Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Conner Chen and Version 10 by Conner Chen.
Shiga toxin-producing Escherichia coli (STEC) are zoonotic Gram-negative bacteria. While raw milk cheese consumption is healthful, contamination with pathogens such as STEC can occur due to poor hygiene practices at the farm level. STEC infections cause mild to serious symptoms in humans. The raw milk cheese-making process concentrates certain milk macromolecules such as proteins and milk fat globules (MFGs), allowing the intrinsic beneficial and pathogenic microflora to continue to thrive. MFGs are surrounded by a biological membrane, the milk fat globule membrane (MFGM), which has a globally positive health effect, including inhibition of pathogen adhesion.
- STEC
- MFGM
- adhesion
- raw milk
- Milk fat globule membrane proteins
1. The Mechanism of STEC-MFG Association: What Do We Know?
1.1. General Information on Bacterial Adhesion
Bacterial adhesion is a complex process involving several factors, including: (i) surface-related properties (hydrophobicity, electrical charge, roughness, and topology); (ii) cell morphological properties (size, volume, dimension, and shape); (iii) the cell surface (chemical properties, envelope type, exposed proteins, and exopolysaccharide (EPS)); and (iv) the cell’s ability to move [1]. Adhesion is a key step for bacteria (pathogenic or not), allowing colonization and growth at a host-specific site [2]. The bacterial adhesion process consists of two phases: a non-specific phase, involving physicochemical bonds, and a specific phase involving molecular factors exposed on both host and bacterial cell surface [3]. The STEC–MFG association can be seen as a host–bacteria adhesion facilitated by the origin of the MFGM and its similarities with the membrane of intestinal cells [4][5]. In this context, both biological membranes will interact together through surface components. Various glycoconjugates are anchored on the MFGM surface and can act as ligands.
1.2. Physicochemical Interactions
Non-specific interactions have been described as the first step of adhesion, which is reversible and occurs rapidly (in the order of ~1 min) [6]. The process of initial bacterial adhesion is still not clearly understood, and physicians and microbiologists are working together to clarify the mechanisms. It is widely accepted that bacterial interaction is conducted according to the Derjaguin, Landau, Verwey, and Overbeek (DLVO) theory [7] and the extended DLVO theory [8]. The DLVO theory describes the force between charged surfaces interacting through a liquid. However, this theory may not be appropriate for modeling bacterial adhesion owing to the numerous processes involved and the influence of both biological and environmental factors (pH, ionic strength, and temperature) [9][10]. The non-specific phase of adhesion is a consequence of the balance between attractive and repulsive forces that are set up between the bacterium and the surface where it could adhere [11][12]. These forces include non-covalent interactions such as electrostatic interactions or surface charges, van der Waals forces, and Lewis acid/base interactions, as well as hydrophobic interactions [13][14]. Hydrophobic interactions and surface charges are the primary forces influencing bacterial adhesion [15].
1.2.1. Cell Surface Hydrophobicity
Bacterial cell surface hydrophobicity (CSH) is probably one of the major phenomena that governs bacterial attachment to a surface [13][14]. Hydrophobic interactions are defined as the ability of two elements of similar hydrophobicity to attract each other [16]. These forces are affected by the nature of their membrane-anchored components, including amino residues that are exposed to the extracellular environment [17]. In the context of STEC and MFGs, both are surrounded by a protein-rich membrane whose anchored surface components have polar properties (e.g., proteins and phospholipids) leading to weak hydrophobic repulsions [18][19]. Interestingly, Brisson et al. showed that the adhesion of
1.1. General Information on Bacterial Adhesion
Bacterial adhesion is a complex process involving several factors, including: (i) surface-related properties (hydrophobicity, electrical charge, roughness, and topology); (ii) cell morphological properties (size, volume, dimension, and shape); (iii) the cell surface (chemical properties, envelope type, exposed proteins, and exopolysaccharide (EPS)); and (iv) the cell’s ability to move [99]. Adhesion is a key step for bacteria (pathogenic or not), allowing colonization and growth at a host-specific site [100]. The bacterial adhesion process consists of two phases: a non-specific phase, involving physicochemical bonds, and a specific phase involving molecular factors exposed on both host and bacterial cell surface [101]. The STEC–MFG association can be seen as a host–bacteria adhesion facilitated by the origin of the MFGM and its similarities with the membrane of intestinal cells [29,68]. In this context, both biological membranes will interact together through surface components. Various glycoconjugates are anchored on the MFGM surface and can act as ligands.
1.2. Physicochemical Interactions
Non-specific interactions have been described as the first step of adhesion, which is reversible and occurs rapidly (in the order of ~1 min) [102]. The process of initial bacterial adhesion is still not clearly understood, and physicians and microbiologists are working together to clarify the mechanisms. It is widely accepted that bacterial interaction is conducted according to the Derjaguin, Landau, Verwey, and Overbeek (DLVO) theory [103] and the extended DLVO theory [104]. The DLVO theory describes the force between charged surfaces interacting through a liquid. However, this theory may not be appropriate for modeling bacterial adhesion owing to the numerous processes involved and the influence of both biological and environmental factors (pH, ionic strength, and temperature) [105,106]. The non-specific phase of adhesion is a consequence of the balance between attractive and repulsive forces that are set up between the bacterium and the surface where it could adhere [107,108]. These forces include non-covalent interactions such as electrostatic interactions or surface charges, van der Waals forces, and Lewis acid/base interactions, as well as hydrophobic interactions [109,110]. Hydrophobic interactions and surface charges are the primary forces influencing bacterial adhesion [111].
