR - Product Information. . European Medicines Agency. Retrieved 2022-4-21
- Martin Krug; Recent Advances in the Development of Multi-Kinase Inhibitors. Mini-Reviews in Medicinal Chemistry 2008, 8, 1312-1327, 10.2174/138955708786369591.
- Fleur Broekman; Tyrosine kinase inhibitors: Multi-targeted or single-targeted?. World Journal of Clinical Oncology 2011, 2, 80-93, 10.5306/wjco.v2.i2.80.
- Elena Ardini; Maria Menichincheri; Patrizia Banfi; Roberta Bosotti; Cristina De Ponti; Romana Pulci; Dario Ballinari; Marina Ciomei; Gemma Texido; Anna Degrassi; et al.Nilla AvanziNadia AmboldiMaria Beatrice SaccardoDaniele CaseroPaolo OrsiniTiziano BandieraLuca MologniDavid AndersonGe WeiJason HarrisJean-Michel VernierGang LiEduard FelderDaniele DonatiAntonella IsacchiEnrico PesentiPaola MagnaghiArturo Galvani Entrectinib, a Pan–TRK, ROS1, and ALK Inhibitor with Activity in Multiple Molecularly Defined Cancer Indications. Molecular Cancer Therapeutics 2016, 15, 628-639, 10.1158/1535-7163.mct-15-0758.
- Shouzheng Wang; Junling Li; Second-generation EGFR and ErbB tyrosine kinase inhibitors as first-line treatments for non-small cell lung cancer.. OncoTargets and Therapy 2019, ume 12, 6535-6548, 10.2147/OTT.S198945.
- Yohann Loriot; Andrea Necchi; Se Hoon Park; Jesus Garcia-Donas; Robert Huddart; Earle Burgess; Mark Fleming; Arash Rezazadeh; Begoña Mellado; Sergey Varlamov; et al.Monika JoshiIgnacio DuranScott T. TagawaYousef ZakhariaBob ZhongKim StuyckensAdemi Santiago-WalkerPeter De PorreAnne O’HaganAnjali AvadhaniArlene Siefker-Radtke Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. New England Journal of Medicine 2019, 381, 338-348, 10.1056/nejmoa1817323.
- Anthony Markham; Erdafitinib: First Global Approval. Drugs 2019, 79, 1017-1021, 10.1007/s40265-019-01142-9.
- William D. Tap; Zev A. Wainberg; Stephen P. Anthony; Prabha N. Ibrahim; Chao Zhang; John Healey; Bartosz Chmielowski; Arthur P. Staddon; Allen Lee Cohn; Geoffrey I. Shapiro; et al.Vicki L. KeedyArun S. SinghIgor PuzanovEunice L. KwakAndrew J. WagnerDaniel D. Von HoffGlen J. WeissRamesh K. RamanathanJiazhong ZhangGaston HabetsYing ZhangElizabeth A. BurtonGary VisorLaura SanftnerPaul SeversonHoa NguyenMarie J. KimAdhirai MarimuthuGarson TsangRafe ShellooeCarolyn GeeBrian WestPeter HirthKeith NolopMatt Van De RijnHenry H. HsuCharles PeterfyPaul S. LinSandra Tong-StarksenGideon Bollag Structure-Guided Blockade of CSF1R Kinase in Tenosynovial Giant-Cell Tumor. New England Journal of Medicine 2015, 373, 428-437, 10.1056/nejmoa1411366.
- Rajmohan Murali; Alexander M Menzies; Gv Long; Dabrafenib and its potential for the treatment of metastatic melanoma. Drug Design, Development and Therapy 2012, 6, 391-405, 10.2147/dddt.s38998.
- James Sun; Jonathan S. Zager; Zeynep Eroglu; Encorafenib/binimetinib for the treatment of BRAF-mutant advanced, unresectable, or metastatic melanoma: design, development, and potential place in therapy. OncoTargets and Therapy 2018, ume 11, 9081-9089, 10.2147/ott.s171693.
