The presence of food contaminants can cause foodborne illnesses, posing a severe threat to human health. Therefore, a rapid, sensitive, and convenient method for monitoring food contaminants is eagerly needed. The complex matrix interferences of food samples and poor performance of existing sensing probes bring significant challenges to improving detection performances. Nanocomposites with multifunctional features provide a solution to these problems. The combination of the superior characteristics of magnetic nanoparticles (MNPs) and quantum dots (QDs) to fabricate magnetic fluorescent quantum dots (MNPs@QDs) nanocomposites are regarded as an ideal multifunctional probe for food contaminants analysis.
- MNPs@QDs
1. Introduction
Currently, the preparation methods of MNPs@QDs have been reported with numerous literature [1][2]. The key points to consider in the reasonable integration of MNPs and QDs into multifunctional MNPs@QDs nanocomposites is whether the two components are firmly combined, and interact without causing loss of magnetic and fluorescent performance. Furthermore, its properties such as dispersibility, stability, biocompatibility and desirable surface functionalization are also related to its practical applications. Although the compositions and morphologies of these nanocomposites perform differently, most strategies can be divided into five categories: heterocrystalline growth, template embedding, LBL assembly, microemulsion technique and one-pot method.
2. Hetero-Crystalline Growth
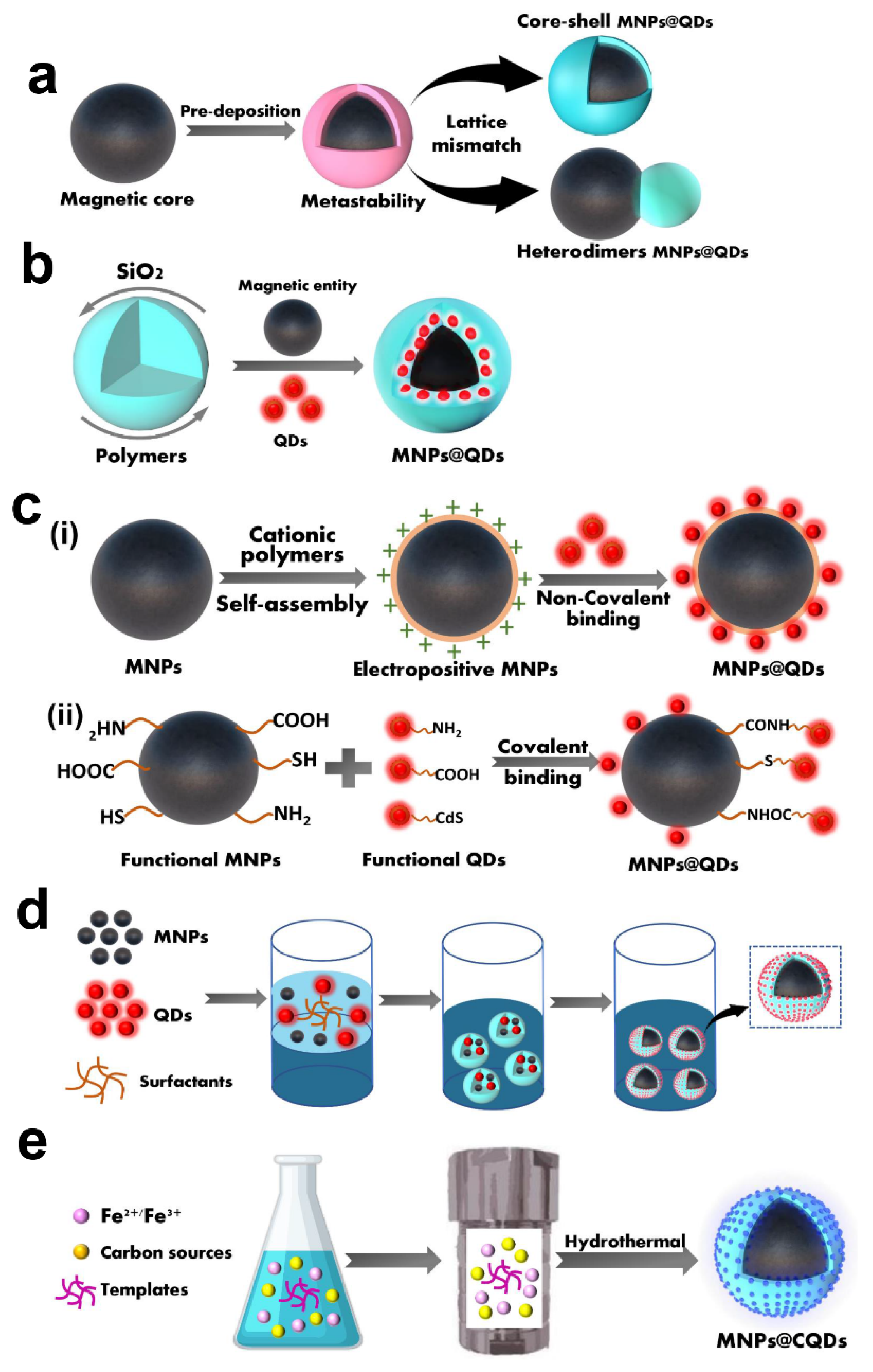
3. Template Embedding
3. Template Embedding
4. Layer-by-Layer Assembly
4. Layer-by-Layer Assembly
5. Microemulsion Technique
5. Microemulsion Technique
6. One-Pot Method
6. One-Pot Method
References
- Mahajan, K.D.; Fan, Q.; Dorcena, J.; Ruan, G.; Winter, J.O. Magnetic quantum dots in biotechnology–synthesis and applications. Biotechnol. J. 2013, 8, 1424–1434.