1.2.1. Cell Surface Hydrophobicity
Bacterial cell surface hydrophobicity (CSH) is probably one of the major phenomena that governs bacterial attachment to a surface [109,110]. Hydrophobic interactions are defined as the ability of two elements of similar hydrophobicity to attract each other [112]. These forces are affected by the nature of their membrane-anchored components, including amino residues that are exposed to the extracellular environment [113]. In the context of STEC and MFGs, both are surrounded by a protein-rich membrane whose anchored surface components have polar properties (e.g., proteins and phospholipids) leading to weak hydrophobic repulsions [114,115]. Interestingly, Brisson et al. showed that the adhesion of
Lactobacillus reuteri to MFGs was strain-dependent, and the more the strain was hydrophobic, the more it adhered [20].
1.2.2. Electrostatic Forces
Electrostatic forces result from the presence of a double ionic layer at the surface of a particle. The bacterial cell surface is generally negatively charged because of the carboxyl and phosphate core as well as the lipopolysaccharide (LPS) located at the surface [21].
1.2.2. Electrostatic Forces
Electrostatic forces result from the presence of a double ionic layer at the surface of a particle. The bacterial cell surface is generally negatively charged because of the carboxyl and phosphate core as well as the lipopolysaccharide (LPS) located at the surface [116].
E. coli surface charge is between −30 and −45 mV at milk pH [22][23]. While there are no published STEC-specific surface charge data generated with a modern instrument, some studies have shown that STEC isolates or reference strains are weakly negative [24][25]. Native MFGs are negatively charged due to the high phospholipid content of the outer layer of the MFGM [26]. The ζ-potential of native MFGs is close to −13 mV [27][28][29]. Furthermore, Malik et al. showed that the MFGM fraction could reach −20 mV at pH 6.5 [30]. When a negative surface meets another negative surface, repulsive forces are produced. Thus, in theory, MFGs and STEC should repel each other. However, it is important to note that the bacterial surface charge should be measured in an appropriate buffer that mimics the properties of raw milk, such as milk ultrafiltration permeate. There is a lack of recent experimental data with appropriate physicochemical conditions to assess the involvement of these forces in the association of STEC with MFGs.
1.2.3. Van der Waals Forces
Van der Waals interactions are long-range attractive forces present in both polar and non-polar molecules and come mainly from the fluctuation of the internal charge of a particle. These forces are generally attractive and result from induced dipole interactions between molecules in a colloidal particle and a substrate [31]. Attractive van der Waals forces are ubiquitous between molecules [32] and could explain part of the interaction between bacteria and the MFGM. However, van der Waals interactions in MFGM–bacteria adhesion have not been studied.
1.2.4. Lewis Acid/Base Interactions
The Lewis acid/base interaction is a polar interaction where acceptor/donor electrons enable the formation of hydrogen bonds also known as Lewis bonds [33]. This link occurs whenever ligands of strong electronegativity are associated with hydrogen. These short-range bonds are strong electrostatic interactions. Kiely and Olson showed that
surface charge is between −30 and −45 mV at milk pH [117,118]. While there are no published STEC-specific surface charge data generated with a modern instrument, some studies have shown that STEC isolates or reference strains are weakly negative [119,120]. Native MFGs are negatively charged due to the high phospholipid content of the outer layer of the MFGM [121]. The ζ-potential of native MFGs is close to −13 mV [122,123,124]. Furthermore, Malik et al. showed that the MFGM fraction could reach −20 mV at pH 6.5 [125]. When a negative surface meets another negative surface, repulsive forces are produced. Thus, in theory, MFGs and STEC should repel each other. However, it is important to note that the bacterial surface charge should be measured in an appropriate buffer that mimics the properties of raw milk, such as milk ultrafiltration permeate. There is a lack of recent experimental data with appropriate physicochemical conditions to assess the involvement of these forces in the association of STEC with MFGs.
1.2.3. Van der Waals Forces
Van der Waals interactions are long-range attractive forces present in both polar and non-polar molecules and come mainly from the fluctuation of the internal charge of a particle. These forces are generally attractive and result from induced dipole interactions between molecules in a colloidal particle and a substrate [126]. Attractive van der Waals forces are ubiquitous between molecules [127] and could explain part of the interaction between bacteria and the MFGM. However, van der Waals interactions in MFGM–bacteria adhesion have not been studied.
1.2.4. Lewis Acid/Base Interactions
The Lewis acid/base interaction is a polar interaction where acceptor/donor electrons enable the formation of hydrogen bonds also known as Lewis bonds [128]. This link occurs whenever ligands of strong electronegativity are associated with hydrogen. These short-range bonds are strong electrostatic interactions. Kiely and Olson showed that
L. casei strains and MFGs behaved as electron donors and could mediate bonds [34][35]. However, the role of Lewis bonds in MFGM–bacteria adhesion was not fully investigated.
1.3. Specific Molecular Interactions
Bacterial molecules involved in adhesion, called adhesins, recognize specific oligosaccharide moieties or peptide residues on the surface of target cells. There are many different adhesins, including porins, complex protein structures, glycoproteins, and glycolipids. Three main types of adhesin–receptor interactions have been described: lectin–glycan; protein–protein; and hydrophobin–protein [36]. Lectins are key factors in bacterial adhesion mechanisms [37][38][39]. Lectins are adhesins that recognize glycoconjugates, the sugar epitopes generally associated with proteins or lipids. Glycoconjugates are polymeric carbohydrates composed of monosaccharides arranged in chains and preferentially present on the external leaflet either attached to lipids or proteins [40]. Commonly, the polysaccharides of glycoconjugates are referred to as the ‘glycan layer’ or ‘glycocalyx’ [41]. The glycocalyx is directly exposed to the environment, allowing interactions with other cells to facilitate cell communication, immune regulation, and adhesion [42].