- Reinhard Dummer; Paolo A Ascierto; Helen J Gogas; Ana Arance; Mario Mandala; Gabriella Liszkay; Claus Garbe; Dirk Schadendorf; Ivana Krajsova; Ralf Gutzmer; et al.Vanna Chiarion SileniCaroline DutriauxJan Willem B de GrootNaoya YamazakiCarmen LoquaiLaure A Moutouh-De ParsevalMichael D PickardVictor SandorCaroline RobertKeith T Flaherty Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF -mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. The Lancet Oncology 2018, 19, 603-615, 10.1016/s1470-2045(18)30142-6.
- Robert Roskoski; Targeting oncogenic Raf protein-serine/threonine kinases in human cancers. Pharmacological Research 2018, 135, 239-258, 10.1016/j.phrs.2018.08.013.
- Andrea M. Gross; Pamela L. Wolters; Eva Dombi; Andrea Baldwin; Patricia Whitcomb; Michael J. Fisher; Brian Weiss; AeRang Kim; Miriam Bornhorst; Amish C. Shah; et al.Staci MartinMarie C. RoderickDominique C. PichardAmanda CarbonellScott M. PaulJanet TherrienOxana KapustinaKara HeiseyD. Wade ClappChi ZhangCody J. PeerWilliam D. FiggMalcolm SmithJohn GlodJaishri O. BlakeleySeth M. SteinbergDavid J. VenzonL. Austin DoyleBrigitte C. Widemann Selumetinib in Children with Inoperable Plexiform Neurofibromas. New England Journal of Medicine 2020, 382, 1430-1442, 10.1056/nejmoa1912735.
- Scott Kopetz; Axel Grothey; Rona Yaeger; Eric Van Cutsem; Jayesh Desai; Takayuki Yoshino; Harpreet Wasan; Fortunato Ciardiello; Fotios Loupakis; Yong Sang Hong; et al.Neeltje SteeghsTormod K. GurenHendrik-Tobias ArkenauPilar Garcia-AlfonsoPer PfeifferSergey OrlovSara LonardiElena ElezTae-Won KimJan H.M. SchellensChristina GuoAsha KrishnanJeroen DekervelVan MorrisAitana Calvo FerrandizL.S. TarpgaardMichael BraunAshwin GollerkeriChristopher KeirKati MaharryMichael PickardJanna Christy-BittelLisa AndersonVictor SandorJosep Tabernero Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–Mutated Colorectal Cancer. New England Journal of Medicine 2019, 381, 1632-1643, 10.1056/nejmoa1908075.
- Dirk Strumberg; Beate Schultheis; Regorafenib for cancer. Expert Opinion on Investigational Drugs 2012, 21, 879-889, 10.1517/13543784.2012.684752.
- Jean-Yves Blay; César Serrano; Michael C Heinrich; John Zalcberg; Sebastian Bauer; Hans Gelderblom; Patrick Schöffski; Robin L Jones; Steven Attia; Gina D'Amato; et al.Ping ChiPeter ReichardtJulie MeadeKelvin ShiRodrigo Ruiz-SotoSuzanne GeorgeMargaret von Mehren Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. The Lancet Oncology 2020, 21, 923-934, 10.1016/s1470-2045(20)30168-6.
- Bryan D. Smith; Michael D. Kaufman; Wei-Ping Lu; Anu Gupta; Cynthia B. Leary; Scott C. Wise; Thomas J. Rutkoski; Yu Mi Ahn; Gada Al-Ani; Stacie L. Bulfer; et al.Timothy M. CaldwellLawrence ChunCarol L. EnsingerMolly M. HoodArin McKinleyWilliam C. PattRodrigo Ruiz-SotoYing SuHanumaiah TelikepalliAjia TownBenjamin A. TurnerLakshminarayana VogetiSubha VogetiKaren YatesFilip JankuAlbiruni Ryan Abdul RazakOliver RosenMichael HeinrichDaniel L. Flynn Ripretinib (DCC-2618) Is a Switch Control Kinase Inhibitor of a Broad Spectrum of Oncogenic and Drug-Resistant KIT and PDGFRA Variants. Cancer Cell 2019, 35, 738-751.e9, 10.1016/j.ccell.2019.04.006.