- Jing, L.; Ding, K.; Kershaw, S.V.; Kempson, I.M.; Rogach, A.L.; Gao, M. Magnetically Engineered Semiconductor Quantum Dots as Multimodal Imaging Probes. Adv. Mater. 2014, 26, 6367–6386.
- Kim, H.; Achermann, M.; Balet, L.P.; Hollingsworth, J.A.; Klimov, V.I. Synthesis and characterization of Co/CdSe core/shell nanocomposites: Bifunctional magnetic-optical nanocrystals. J. Am. Chem. Soc. 2005, 127, 544–546.
- Zhou, S.; Chen, Q.; Hu, X.; Zhao, T. Bifunctional luminescent superparamagnetic nanocomposites of CdSe/CdS-Fe3O4 synthesized via a facile method. J. Mater. Chem. 2012, 22, 8263–8270.
- Bhandari, S.; Khandelia, R.; Pan, U.N.; Chattopadhyay, A. Surface Complexation-Based Biocompatible Magnetofluorescent Nanoprobe for Targeted Cellular Imaging. ACS Appl. Mater. Interfaces 2015, 7, 17552–17557.
- Wu, Y.; Zou, H.; Zhang, Y.; Mou, M.; Niu, Q.; Yan, Z.; Liao, S. The Loading of Luminescent Magnetic Nanocomposites Fe3O4@Polyaniline/Carbon Dots for Methotrexate and Its Release Behavior In Vitro. J. Nanosci. Nanotechnol. 2020, 20, 701–708.
- Lee, J.-S.; Bodnarchuk, M.I.; Shevchenko, E.V.; Talapin, D.V. “Magnet-in-the-Semiconductor” FePt-PbS and FePt-PbSe Nanostructures: Magnetic Properties, Charge Transport, and Magnetoresistance. J. Am. Chem. Soc. 2010, 132, 6382–6391.
- Gu, H.W.; Zheng, R.K.; Zhang, X.X.; Xu, B. Facile one-pot synthesis of bifunctional heterodimers of nanoparticles: A conjugate of quantum dot and magnetic nanoparticles. J. Am. Chem. Soc. 2004, 126, 5664–5665.
- Selvan, S.T.; Patra, P.K.; Ang, C.Y.; Ying, J.Y. Synthesis of silica-coated semiconductor and magnetic quantum dots and their use in the imaging of live cells. Angew. Chem. Int. Ed. 2007, 46, 2448–2452.
- Lin, A.W.H.; Ang, C.Y.; Patra, P.K.; Han, Y.; Gu, H.; Le Breton, J.-M.; Juraszek, J.; Chiron, H.; Papaefthymiou, G.C.; Selvan, S.T.; et al. Seed-mediated synthesis, properties and application of gamma-Fe2O3-CdSe magnetic quantum dots. J. Solid State Chem. 2011, 184, 2150–2158.