A wide range of STEC isolates can be responsible for human infections, and these can be genetically different [43]. However, regardless of the strain or serogroup, STEC possess virulence factors that allow attachment to intestinal epithelial cells (IECs), and these adhesion factors are generally considered essential for infection. A large range of polysaccharides exists, but only a subset is exposed at the cell surface where they can be recognized by complementary receptors. Adhesins can be found at the distal end of bacterial pili (or fimbriae). These are bacterial extracellular appendages approximately 1 to 20 μm long and <2 to 10 nm in diameter [44]. Other adhesins are anchored directly in the biological membrane of bacteria and are referred to as afimbrial adhesins [45][46].
1.3.1. MFGM as a Decoy Receptor for STEC
Douëllou et al. showed that raw milk reduced the adhesion of two STEC strains (O157:H7 str. EDL933 and O26:H11 str. 21765) to intestinal cells in vitro and in vivo, whereas pasteurized milk did not [47]. Furthermore, Brewster and Paul showed that more than 98% of the pathogenic bacteria (including STEC) added to pasteurized or homogenized milk were recovered in the pellet after centrifugation, while less than 7% were recovered from raw milk, suggesting that processing could weaken the MFG–bacteria association [48]. Another study demonstrated that only MFGM proteins and glycoproteins inhibited
strains and MFGs behaved as electron donors and could mediate bonds [129,130]. However, the role of Lewis bonds in MFGM–bacteria adhesion was not fully investigated.
1.3. Specific Molecular Interactions
Bacterial molecules involved in adhesion, called adhesins, recognize specific oligosaccharide moieties or peptide residues on the surface of target cells. There are many different adhesins, including porins, complex protein structures, glycoproteins, and glycolipids. Three main types of adhesin–receptor interactions have been described: lectin–glycan; protein–protein; and hydrophobin–protein [131]. Lectins are key factors in bacterial adhesion mechanisms [132,133,134]. Lectins are adhesins that recognize glycoconjugates, the sugar epitopes generally associated with proteins or lipids. Glycoconjugates are polymeric carbohydrates composed of monosaccharides arranged in chains and preferentially present on the external leaflet either attached to lipids or proteins [135]. Commonly, the polysaccharides of glycoconjugates are referred to as the ‘glycan layer’ or ‘glycocalyx’ [136]. The glycocalyx is directly exposed to the environment, allowing interactions with other cells to facilitate cell communication, immune regulation, and adhesion [137].
A wide range of STEC isolates can be responsible for human infections, and these can be genetically different [138]. However, regardless of the strain or serogroup, STEC possess virulence factors that allow attachment to intestinal epithelial cells (IECs), and these adhesion factors are generally considered essential for infection. A large range of polysaccharides exists, but only a subset is exposed at the cell surface where they can be recognized by complementary receptors. Adhesins can be found at the distal end of bacterial pili (or fimbriae). These are bacterial extracellular appendages approximately 1 to 20 μm long and <2 to 10 nm in diameter [139]. Other adhesins are anchored directly in the biological membrane of bacteria and are referred to as afimbrial adhesins [59,63].
1.3.1. MFGM as a Decoy Receptor for STEC
Douëllou et al. showed that raw milk reduced the adhesion of two STEC strains (O157:H7 str. EDL933 and O26:H11 str. 21765) to intestinal cells in vitro and in vivo, whereas pasteurized milk did not [88]. Furthermore, Brewster and Paul showed that more than 98% of the pathogenic bacteria (including STEC) added to pasteurized or homogenized milk were recovered in the pellet after centrifugation, while less than 7% were recovered from raw milk, suggesting that processing could weaken the MFG–bacteria association [89]. Another study demonstrated that only MFGM proteins and glycoproteins inhibited
E. coli adhesion in the Caco-2/HT-28 model [49]. In addition, Ross et al. suggested that the anti-infective activity of MFGM is due to the interaction of bacteria with MFGM proteins and glycoproteins rather than the interaction between MFGM and host cell receptors. In addition, modifications to MFGM surfaces such as surface roughness, zeta potential, MFG size, and phospholipid content can drastically impair the adhesive proprieties of
adhesion in the Caco-2/HT-28 model [140]. In addition, Ross et al. suggested that the anti-infective activity of MFGM is due to the interaction of bacteria with MFGM proteins and glycoproteins rather than the interaction between MFGM and host cell receptors. In addition, modifications to MFGM surfaces such as surface roughness, zeta potential, MFG size, and phospholipid content can drastically impair the adhesive proprieties of
L. fermatum [29]. The MFGM can also inhibit ETEC hemagglutination, suggesting that similar motifs are present on both membranes [50].
1.3.2. MFGM Proteins and Glycoproteins Potentially Targeted by STEC
No published studies have focused on which MFGM proteins are recognized by STEC or which adhesins are involved. However, studies have been conducted on other bacterial models (mostly beneficial). Guerin et al. used atomic force microscopy (AFM) to show that the spaCBA pili of
[124]. The MFGM can also inhibit ETEC hemagglutination, suggesting that similar motifs are present on both membranes [141].