- Maria E Cabanillas; Mabel Ryder; Camilo Jimenez; Targeted Therapy for Advanced Thyroid Cancer: Kinase Inhibitors and Beyond. Endocrine Reviews 2019, 40, 1573-1604, 10.1210/er.2019-00007.
- Lori J. Wirth; Eric Sherman; Bruce Robinson; Benjamin Solomon; Hyunseok Kang; Jochen Lorch; Francis Worden; Marcia Brose; Jyoti Patel; Sophie Leboulleux; et al.Yann GodbertFabrice BarlesiJohn C. MorrisTaofeek K. OwonikokoDaniel S.W. TanOliver GautschiJared WeissChristelle de la FouchardièreMark E. BurkardJanessa LaskinMatthew H. TaylorMatthias KroissJacques MedioniJonathan W. GoldmanTodd M. BauerBenjamin LevyViola W. ZhuNehal LakhaniVictor MorenoKevin EbataMichele NguyenDana HeirichEdward Y. ZhuXin HuangLuxi YangJennifer KheraniS. Michael RothenbergAlexander DrilonVivek SubbiahManisha H. ShahMaria E. Cabanillas Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. New England Journal of Medicine 2020, 383, 825-835, 10.1056/nejmoa2005651.
- Vivek Subbiah; Robert J. Kreitman; Zev A. Wainberg; Jae Yong Cho; Jan H.M. Schellens; Jean Charles Soria; Patrick Y. Wen; Christoph Zielinski; Maria E. Cabanillas; Gladys Urbanowitz; et al.Bijoyesh MookerjeeDazhe WangFatima RangwalaBhumsuk Keam Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600–Mutant Anaplastic Thyroid Cancer. Journal of Clinical Oncology 2018, 36, 7-13, 10.1200/jco.2017.73.6785.
- Theodore W. Laetsch; Douglas S. Hawkins; Larotrectinib for the treatment of TRK fusion solid tumors. Expert Review of Anticancer Therapy 2018, 19, 1-10, 10.1080/14737140.2019.1538796.
- Stephanie Berger; Uwe M. Martens; Sylvia Bochum; Larotrectinib (LOXO-101). Computational Biology 2018, 211, 141-151, 10.1007/978-3-319-91442-8_10.
- Alexander Drilon; Salvatore Siena; Sai-Hong Ignatius Ou; Manish Patel; Myung Ju Ahn; Jeeyun Lee; Todd M. Bauer; Anna F. Farago; Jennifer J. Wheler; Stephen V. Liu; et al.Robert DoebeleLaura GiannettaGiulio CereaGiovanna MarrapeseMichele SchirruAlessio AmatuKatia BencardinoLaura PalmeriAndrea Sartore-BianchiAngelo VanzulliSara CrestaSilvia DamianMatteo DucaElena ArdiniGang LiJason ChristiansenKarey KowalskiAnn D. JohnsonRupal PatelDavid LuoEdna Chow-ManevalZachary HornbyPratik S. MultaniAlice T. ShawFilippo G. De Braud Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discovery 2017, 7, 400-409, 10.1158/2159-8290.cd-16-1237.
- Maria E. Cabanillas; Mouhammed Amir Habra; Lenvatinib: Role in thyroid cancer and other solid tumors. Cancer Treatment Reviews 2015, 42, 47-55, 10.1016/j.ctrv.2015.11.003.
- Vincent Chau; Marijo Bilusic; Pembrolizumab in Combination with Axitinib as First-Line Treatment for Patients with Renal Cell Carcinoma (RCC): Evidence to Date. Cancer Management and Research 2020, ume 12, 7321-7330, 10.2147/cmar.s216605.