- McDaniel, H.; Shim, M. Size and Growth Rate Dependent Structural Diversification of Fe3O4/CdS Anisotropic Nanocrystal Heterostructures. ACS Nano 2009, 3, 434–440.
- Gao, J.; Zhang, W.; Huang, P.; Zhang, B.; Zhang, X.; Xu, B. Intracellular spatial control of fluorescent magnetic nanoparticles. J. Am. Chem. Soc. 2008, 130, 3710–3711.
- Du, G.H.; Liu, Z.L.; Lu, Q.H.; Xia, X.; Jia, L.H.; Yao, K.L.; Chu, Q.; Zhang, S.M. Fe3O4/CdSe/ZnS magnetic fluorescent bifunctional nanocomposites. Nanotechnology 2006, 17, 2850–2854.
- Hines, M.A.; Guyot-Sionnest, P. Synthesis and characterization of strongly luminescing ZnS-Capped CdSe nanocrystals. J. Phys. Chem. 1996, 100, 468–471.
- Deng, S.; Ruan, G.; Han, N.; Winter, J.O. Interactions in fluorescent-magnetic heterodimer nanocomposites. Nanotechnology 2010, 21, 145605.
- Beaune, G.; Dubertret, B.; Clement, O.; Vayssettes, C.; Cabuil, V.; Menager, C. Giant vesicles containing magnetic nanoparticles and quantum dots: Feasibility and tracking by fiber confocal fluorescence microscopy. Angew. Chem. Int. Ed. 2007, 46, 5421–5424.
- Ruan, G.; Vieira, G.; Henighan, T.; Chen, A.; Thakur, D.; Sooryakumar, R.; Winter, J.O. Simultaneous Magnetic Manipulation and Fluorescent Tracking of Multiple Individual Hybrid Nanostructures. Nano Lett. 2010, 10, 2220–2224.
- Park, J.-H.; von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Micellar hybrid nanoparticles for simultaneous magnetofluorescent imaging and drug delivery. Angew. Chem. Int. Ed. 2008, 47, 7284–7288.
- Kim, J.; Lee, J.E.; Lee, J.; Yu, J.H.; Kim, B.C.; An, K.; Hwang, Y.; Shin, C.H.; Park, J.G.; Kim, J.; et al. Magnetic fluorescent delivery vehicle using uniform mesoporous silica spheres embedded with monodisperse magnetic and semiconductor nanocrystals. J. Am. Chem. Soc. 2006, 128, 688–689.
- Sun, L.; Zang, Y.; Sun, M.D.; Wang, H.G.; Zhu, X.J.; Xu, S.F.; Yang, Q.B.; Li, Y.X.; Shan, Y.M. Synthesis of magnetic and fluorescent multifunctional hollow silica nanocomposites for live cell imaging. J. Colloid Interface Sci. 2010, 350, 90–98.
- Xiao, Q.; Xiao, C. Preparation and Characterization of Silica-Coated Magnetic-Fluorescent Bifunctional Microspheres. Nanoscale Res. Lett. 2009, 4, 1078–1084.
- Yi, D.K.; Selvan, S.T.; Lee, S.S.; Papaefthymiou, G.C.; Kundaliya, D.; Ying, J.Y. Silica-coated nanocomposites of magnetic nanoparticles and quantum dots. J. Am. Chem. Soc. 2005, 127, 4990–4991.
- Ruan, J.; Wang, K.; Song, H.; Xu, X.; Ji, J.J.; Cui, D.X. Biocompatibility of hydrophilic silica-coated CdTe quantum dots and magnetic nanoparticles. Nanoscale Res. Lett. 2011, 6, 13.
- Kyeong, S.; Jeong, C.; Kim, H.Y.; Hwang, D.W.; Kang, H.; Yang, J.-K.; Lee, D.S.; Jun, B.-H.; Lee, Y.-S. Fabrication of mono-dispersed silica-coated quantum dot-assembled magnetic nanoparticles. RSC Adv. 2015, 5, 32072–32077.
