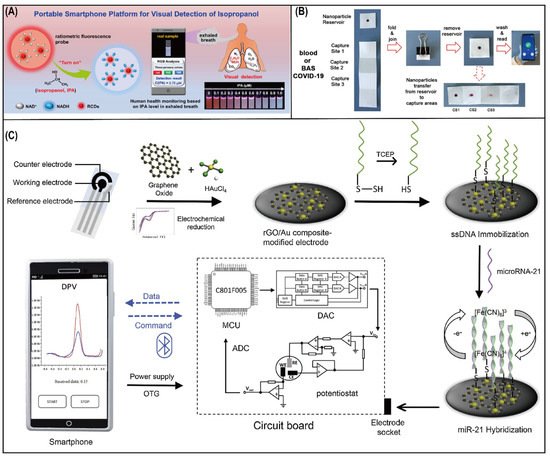
Lung cancer has been studied for decades because of its high morbidity and high mortality. Traditional methods involving bronchoscopy and needle biopsy are invasive and expensive, which makes patients suffer more risks and costs. Various noninvasive lung cancer markers, such as medical imaging indices, volatile organic compounds (VOCs), and exhaled breath condensates (EBCs), have been discovered for application in screening, diagnosis, and prognosis. However, the detection of markers still relies on bulky and professional instruments, which are limited to training personnel or laboratories. This seriously hinders population screening for early diagnosis of lung cancer. Advanced smartphones integrated with powerful applications can provide easy operation and real-time monitoring for healthcare, which demonstrates tremendous application scenarios in the biomedical analysis region from medical institutions or laboratories to personalized medicine.
- smartphone
- volatile organic compounds
1. Introduction
1. Introduction
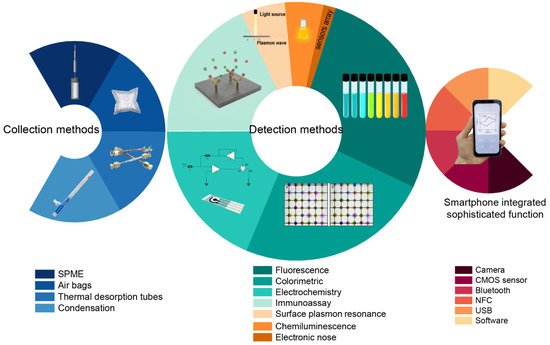
2. Biomarkers in VOCs and EBCs
2.1. Breath Sampling and Preconcentration
Exhaled breath is composed of air from the mouth, nasal cavity, alveoli, and part dead space. Biomarkers associated with different diseases are produced in different locations. Exhaled breath biomarkers that have been fully exchanged in the lung–blood circulatory system will be comparatively accurate in their reflection of lung diseases and metabolic diseases. Although there are high-sensitivity gas sensors [13] that have achieved rapid online detection of components in exhaled breath, the most accurate detection of exhaled breath components still requires enrichment before detection and analysis. Therefore, the collection and storage of respiratory samples have an impact on subsequent procedures. However, there is still no standardized sampling protocol for collecting patient exhalation. In addition, the concentration of exhaled breath markers is between the volume fraction ppb and ppt. This concentration level is commonly lower than the limit of detection of equipment during direct detection of exhaled breath. Furthermore, the complete elimination of gas from unexchanged dead space and the oral and nasal cavities is still a challenge. Thus, researchers have mostly chosen to collect late expiratory samples to eliminate dead space and have used polymer bags as collecting sample containers [14] due to the advantages of low cost and easy operation. Normally, subjects are asked to take a deep breath, and the first collected breath is discarded. Additionally, some researchers stipulated strict sampling procedures. Chang et al. [15] asked subjects not to eat, smoke, brush their teeth, or gargle for a minimum of 2 h before sampling. Ambient air is another interference that can greatly alter analysis results. Di Gilio et al. [16] required that volunteers waited in the room for at least 10 min before breath collection so that equilibrium could be created between the lungs and ambient air. Owing to the low concentration of VOCs in exhaled breath [17], preconcentration is commonly used to improve the accuracy and sensitivity of detection. Preconcentration methods include solid-phase microextraction (SPME), thermal desorption enrichment technology, needle trap microextraction (NTME), and so on. In essence, most methods use the force (such as van der Waals forces) between adsorption materials and gas molecules at the microscopic scale. The flexible use of these enrichment methods can not only eliminate more than 90% of the water vapor in exhaled breath but also greatly enrich the target markers by 10- to 100-fold. SPME is easy to use and widely used in many environmental, clinical, and biological analyses. Normally, SPME fibers are coated with various polymers such as polydimethylsiloxane, divinylbenzene, polyacrylate, and polyethylene glycol [18] to absorb analytes within the ppb range. Thermal desorption enrichment technologies mainly rely on adsorption materials. The adsorbents used in breath testing include carbon nanotubes, Tenax2. Biomarkers in VOCs and EBCs
2.1. Breath Sampling and Preconcentration
2.2. Biomarkers in VOCs