- Toni K Choueiri; James Larkin; Mototsugu Oya; Fiona Thistlethwaite; Marcella Martignoni; Paul Nathan; Thomas Powles; David McDermott; Paul B Robbins; David D Chism; et al.Daniel ChoMichael B AtkinsMichael S GordonSumati GuptaHirotsugu UemuraYoshihiko TomitaAnna CompagnoniCamilla FowstAlessandra di PietroBrian I Rini Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial. The Lancet Oncology 2018, 19, 451-460, 10.1016/s1470-2045(18)30107-4.
- Guru Sonpavde; Thomas Hutson; Brian I Rini; Axitinib for renal cell carcinoma. Expert Opinion on Investigational Drugs 2008, 17, 741-748, 10.1517/13543784.17.5.741.
- Sohita Dhillon; Ripretinib: First Approval. Drugs 2020, 80, 1133-1138, 10.1007/s40265-020-01348-2.
- Qinlock: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Anthony Markham; Selpercatinib: First Approval. Drugs 2020, 80, 1119-1124, 10.1007/s40265-020-01343-7.
- Retsevmo: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Anthony Markham; Susan J. Keam; Selumetinib: First Approval. Drugs 2020, 80, 931-937, 10.1007/s40265-020-01331-x.
- Koselugo: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Sohita Dhillon; Avapritinib: First Approval. Drugs 2020, 80, 433-439, 10.1007/s40265-020-01275-2.
- Ayvakyt: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Zaina T. Al-Salama; Susan J. Keam; Entrectinib: First Global Approval. Drugs 2019, 79, 1477-1483, 10.1007/s40265-019-01177-y.
- Rozlytrek: EPAR – Overview. . European Medicines Agency. Retrieved 2022-4-21
- Yvette N. Lamb; Pexidartinib: First Approval. Drugs 2019, 79, 1805-1812, 10.1007/s40265-019-01210-0.
- Refusal of the marketing authorisation for Turalio (pexidartinib). . European Medicines Agency. Retrieved 2022-4-21
- Lesley J. Scott; Larotrectinib: First Global Approval. Drugs 2019, 79, 201-206, 10.1007/s40265-018-1044-x.
- Vitrakvi: EPAR - Product Information. . European Medicines Agency. Retrieved 2022-4-21
- Yahiya Y. Syed; Lorlatinib: First Global Approval. Drugs 2019, 79, 93-98, 10.1007/s40265-018-1041-0.
- Lorviqua: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Matt Shirley; Dacomitinib: First Global Approval. Drugs 2018, 78, 1947-1953, 10.1007/s40265-018-1028-x.
- Vizimpro: EPAR - Product Information. . European Medicines Agency. Retrieved 2022-4-21
- Matt Shirley; Encorafenib and Binimetinib: First Global Approvals. Drugs 2018, 78, 1277-1284, 10.1007/s40265-018-0963-x.
- Braftovi: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Mektovi: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Anthony Markham; Brigatinib: First Global Approval. Drugs 2017, 77, 1131-1135, 10.1007/s40265-017-0776-3.
- Alunbrig: EPAR - Product Information. . European Medicines Agency. Retrieved 2022-4-21
- Julia Paik; Sohita Dhillon; Alectinib: A Review in Advanced, ALK-Positive NSCLC. Drugs 2018, 78, 1247-1257, 10.1007/s40265-018-0952-0.
- Alecensa: EPAR – Summary for the public. . European Medicines Agency. Retrieved 2022-4-21
- Karly P. Garnock-Jones; Cobimetinib: First Global Approval. Drugs 2015, 75, 1823-1830, 10.1007/s40265-015-0477-8.
- Cotellic: EPAR – Summary for the public. . European Medicines Agency. Retrieved 2022-4-21
- Lesley J. Scott; Lenvatinib: First Global Approval. Drugs 2015, 75, 553-560, 10.1007/s40265-015-0383-0.