1.3.2. MFGM Proteins and Glycoproteins Potentially Targeted by STEC
No published studies have focused on which MFGM proteins are recognized by STEC or which adhesins are involved. However, studies have been conducted on other bacterial models (mostly beneficial). Guerin et al. used atomic force microscopy (AFM) to show that the spaCBA pili of
L. rhamnosus engaged with the MFGM. Another experiment conducted by Novakovic et al. demonstrated, by blot overlay, binding of the ETEC F4ac pili to various porcine MFGM or milk proteins, including lactadherin, butyrophilin, adipophilin, acyl-CoA synthetase 3, and fatty acid-binding protein 3 [51]. An extensive literature search highlighted several MFGM proteins or glycoproteins that could interact with bacteria (
engaged with the MFGM. Another experiment conducted by Novakovic et al. demonstrated, by blot overlay, binding of the ETEC F4ac pili to various porcine MFGM or milk proteins, including lactadherin, butyrophilin, adipophilin, acyl-CoA synthetase 3, and fatty acid-binding protein 3 [142]. An extensive literature search highlighted several MFGM proteins or glycoproteins that could interact with bacteria (
Table 1). As an example, Zg16 can bind peptidoglycan [52]. Milk whey proteins such as lactoferrin, β-lactoglobulin, and α-lactalbumin can be adsorbed on the MFGM by heat treatment [53][54] and can be bound by bacteria. Glycoproteins such as mucins (MUC1 and MUC15), CD59, ECM proteins (tenascin, vitronectin), butyrophilin, prolactin-inducible protein (mPIP), CD36, and alpha1-antichymotrypsin can be bacterial lectin targets (
). As an example, Zg16 can bind peptidoglycan [143]. Milk whey proteins such as lactoferrin, β-lactoglobulin, and α-lactalbumin can be adsorbed on the MFGM by heat treatment [144,145] and can be bound by bacteria. Glycoproteins such as mucins (MUC1 and MUC15), CD59, ECM proteins (tenascin, vitronectin), butyrophilin, prolactin-inducible protein (mPIP), CD36, and alpha1-antichymotrypsin can be bacterial lectin targets (
Table 1). Among this non-exhaustive list, mucins could well be potential targets for STEC. Mucins are highly glycosylated proteins known to adhere to bacteria. Mucins constitute mucus, a secreted gel that binds the intestinal microbiota and protects the epithelium from pathogens [55][56]. Additionally, EF-Tu, a ubiquitous bacterial protein that can bind many proteins and mediate adhesion, could potentially interact with the MFGM [57].
). Among this non-exhaustive list, mucins could well be potential targets for STEC. Mucins are highly glycosylated proteins known to adhere to bacteria. Mucins constitute mucus, a secreted gel that binds the intestinal microbiota and protects the epithelium from pathogens [146,147]. Additionally, EF-Tu, a ubiquitous bacterial protein that can bind many proteins and mediate adhesion, could potentially interact with the MFGM [148].
Table 1.
MFGM proteins or glycoproteins that are potentially bound by STEC.
| Bovine MFGM Components | Bacterial Components | References | |||
|---|---|---|---|---|---|
| Adipophilin * (ADPH) | F4ac (E. coli) fimbria | [51] | [142] | ||
| Alpha 1-antichymotrypsin (serpin) | - | [58] | [149] | ||
| Annexins A1, A2, A5 | LPS (lipid A), OmpB, YadC (tip adhesin of Yad fimbriae) | [59][60][61] | [150,151,152] | ||
| Apolipoprotein serum amyloid A protein | OmpA | [62] | [153] | ||
| Apolipoproteins | LPS | [63][64] | [154,155] | ||
| Butyrophilin * | F4ac (E. coli) fimbria | [4][51] | [29,142] | ||
| Calnexin | LPS, peptidoglycan | [65] | [156] | ||
| Cathelicidin 1 | LPS, LTA | [66] | [157] | ||
| CD36 * | LPS, LTA | [4] | [29 | [67] | ,158] |
| CD5L protein | - | [68] | [159] | ||
| Elongation factor thermal unstable Tu (EF-Tu) |
- | [57] | [148] | ||
| Fatty acid-binding protein * | F4ac (E. coli) fimbria | [51] | [142] | ||
| Fibrinogen | Fibrinogen-binding protein (MSCRAMMs), curli | [69][70][71][72][73][74] | [160,161,162,163,164,165] | ||
| Galectin 7 | LPS | [75] | [166] | ||
| Gelsolin | LPS, LTA | [76] | [167] | ||
| Immunoglobulins | Many bacterial proteins | - | |||
| Integrin | Many bacterial proteins | [74][77][78][79][80][81] | [52,165,168,169,170,171] | ||
| Lactadherin * | F4ac (E. coli) fimbria | [51][82] | [142,172] | ||
| Lactoferrin | OMPs | [83] | [173] | ||
| Macrophage scavenger receptor | LPS, LTA | [84] | [174] | ||
| MUC1 *, MUC15 * | Many bacterial proteins | [85] | [175] | ||
| Polymeric immunoglobulin receptor (PIgR) | Ig-mediated adhesion, direction interaction via adhesin | [86][87] | [176,177] | ||
| Prolactin-inducible protein (mPIP) | - | [88][89] | [178,179] | ||
| Peptidoglycan recognition protein 1 | - | [90] | [180] | ||
| Protein disulfide-isomerase (PDI) | - | [91] | [181] | ||
| Toll-like receptor 4, 2 | Many bacterial proteins | [92][93][94][95] | [182,183,184,185] | ||
| Uromodulin | Surface layer protein A, FimH | [96] | [186 | [97] | ,187] |
| Vimentin | Many bacterial proteins | [98] | [188] | ||
| Vitronectin | Many bacterial proteins | [99] | [189] | ||
| Zymogen granule protein 16 homolog B | LTA, peptidoglycan | [100] | [190] | ||
| β-lactoglobulin | Spa pili | [101][102] | [191,192] |
Besides proteins, a strain-specific adhesion between milk phospholipids (MPLs) and lactic acid bacteria (LAB) has been shown [106][107]. D’Incecco et al. showed that in the case of the presence of
Clostridium tyrobutyricum spores in raw milk, these spores can be localized at the proximity of MFGs [108]. Like bacteria, the spore’s surface is decorated by polysaccharides and anchored extracellular appendages that mediate lectin–carbohydrate interactions [109][110]. However, the surface structure of
Clostridium tyrobutyricum