- Huang, L.; Zhang, Y.; Liao, T.; Xu, K.; Jiang, C.; Zhuo, D.; Wang, Y.; Wen, H.-M.; Wang, J.; Ao, L.; et al. Compact Magneto-Fluorescent Colloids by Hierarchical Assembly of Dual-Components in Radial Channels for Sensitive Point-of-Care Immunoassay. Small 2021, 17, 2100862.
- Tan, Y.F.; Chandrasekharan, P.; Maity, D.; Yong, C.X.; Chuang, K.-H.; Zhao, Y.; Wang, S.; Ding, J.; Feng, S.-S. Multimodal tumor imaging by iron oxides and quantum dots formulated in poly (lactic acid)-D-alpha-tocopheryl polyethylene glycol 1000 succinate nanoparticles. Biomaterials 2011, 32, 2969–2978.
- Xie, H.Y.; Zuo, C.; Liu, Y.; Zhang, Z.L.; Pang, D.W.; Li, X.L.; Gong, J.P.; Dickinson, C.; Zhou, W.Z. Cell-targeting multifunctional nanospheres with both fluorescence and magnetism. Small 2005, 1, 506–509.
- Li, Y.-H.; Song, T.; Liu, J.-Q.; Zhu, S.-J.; Chang, J. An efficient method for preparing high-performance multifunctional polymer beads simultaneously incorporated with magnetic nanoparticles and quantum dots. J. Mater. Chem. 2011, 21, 12520–12528.
- Wilson, R.; Spiller, D.G.; Prior, I.A.; Veltkamp, K.J.; Hutchinson, A. A Simple Method for Preparing Spectrally Encoded Magnetic Beads for Multiplexed Detection. ACS Nano 2007, 1, 487–493.
- Wang, C.; Wang, L.; Yang, W. Preparation and characterization of functional inorganic/organic composite microspheres via electrostatic interaction. J. Colloid Interface Sci. 2009, 333, 749–756.
- Li, L.; Choo, E.S.G.; Liu, Z.; Ding, J.; Xue, J. Double-layer silica core-shell nanospheres with superparamagnetic and fluorescent functionalities. Chem. Phys. Lett. 2008, 461, 114–117.
- Yin, N.; Wang, X.; Yang, T.; Ding, Y.; Li, L.; Zhao, S.; Li, P.; Xu, X.; Zhu, L. Multifunctional Fe3O4 quantum dot-embedded mesoporous SiO2 nanoplatform probe for cancer cell fluorescence-labelling detection and photothermal therapy. Ceram. Int. 2021, 47, 8271–8278.
- Yoo, J.H.; Kim, J.S. The Preparation of Core-Shell Magnetic Silica Nanospheres for Enhancing Magnetism and Fluorescence Intensity. J. Nanosci. Nanotechnol. 2013, 13, 7615–7619.
- Sathe, T.R.; Agrawal, A.; Nie, S. Mesoporous silica beads embedded with semiconductor quantum dots and iron oxide nanocrystals: Dual-function microcarriers for optical encoding and magnetic separation. Anal. Chem. 2006, 78, 5627–5632.
- Xie, H.-Y.; Xie, M.; Zhang, Z.-L.; Long, Y.-M.; Liu, X.; Tang, M.-L.; Pang, D.-W.; Tan, Z.; Dickinson, C.; Zhou, W. Wheat germ agglutinin-modified trifunctional nanospheres for cell recognition. Bioconjugate Chem. 2007, 18, 1749–1755.
- Zebli, B.; Susha, A.S.; Sukhorukov, G.B.; Rogach, A.L.; Parak, W.J. Magnetic Targeting and Cellular Uptake of Polymer Microcapsules Simultaneously Functionalized with Magnetic and Luminescent Nanocrystals. Langmuir 2005, 21, 4262–4265.
- Li, Z.; Wang, G.; Shen, Y.; Guo, N.; Ma, N. DNA-Templated Magnetic Nanoparticle-Quantum Dot Polymers for Ultrasensitive Capture and Detection of Circulating Tumor Cells. Adv. Funct. Mater. 2018, 28, 1707152.
- Zou, W.-S.; Yang, J.; Yang, T.-T.; Hu, X.; Lian, H.-Z. Magnetic-room temperature phosphorescent multifunctional nanocomposites as chemosensor for detection and photo-driven enzyme mimetics for degradation of 2,4,6-trinitrotoluene. J. Mater. Chem. 2012, 22, 4720–4727.