More than 1000 volatile organic compounds (VOCs) have been identified in human breath [20]. Lung-cancer-correlated VOCs have been sought in case–control studies and verified by cohort studies. To date, seven categories of VOCs have been found to serve as biomarkers of lung cancer in exhaled breath. The categories are alkanes, alkanols, aldehydes, ketones, lipids, nitriles, and aromatics. Researchers thought that alkanes such as ethane and pentane were generated by the lipid peroxidation of polyunsaturated fatty acids in cell membranes [21][22][23]. Breath methyl alkanes may also be products of the same process [24]. The first report on exhaled VOCs in lung cancer dates back to 1985, when Gordon et al. identified that several VOCs in exhaled breath were associated with lung cancer [25]. In 1999, Phillips et al. used a combination of 22 VOCs in breath samples to distinguish between patients with and without lung cancer [26]. Poli et al. demonstrated that a combination of 13 VOCs could be used to correctly classify lung cancer patients, chronic obstructive pulmonary disease (COPD) patients, asymptomatic smokers, and healthy subjects into defined groups [27]. Additionally, it is worth noting that some pulmonary diseases, especially COPD or emphysema, could be a major risk factor for lung cancer. This is because COPD and lung cancer share common risk factors such as smoking [28]. Wehinger et al. reported that the concentration of mass-to-charge ratios 31 and 43 were increased in lung cancer patients in a study using proton-transfer-reaction mass spectrometry (PTR-MS) [29].2.2. Biomarkers in VOCs
| Years | Author | Collection Method | Sample | VOCs | |||
|---|---|---|---|---|---|---|---|
| 1985 | Gordon [25] | Gordon [47] | Tenax GC sorbent cartridges | Expired breath | Acetone, 2-butanone, n-propanol | ||
| 1999 | Phillips [26] | Phillips [48] | Sorbent trap | Alveolar breath | Styrene, 2,2,4,6,6-pentamethylheptane, 2-methylheptane, decane, n-propylbenzene undecane, methyl cyclopentane, 1-methyl-2-pentylcyclopropane, trichlorofluoromethane, benzene, 1,2,4-trimethylbenzene, isoprene, 3-methyloctane, 1-hexene, 3-methylnonane, 1-heptene, 1,4-dimethylbenzene, 2,4-dimethylheptane, hexanal, cyclohexane, 1-methylethenylbenzene, heptanal | ||
| 2005 | Poli [27] | Poli [49] | Teflon | ® | bulb; SPME | Mixed expiratory samples | Isoprene; methylpentane; pentane; ethylbenzene; xylenes; trimethylbenzene; toluene; benzene; heptane; decane, styrene; octane; pentamethylheptane |
| 2007 | Wehinger [29] | Wehinger [51] | Tedlar | ® | bags | Alveolar breath | Formaldehyde, isopropanol |
| 2009 | Bajtarevic [30] | Bajtarevic [52] | Tedlar | ® | bags | Mixed expiratory and indoor air | Isoprene, acetone, methanol; 2-butanone, benzaldehyde, 2,3-butanedione, 1-propanol, 2-butanone, 3-hydroxy-, 3-butyn-2-ol, butane, 2-methyl-, 2-butene, 2-methyl-, acetophenone, 1-cyclopentene, methyl propyl sulfide, urea, tetramethyl-, n-pentanal, 1,3-cyclopentadiene, 1-methyl-, 2-butanol, 2,3-dimethyl-, isoquinoline, 1,2,3,4-tetrahydro-, undecane, 3,7-dimethyl-, benzene, cyclobutyl-, butyl acetate, ethylenimine, n-undecane, |
| 2010 | Fuchs [31] | Fuchs [53] | Mylar sampling bag | Alveolar breath | p-Cymene, toluene, dodecane, 3,3-dimethylpentane, 2,3,4-trimethylhexane, (1-phenyl-1-butenyl)benzene 1,3-dimethylbenzene, 1-iodononane, [(1,1-dimethylethyl) thiol]acetic acid, 4-(4-propylcyclohexyl)-4′-cyano [1,1′-biphenyl]4-yl ester benzoic acid, 2-amino-5-isopropyl-8-methyl-1-azulenecarbonitrile, 5-(2-methylpropyl)nonane, 2,3,4-trimethyldecane, 6-ethyl-3-octanyl 2-(trifluoromethyl)benzoate, p-xylene, and 2,2-dimethyldecane | ||
| 2010 | Song [32] | Song [54] | Tedlar | ® | gas bags; SPME | Mixed expiratory samples | 1-Butanol and 3-hydroxy-2-butanone |
| 2011 | Ulanowska [33] | Ulanowska [55] | Tedlar | ® | bags; SPME | Alveolar breath | Ethanol, acetone, butane, dimethyl sulfide, isoprene, propanal, 1-propanol, 2-pentanone, furan, o-xylene, ethylbenzene, pentanal, hexanal, nonane |
| 2012 | Buszewski [34] | Buszewski [56] | Tedlar | ® | bags; SPME | Alveolar breath | Butanal, ethyl acetate, 2-pentanone, ethylbenzene, 1-propanol, 2-propanol |
| 2015 | Kumar [35] | Kumar [57] | Nalophan bag | Mixed alveolar breath | Pentanoic acid; hexanoic acid; phenol; methyl phenol; ethyl phenol; butanal; pentanal; hexanal; heptanal; octanal; nonanal; decanal | ||
| 2016 | Schallschmidt [36] | Schallschmidt [58] | Gas bulbs; SPME | Tidal breath | Propanal, butanal, decanal, butanal, 2-butanone, ethylbenzene | ||
| 2017 | Sakumura [37] | Sakumura [59] | Analytic Barrier Bag | Alveolar breath | Hydrogen cyanide, methanol, acetonitrile, isoprene, 1-propanol | ||
| 2019 | Phillips [38] | Phillips [60] | Carbotrap C and Carbopack C | Alveolar breath | 1,4-Butanediol, 2-pentanamine, 4-methyl-, 2-propanamine, 3-butenamide, 4-penten-2-ol, acetamide, 2-cyanoalanine, n-methylglycine, octodrine | ||
| 2019 | Li [39] | Li [61] | Tedlar | ® | bags; SPME | End-tidal breath | Isopropanol, n-butanol, n-heptanol, n-hexanal, n-heptanal, n-decanal |