spores involved in the association with MFG was not identified in the study. Interestingly, further experiments conducted by D’Incecco et al. used transmission electron microscopy (TEM) to show that
Milk provides not only nutrients but also protection to newborns through immunocompetent cells, antimicrobial peptides, oligosaccharides, immunoglobulins (Igs), cytokines, growth factors, and lysosomes [112]. Bovine MFGs contain numerous immune-related proteins including proteins with bacterial binding capacities. Immune proteins are well characterized and known to recognize specific epitopes on pathogens. Immunoglobulins and immune cells in milk reflect the mother’s pathogen exposure and can provide immunity against some pathogens. Studies have shown that IgA, secreted-IgA (sIgA), and IgM are concentrated in the cream layer and can adsorb onto human [113][114] or bovine [108] MFGM surfaces. These adsorbed Igs may act as mediators of bacterial adherence to MFGs. Other studies have demonstrated the efficacy of bovine Igs against various human pathogens related to diarrhea [115][116][117]. Antibodies against pathogenic
Milk provides not only nutrients but also protection to newborns through immunocompetent cells, antimicrobial peptides, oligosaccharides, immunoglobulins (Igs), cytokines, growth factors, and lysosomes [200]. Bovine MFGs contain numerous immune-related proteins including proteins with bacterial binding capacities. Immune proteins are well characterized and known to recognize specific epitopes on pathogens. Immunoglobulins and immune cells in milk reflect the mother’s pathogen exposure and can provide immunity against some pathogens. Studies have shown that IgA, secreted-IgA (sIgA), and IgM are concentrated in the cream layer and can adsorb onto human [201,202] or bovine [90] MFGM surfaces. These adsorbed Igs may act as mediators of bacterial adherence to MFGs. Other studies have demonstrated the efficacy of bovine Igs against various human pathogens related to diarrhea [203,204,205]. Antibodies against pathogenic
E. coli are common in samples of human milk [118][119]. Several studies have also shown that bovine colostrum contains antibodies to
E. coli O157:H7 and other pathogens, regardless of whether the animals were immunized (vaccinated) or not. These antibodies can confer protection against relevant pathogens to humans [120][121][122]. Oliveira et al. showed that Igs could interact with ETEC fimbrial proteins and block adhesion to host receptors [123]. It has also been reported that K88-positive
O157:H7 and other pathogens, regardless of whether the animals were immunized (vaccinated) or not. These antibodies can confer protection against relevant pathogens to humans [208,209,210]. Oliveira et al. showed that Igs could interact with ETEC fimbrial proteins and block adhesion to host receptors [211]. It has also been reported that K88-positive
The spontaneous agglutination of MFGs in cold milk is due to the presence of immunoglobulins, called cryoglobulins [125]. Cryoglobulins are large molecules that precipitate at low temperatures (<37 °C) and disperse again on warming. Cryoglobulins are probably involved in bacterial clarification during natural creaming [126]. Immunoglobulin cell receptors are present on both the bacterial surface and MFGMs and, therefore, could act as a bridge. A generic IgG receptor is present in cold-stored MFGM preparations, but bacterial interaction has not been shown [127]. The polymeric immunoglobulin receptor (pIgR) is present on intestinal epithelial cells and facilitates the transcytosis of Igs, especially IgA, and immune complexes [87][128].
Toll-like receptors 2 and 4 (TLR2 and TLR4), which recognize foreign antigens, are present at low levels on MFGMs [103]. For example, FimH, the adhesive tip from the Type 1 fimbriae of
The spontaneous agglutination of MFGs in cold milk is due to the presence of immunoglobulins, called cryoglobulins [213]. Cryoglobulins are large molecules that precipitate at low temperatures (<37 °C) and disperse again on warming. Cryoglobulins are probably involved in bacterial clarification during natural creaming [214]. Immunoglobulin cell receptors are present on both the bacterial surface and MFGMs and, therefore, could act as a bridge. A generic IgG receptor is present in cold-stored MFGM preparations, but bacterial interaction has not been shown [215]. The polymeric immunoglobulin receptor (pIgR) is present on intestinal epithelial cells and facilitates the transcytosis of Igs, especially IgA, and immune complexes [177,216].
Toll-like receptors 2 and 4 (TLR2 and TLR4), which recognize foreign antigens, are present at low levels on MFGMs [33]. For example, FimH, the adhesive tip from the Type 1 fimbriae of
E. coli, binds to mannose, TLR4, and CD48 [93][129]. Furthermore, TLR2 recognizes lipoteichoic acid (LTA), peptidoglycan, lipoprotein, curli, and other pathogen-associated molecular patterns (PAMPs) [94][95][130]. CD36 is a scavenger receptor that binds lipopolysaccharide (LPS) and other ligands [131]. Cathelicidins are antimicrobial peptides that can bind LPS [66]. Peptidoglycan recognition protein 1 (PRP1) is an antibacterial protein that can kill Gram-positive bacteria by binding to peptidoglycans and interfering with peptidoglycan biosynthesis [90].
, binds to mannose, TLR4, and CD48 [183,217]. Furthermore, TLR2 recognizes lipoteichoic acid (LTA), peptidoglycan, lipoprotein, curli, and other pathogen-associated molecular patterns (PAMPs) [184,185,218]. CD36 is a scavenger receptor that binds lipopolysaccharide (LPS) and other ligands [219]. Cathelicidins are antimicrobial peptides that can bind LPS [157]. Peptidoglycan recognition protein 1 (PRP1) is an antibacterial protein that can kill Gram-positive bacteria by binding to peptidoglycans and interfering with peptidoglycan biosynthesis [180].