- Lenvima: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Rosselle T. Dungo; Gillian M. Keating; Afatinib: First Global Approval. Drugs 2013, 73, 1503-1515, 10.1007/s40265-013-0111-6.
- Giotrif: EPAR – Summary for the public. . European Medicines Agency. Retrieved 2022-4-21
- Cameron J. M. Wright; Paul L. McCormack; Trametinib: First Global Approval. Drugs 2013, 73, 1245-1254, 10.1007/s40265-013-0096-1.
- Mekinist: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Anita D. Ballantyne; Karly P. Garnock-Jones; Dabrafenib: First Global Approval. Drugs 2013, 73, 1367-1376, 10.1007/s40265-013-0095-2.
- Tafinlar: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Harpreet Singh; Michael Brave; Julia A. Beaver; Joyce Cheng; Shenghui Tang; Eias Zahalka; Todd R. Palmby; Rajesh Venugopal; Pengfei Song; Qi Liu; et al.Chao LiuJingyu YuXiao Hong ChenXing WangYaning WangPaul G. KluetzSelena R. DanielsElektra J. PapadopoulosRajeshwari SridharaAmy E. McKeeAmna IbrahimGeoffrey KimRichard Pazdur U.S. Food and Drug Administration Approval: Cabozantinib for the Treatment of Advanced Renal Cell Carcinoma. Clinical Cancer Research 2016, 23, 330-335, 10.1158/1078-0432.ccr-16-1073.
- CABOMETYX (cabozantinib). Prescribing Information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- Cabometyx: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Subhajit Roy; Bawneet K Narang; Shiva K Rastogi; Ravindra K Rawal; A Novel Multiple Tyrosine-kinase Targeted Agent to Explore the Future Perspectives of Anti-Angiogenic Therapy for the Treatment of Multiple Solid Tumors: Cabozantinib. Anti-Cancer Agents in Medicinal Chemistry 2014, 15, 37-47, 10.2174/1871520614666140902153840.
- COMETRIQ (cabozantinib). Prescribing Information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- Cometriq: EPAR – Summary for the public. . European Medicines Agency. Retrieved 2022-4-21
- Thomas J. Ettrich; Thomas Seufferlein; Regorafenib. Methods in Pharmacology and Toxicology 2014, 201, 185-196, 10.1007/978-3-642-54490-3_10.
- STIVARGA (regorafenib). Prescribing Information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- Stivarga: EPAR – Summary for the public. . European Medicines Agency. Retrieved 2022-4-21
- Audrey Bellesoeur; Edith Carton; Jerome Alexandre; Francois Goldwasser; Olivier Huillard; Axitinib in the treatment of renal cell carcinoma: design, development, and place in therapy. Drug Design, Development and Therapy 2017, ume 11, 2801-2811, 10.2147/dddt.s109640.
- INLYTA (axitinib). Prescribing Information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- Inlyta: EPAR – Summary for the public. . European Medicines Agency. Retrieved 2022-4-21
- Alice T. Shaw; Uma Yasothan; Peter Kirkpatrick; Crizotinib. Nature Reviews Drug Discovery 2011, 10, 897-898, 10.1038/nrd3600.
- XALKORI (crizotinib). Prescribing Information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- Xalkori: EPAR – Summary for the public. . European Medicines Agency. Retrieved 2022-4-21
- Keith. T Flaherty; Uma Yasothan; Peter Kirkpatrick; Vemurafenib. Nature Reviews Drug Discovery 2011, 10, 811-812, 10.1038/nrd3579.
- ZELBORAF (vemurafenib). Prescribing Information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- Zelboraf: EPAR – Summary for the public. . European Medicines Agency. Retrieved 2022-4-21
- James E. Frampton; Vandetanib. Drugs 2012, 72, 1423-1436, 10.2165/11209300-000000000-00000.
- Caprelsa: EPAR – Summary for the public. . European Medicines Agency. Retrieved 2022-4-21