- Ortgies, D.H.; de la Cueva, L.; del Rosal, B.; Sanz-Rodríguez, F.; Fernández, N.; Iglesias-de la Cruz, M.C.; Salas, G.; Cabrera, D.; Teran, F.J.; Jaque, D.; et al. In Vivo Deep Tissue Fluorescence and Magnetic Imaging Employing Hybrid Nanostructures. ACS Appl. Mater. Interfaces 2016, 8, 1406–1414.
- Xiao, L.-H.; Wang, T.; Zhao, T.-Y.; Zheng, X.; Sun, L.-Y.; Li, P.; Liu, F.-Q.; Gao, G.; Dong, A. Fabrication of Magnetic-Antimicrobial-Fluorescent Multifunctional Hybrid Microspheres and Their Properties. Int. J. Mol. Sci. 2013, 14, 7391–7404.
- Fan, H.-M.; Olivo, M.; Shuter, B.; Yi, J.-B.; Bhuvaneswari, R.; Tan, H.-R.; Xing, G.-C.; Ng, C.-T.; Liu, L.; Lucky, S.S.; et al. Quantum Dot Capped Magnetite Nanorings as High Performance Nanoprobe for Multiphoton Fluorescence and Magnetic Resonance Imaging. J. Am. Chem. Soc. 2010, 132, 14803–14811.
- Hong, X.; Li, J.; Wang, M.J.; Xu, J.J.; Guo, W.; Li, J.H.; Bai, Y.B.; Li, T.J. Fabrication of magnetic luminescent nanocomposites by a layer-by-layer self-assembly approach. Chem. Mater. 2004, 16, 4022–4027.
- Wang, C.W.; Shen, W.Z.; Rong, Z.; Liu, X.X.; Gu, B.; Xiao, R.; Wang, S.Q. Layer-by-layer assembly of magnetic-core dual quantum dot-shell nanocomposites for fluorescence lateral flow detection of bacteria. Nanoscale 2020, 12, 795–807.
- Chen, B.; Zhang, H.; Zhai, C.; Du, N.; Sun, C.; Xue, J.; Yang, D.; Huang, H.; Zhang, B.; Xie, Q.; et al. Carbon nanotube-based magnetic-fluorescent nanohybrids as highly efficient contrast agents for multimodal cellular imaging. J. Mater. Chem. 2010, 20, 9895–9902.
- Sun, X.; Ding, K.; Hou, Y.; Gao, Z.; Yang, W.; Jing, L.; Gao, M. Bifunctional Superparticles Achieved by Assembling Fluorescent CuInS2@ZnS Quantum Dots and Amphibious Fe3O4 Nanocrystals. J. Phys. Chem. C 2013, 117, 21014–21020.
- Wang, D.; He, J.; Rosenzweig, N.; Rosenzweig, Z. Superparamagnetic Fe2O3 Beads−CdSe/ZnS Quantum Dots Core−Shell Nanocomposite Particles for Cell Separation. Nano Lett. 2004, 4, 409–413.
- Nikitin, M.P.; Zdobnova, T.A.; Lukash, S.V.; Stremovskiy, O.A.; Deyev, S.M. Protein-assisted self-assembly of multifunctional nanoparticles. Proc. Natl. Acad. Sci. USA 2010, 107, 5827.
- Shibu, E.S.; Ono, K.; Sugino, S.; Nishioka, A.; Yasuda, A.; Shigeri, Y.; Wakida, S.-i.; Sawada, M.; Biju, V. Photouncaging Nanoparticles for MRI and Fluorescence Imaging in Vitro and in Vivo. ACS Nano 2013, 7, 9851–9859.
- Song, E.; Han, W.; Xu, H.; Jiang, Y.; Cheng, D.; Song, Y.; Swihart, M.T. Magnetically Encoded Luminescent Composite Nanoparticles through Layer-by-Layer Self-Assembly. Chem.—Eur. J. 2014, 20, 14642–14649.
- Rong, Z.; Bai, Z.K.; Li, J.N.; Tang, H.; Shen, T.Y.; Wang, Q.; Wang, C.W.; Xiao, R.; Wang, S.Q. Dual-color magnetic-quantum dot nanobeads as versatile fluorescent probes in test strip for simultaneous point-of-care detection of free and complexed prostate-specific antigen. Biosens. Bioelectron. 2019, 145, 8.