2. Consequences of the STEC–MFG Association
2.1. Difficulties in Detecting STEC in Raw Milk Products
STEC detection in food matrices classically relies on four different steps: sample preparation; enrichment; detection; and confirmation by bacterial isolation. The enrichment step consists of adding an enrichment broth to the matrix to enable growth of the target bacteria. In the detection step, a genetic method is implemented to detect the presence of target bacteria by PCR screening. Finally, the confirmation step is carried out. This confirmation is based on isolation of target bacteria grown on selective media. Immunoconcentration tools using magnetic beads spiked with antibodies can also be used in this step. The ISO TS13136:2012 is the standard currently used to detect and isolate STEC belonging to O157, O26, O103, O111, and O145 serogroups and carrying
2.1. Difficulties in Detecting STEC in Raw Milk Products
STEC detection in food matrices classically relies on four different steps: sample preparation; enrichment; detection; and confirmation by bacterial isolation. The enrichment step consists of adding an enrichment broth to the matrix to enable growth of the target bacteria. In the detection step, a genetic method is implemented to detect the presence of target bacteria by PCR screening. Finally, the confirmation step is carried out. This confirmation is based on isolation of target bacteria grown on selective media. Immunoconcentration tools using magnetic beads spiked with antibodies can also be used in this step. The ISO TS13136:2012 is the standard currently used to detect and isolate STEC belonging to O157, O26, O103, O111, and O145 serogroups and carrying
eae
and
stx
genes in food samples.
STEC detection in raw milk cheeses is particularly challenging. First, bacterial DNA is extracted from a specific volume of enrichment broth. Then, STEC target genes (
eae
, stx, and genes encoding one of the five somatic antigens) are detected. However, the microflora of cheese contains bacteria that carry some of the genes used to screen for STEC. For example, some non-STEC strains of
E. coli
(such as enteropathogenic
E. coli
(EPEC)) carry the
eae
gene or contain phages carrying the
stx
gene. Some other bacterial strains can also carry the
stx
gene, including
Citrobacter freundi
,
Shigella
spp.,
Acinetobacter
,
Aeromonas
spp.,
Hafnia alvei
,
Escherichia albertii
, Escherichia cloacae, and enterotoxigenic
E. coli (ETEC) [132].The presence of these bacteria in raw milk cheese can lead to positive PCR results even though STEC isolates are not present in the enrichment broth (false positives). Furthermore, the performance of the methods (LOD) varies depending on the methods used and the matrices analyzed.
In general, STEC detection is more difficult in cheese than in meat. The LODs of the different detection methods are approximately 5–10 cells/g and 10–50 cells/g for bovine meat and raw milk cheese, respectively [133][134]. The presence of a richer flora and a higher amount of fat in cheese compared with meat may be an explanation. As discussed above, bacteria are preferentially found in contact with MFGs in raw milk products; however, the available detection kits perform DNA extraction on the pelleted fraction. Moreover, MFGs can interfere with DNA extraction methods by blocking spin column filters and acting as a PCR inhibitor [135][136]. Lower efficiency of bacterial DNA extraction can lead to false negative PCR results from enrichment broth samples. Several authors have described this phenomenon for various milk origins and suggest performing the extraction on both the cream and pelleted fraction. Sun et al. showed that cream harbors bacterial species that may be underestimated when skimmed milk, rather than whole milk, is used for DNA extraction [137]. Stinson et al. showed that a significant amount of human and bacterial cells remains with the cream and that bacterial DNA profiles can vary between fractions, especially for staphylococcal species [136]. The authors suggested that high-speed centrifugation may be insufficient to pellet bacterial or eukaryotic cells from milk. Furthermore, MFGs and proteins such as caseins can disrupt the interaction between immunomagnetic beads and STEC during the confirmation step. Tween 20 can be added at this stage to improve sample homogenization and block non-specific interactions [138].
Finally, STEC isolation to confirm the presence of the bacteria is also very difficult in raw milk cheese because the cheese microbiota limits STEC growth on agar plates. In addition, the different challenges encountered during cheese processing as well as the stresses suffered by STEC during the detection protocol can lead to viable but nonculturable (VBNC) isolates [139].
These studies emphasize the importance of using whole milk instead of skimmed milk for DNA extraction, but MFGs can perturb downstream applications. To improve the recovery rate of STEC in raw milk products, it seems essential to identify the nature of the STEC–MFG association in order to dissociate the two before performing the detection process. The identification of milk components involved in PCR inhibition and the improvement of DNA purification methods would allow the development of new kits to extract bacterial DNA from milk and cream. It should be noted that, despite these limitations, available DNA kits are still very effective. Quigley et al. showed that commercial kits provided very pure DNA suitable for PCR amplification from raw milk and raw milk cheese [140]. Furthermore, ISO 6887 recommends the addition of Tween 80 when enriching high-fat matrix to improve detection and bacterial isolation. However, no formal study has shown a significant effect due to the addition of Tween 80. Finally, at the enrichment temperature (37 °C or 41.5 °C), the lipids form a surface layer that may contain the desired bacterial cells and contribute to the reduction of available oxygen and thus modify the physiological state of the bacteria (
(ETEC) [44].The presence of these bacteria in raw milk cheese can lead to positive PCR results even though STEC isolates are not present in the enrichment broth (false positives). Furthermore, the performance of the methods (LOD) varies depending on the methods used and the matrices analyzed.
In general, STEC detection is more difficult in cheese than in meat. The LODs of the different detection methods are approximately 5–10 cells/g and 10–50 cells/g for bovine meat and raw milk cheese, respectively [220,221]. The presence of a richer flora and a higher amount of fat in cheese compared with meat may be an explanation. As discussed above, bacteria are preferentially found in contact with MFGs in raw milk products; however, the available detection kits perform DNA extraction on the pelleted fraction. Moreover, MFGs can interfere with DNA extraction methods by blocking spin column filters and acting as a PCR inhibitor [222,223]. Lower efficiency of bacterial DNA extraction can lead to false negative PCR results from enrichment broth samples. Several authors have described this phenomenon for various milk origins and suggest performing the extraction on both the cream and pelleted fraction. Sun et al. showed that cream harbors bacterial species that may be underestimated when skimmed milk, rather than whole milk, is used for DNA extraction [96]. Stinson et al. showed that a significant amount of human and bacterial cells remains with the cream and that bacterial DNA profiles can vary between fractions, especially for staphylococcal species [223]. The authors suggested that high-speed centrifugation may be insufficient to pellet bacterial or eukaryotic cells from milk. Furthermore, MFGs and proteins such as caseins can disrupt the interaction between immunomagnetic beads and STEC during the confirmation step. Tween 20 can be added at this stage to improve sample homogenization and block non-specific interactions [224].
Finally, STEC isolation to confirm the presence of the bacteria is also very difficult in raw milk cheese because the cheese microbiota limits STEC growth on agar plates. In addition, the different challenges encountered during cheese processing as well as the stresses suffered by STEC during the detection protocol can lead to viable but nonculturable (VBNC) isolates [225].
These studies emphasize the importance of using whole milk instead of skimmed milk for DNA extraction, but MFGs can perturb downstream applications. To improve the recovery rate of STEC in raw milk products, it seems essential to identify the nature of the STEC–MFG association in order to dissociate the two before performing the detection process. The identification of milk components involved in PCR inhibition and the improvement of DNA purification methods would allow the development of new kits to extract bacterial DNA from milk and cream. It should be noted that, despite these limitations, available DNA kits are still very effective. Quigley et al. showed that commercial kits provided very pure DNA suitable for PCR amplification from raw milk and raw milk cheese [226]. Furthermore, ISO 6887 recommends the addition of Tween 80 when enriching high-fat matrix to improve detection and bacterial isolation. However, no formal study has shown a significant effect due to the addition of Tween 80. Finally, at the enrichment temperature (37 °C or 41.5 °C), the lipids form a surface layer that may contain the desired bacterial cells and contribute to the reduction of available oxygen and thus modify the physiological state of the bacteria (
Figure 1) .
2) .
Figure 1 brings together the different concepts discussed in this section.
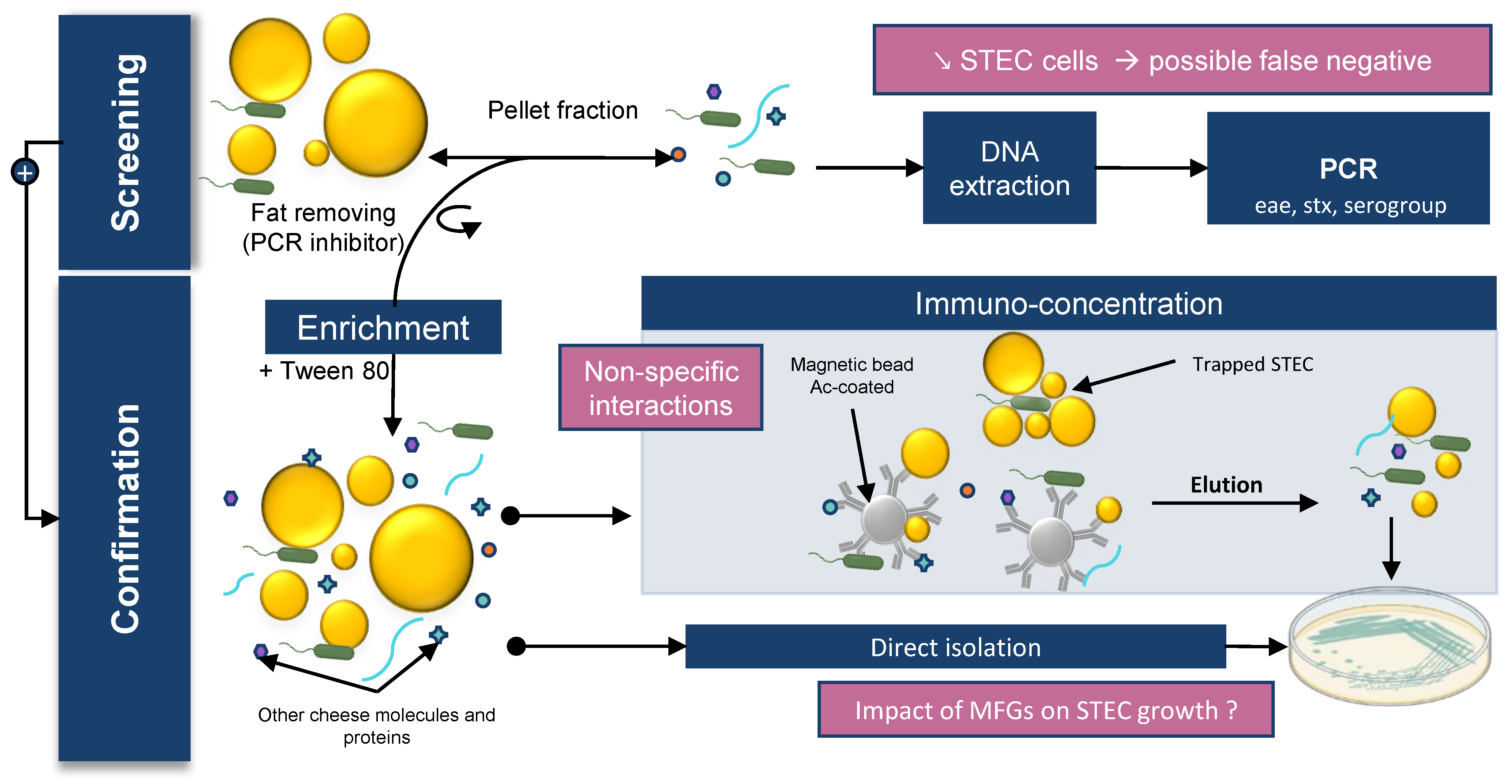
2 brings together the different concepts discussed in this section.