- Wang, C.; Cheng, X.; Liu, L.; Zhang, X.; Yang, X.; Zheng, S.; Rong, Z.; Wang, S. Ultrasensitive and Simultaneous Detection of Two Specific SARS-CoV-2 Antigens in Human Specimens Using Direct/Enrichment Dual-Mode Fluorescence Lateral Flow Immunoassay. ACS Appl. Mater. Interfaces 2021, 13, 40342–40353.
- Dong, S.; Wang, S.; Wang, X.; Zhai, L. Superparamagnetic nanocomposite Fe3O4@SiO2-NH2/CQDs as fluorescent probe for copper (II) detection. Mater. Lett. 2020, 278, 128404.
- Zhang, X.; Tang, B.; Li, Y.; Liu, C.; Jiao, P.; Wei, Y. Molecularly Imprinted Magnetic Fluorescent Nanocomposite-Based Sensor for Selective Detection of Lysozyme. Nanomaterials 2021, 11, 1575.
- Li, C.-L.; Huang, B.-R.; Chang, J.-Y.; Chen, J.-K. Bifunctional superparamagnetic-luminescent core-shell-satellite structured microspheres: Preparation, characterization, and magnetodisplay application. J. Mater. Chem. C 2015, 3, 4603–4615.
- Koc, K.; Karakus, B.; Rajar, K.; Alveroglu, E. Synthesis and characterization. of 3O4 fluorescent-magnetic bifunctional nanospheres. Superlattices Microstruct. 2017, 110, 198–204.
- Wang, K.; Xu, X.; Li, Y.; Rong, M.; Wang, L.; Lu, L.; Wang, J.; Zhao, F.; Sun, B.; Jiang, Y. Preparation Fe3O4@chitosan-graphene quantum dots nanocomposites for fluorescence and magnetic resonance imaging. Chem. Phys. Lett. 2021, 783, 139060.
- Wang, Z.; Jiang, X.; Liu, W.; Lu, G.; Huang, X. A rapid and operator-safe powder approach for latent fingerprint detection using hydrophilic Fe3O4@SiO2-CdTe nanoparticles. Sci. China Chem. 2019, 62, 889–896.
- Wang, M.; Fei, X.F.; Lv, S.W.; Sheng, Y.; Zou, H.F.; Song, Y.H.; Yan, F.; Zhu, Q.L.; Zheng, K.Y. Synthesis and characterization of a flexible fluorescent magnetic Fe3O4@SiO2/CdTe-NH2 nanoprobe. J. Inorg. Biochem. 2018, 186, 307–316.
- Zhang, Y.; Wang, S.-N.; Ma, S.; Guan, J.-J.; Li, D.; Zhang, X.-D.; Zhang, Z.-D. Self-assembly multifunctional nanocomposites with Fe3O4 magnetic core and CdSe/ZnS quantum dots shell. J. Biomed. Mater. Res. Part A 2008, 85A, 840–846.
- Chen, O.; Riedemann, L.; Etoc, F.; Herrmann, H.; Coppey, M.; Barch, M.; Farrar, C.T.; Zhao, J.; Bruns, O.T.; Wei, H.; et al. Magneto-fluorescent core-shell supernanoparticles. Nat. Commun. 2014, 5, 5093.
- Piao, Y.; Burns, A.; Kim, J.; Wiesner, U.; Hyeon, T. Designed Fabrication of Silica-Based Nanostructured Particle Systems for Nanomedicine Applications. Adv. Funct. Mater. 2008, 18, 3745–3758.
- Guo, L.; Shao, Y.; Duan, H.; Ma, W.; Leng, Y.; Huang, X.; Xiong, Y. Magnetic Quantum Dot Nanobead-Based Fluorescent Immunochromatographic Assay for the Highly Sensitive Detection of Aflatoxin B1 in Dark Soy Sauce. Anal. Chem. 2019, 91, 4727–4734.
- Guo, S.; Chen, Y.-Q.; Lu, N.-N.; Wang, X.-Y.; Xie, M.; Sui, W.-P. Ultrasonication-assisted one-step self-assembly preparation of biocompatible fluorescent-magnetic nanobeads for rare cancer cell detection. Nanotechnology 2014, 25, 505603.