Figure 12. Impact of MFGs on STEC detection in dairy matrix. STEC detection in food matrices classically relies on 4 different steps: sample preparation; enrichment; detection; and confirmation by bacterial isolation.
2.2. Impact of Creaming on the Presence of STEC in Milk
One of the industry’s goals should be a non-invasive method to eliminate pathogenic bacteria in raw milk without affecting the nutritional qualities and raw milk microbiota of the final product. Many techniques exist, such as bactofugation and microfiltration, [141], but these techniques affect MFG structure [142] and also remove the raw milk microflora. In lab, a raw milk skimming assay was performed by electric centrifuge, and
Impact of MFGs on STEC detection in dairy matrix. STEC detection in food matrices classically relies on 4 different steps: sample preparation; enrichment; detection; and confirmation by bacterial isolation.
2.2. Impact of Creaming on the Presence of STEC in Milk
One of the industry’s goals should be a non-invasive method to eliminate pathogenic bacteria in raw milk without affecting the nutritional qualities and raw milk microbiota of the final product. Many techniques exist, such as bactofugation and microfiltration, [227], but these techniques affect MFG structure [82] and also remove the raw milk microflora. In our lab, we performed a raw milk skimming assay by electric centrifuge, and
E. coli were not found in significant numbers in the cream fraction [143]. Stronger centrifugal forces are applied by the centrifuge; therefore, the STEC–MFG association is probably too weak to overcome the centrifugal forces. However, it was reported that natural creaming produces reduced-fat milk with a lower bacterial count and fewer somatic cells [144]. MFGs spontaneously rise to the surface due to the difference in density between MFGs and the aqueous phase (Stokes’ law) [145]. As previously discussed, STEC were predominantly found in the cream layer after raw milk creaming [47]. Therefore, performing natural creaming methods before cheese transformation could decrease the level of STEC in the final product. However, no study has been conducted in experimental field conditions (with low STEC contamination levels).
2.3. Anti-Adhesive Strategies
As bacterial adhesion is the first step of infection, inhibiting this step is a key strategy for infection control. Competition for the natural binding sites of pathogenic bacteria by mimetic receptors could inhibit pathogen attachment. Several natural food components could act as efficient inhibitors of pathogen adherence [2][146][147][148][149], especially milk components [4][150]. Moreover, numerous experimental studies have shown that the association of bacteria with MFGs could prevent the adhesion of several enteropathogens to enterocytes through mimetic receptors [51][82][151][152][153][154][155][156]. To avoid STEC adhesion to the epithelium of the intestinal mucosa, the STEC–MFG complex must be maintained at the site of STEC adhesion. Therefore, the expression of STEC genes involved in adhesion must be able to occur in product and during the human digestive process.
MFGM glycoconjugates are the main macromolecules involved in the anti-adhesive properties of milk against enteropathogens [4][157]. The MFGM protein fraction shows similarities to intestinal cells. Major MFGM proteins are conserved between species, although there are variations in protein expression levels and molecular functions between species and stages of lactation [158]. The MFGM was recently recognized as a high value-added ingredient, and the valorization of this by-product is constantly increasing. The MFGM, or some of its components, are added to infant milk formulas because of the MFGM’s beneficial properties [158][159][160][161][162]. More studies should be conducted to identify the MFGM surface glyco-epitopes recognized by STEC. These data could lead to pharmaceutical development of a specific drug to treat STEC infections. Currently available therapeutic solutions for STEC are controversial.
were not found in significant numbers in the cream fraction [228]. Stronger centrifugal forces are applied by the centrifuge; therefore, the STEC–MFG association is probably too weak to overcome the centrifugal forces. However, it was reported that natural creaming produces reduced-fat milk with a lower bacterial count and fewer somatic cells [229]. MFGs spontaneously rise to the surface due to the difference in density between MFGs and the aqueous phase (Stokes’ law) [230]. As previously discussed, STEC were predominantly found in the cream layer after raw milk creaming [88]. Therefore, performing natural creaming methods before cheese transformation could decrease the level of STEC in the final product. However, no study has been conducted in experimental field conditions (with low STEC contamination levels).
2.3. Anti-Adhesive Strategies
As bacterial adhesion is the first step of infection, inhibiting this step is a key strategy for infection control. Competition for the natural binding sites of pathogenic bacteria by mimetic receptors could inhibit pathogen attachment. Several natural food components could act as efficient inhibitors of pathogen adherence [30,100,231,232,233], especially milk components [29,234]. Moreover, numerous experimental studies have shown that the association of bacteria with MFGs could prevent the adhesion of several enteropathogens to enterocytes through mimetic receptors [142,172,235,236,237,238,239,240]. To avoid STEC adhesion to the epithelium of the intestinal mucosa, the STEC–MFG complex must be maintained at the site of STEC adhesion. Therefore, the expression of STEC genes involved in adhesion must be able to occur in product and during the human digestive process.
MFGM glycoconjugates are the main macromolecules involved in the anti-adhesive properties of milk against enteropathogens [29,73]. The MFGM protein fraction shows similarities to intestinal cells. Major MFGM proteins are conserved between species, although there are variations in protein expression levels and molecular functions between species and stages of lactation [241]. The MFGM was recently recognized as a high value-added ingredient, and the valorization of this by-product is constantly increasing. The MFGM, or some of its components, are added to infant milk formulas because of the MFGM’s beneficial properties [241,242,243,244,245]. More studies should be conducted to identify the MFGM surface glyco-epitopes recognized by STEC. These data could lead to pharmaceutical development of a specific drug to treat STEC infections. Currently available therapeutic solutions for STEC are controversial.