- Kim, J.; Lee, J.E.; Lee, S.H.; Yu, J.H.; Lee, J.H.; Park, T.G.; Hyeon, T. Designed fabrication of a multifunctional polymer nanomedical platform for simultaneous cancer-targeted imaging and magnetically guided drug delivery. Adv. Mater. 2008, 20, 478–483.
- Leng, Y.; Wu, W.; Li, L.; Lin, K.; Sun, K.; Chen, X.; Li, W. Magnetic/Fluorescent Barcodes Based on Cadmium-Free Near-Infrared-Emitting Quantum Dots for Multiplexed Detection. Adv. Funct. Mater. 2016, 26, 7581–7589.
- Lan, J.; Chen, J.; Li, N.; Ji, X.; Yu, M.; He, Z. Microfluidic generation of magnetic-fluorescent Janus microparticles for biomolecular detection. Talanta 2016, 151, 126–131.
- Zhou, C.; Wang, Z.; Xia, J.; Via, B.K.; Zhang, F.; Xia, Y.; Li, Y. A simplistic one-pot method to produce magnetic graphene-CdS nanocomposites. Comptes Rendus Chim. 2012, 15, 714–718.
- Maleki, S.; Madrakian, T.; Afkhami, A. Magnetic solid-phase extraction of codeine in a biological sample utilizing Fe3O4/CDs/Lys nanocomposite as an efficient adsorbent. J. Iran. Chem. Soc. 2019, 16, 2111–2121.
- Liu, X.; Jiang, H.; Ye, J.; Zhao, C.; Gao, S.; Wu, C.; Li, C.; Li, J.; Wang, X. Nitrogen-Doped Carbon Quantum Dot Stabilized Magnetic Iron Oxide Nanoprobe for Fluorescence, Magnetic Resonance, and Computed Tomography Triple-Modal In Vivo Bioimaging. Adv. Funct. Mater. 2016, 26, 8694–8706.
- Li, B.; Wang, X.; Guo, Y.; Iqbal, A.; Dong, Y.; Li, W.; Liu, W.; Qin, W.; Chen, S.; Zhou, X.; et al. One-pot synthesis of polyamines improved magnetism and fluorescence Fe3O4-carbon dots hybrid NPs for dual modal imaging. Dalton Trans. 2016, 45, 5484–5491.
- Irmania, N.; Dehvari, K.; Gedda, G.; Tseng, P.-J.; Chang, J.-Y. Manganese-doped green tea-derived carbon quantum dots as a targeted dual imaging and photodynamic therapy platform. J. Biomed. Mater. Res. Part B 2020, 108, 1616–1625.
- Ji, Z.; Ai, P.; Shao, C.; Wang, T.; Yan, C.; Ye, L.; Gu, W. Manganese-Doped Carbon Dots for Magnetic Resonance/Optical Dual-Modal Imaging of Tiny Brain Glioma. ACS Biomater. Sci. Eng. 2018, 4, 2089–2094.
- Yao, Y.-Y.; Gedda, G.; Girma, W.M.; Yen, C.-L.; Ling, Y.-C.; Chang, J.-Y. Magnetofluorescent Carbon Dots Derived from Crab Shell for Targeted Dual-Modality Bioimaging and Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 13887–13899.
- Liao, H.; Wang, Z.; Chen, S.; Wu, H.; Ma, X.; Tan, M. One-pot synthesis of gadolinium(III) doped carbon dots for fluorescence/magnetic resonance bimodal imaging. RSC Adv. 2015, 5, 66575–66581.
- Yu, C.; Xuan, T.; Chen, Y.; Zhao, Z.; Liu, X.; Lian, G.; Li, H. Gadolinium-doped carbon dots with high quantum yield as an effective fluorescence and magnetic resonance bimodal imaging probe. J. Alloys Compd. 2016, 688, 611–619.
- Wu, F.; Yue, L.; Yang, L.; Wang, K.; Liu, G.; Luo, X.; Zhu, X. Ln(III) chelates-functionalized carbon quantum dots: Synthesis, optical studies and multimodal bioimaging applications. Colloids Surf. B 2019, 175, 272–280.
