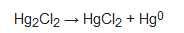Skin lightening is defined as "the practice of using chemicals or any other product with depigmenting potential in an attempt to lighten skin tone or improve the complexion; these goals are achieved by decreasing the concentration of melanin to achieve a reduction in the physiological pigmentation of the skin". Products used to achieve this purpose are known as depigmenting, skin-lightening, skin-bleaching, skin-brightening or skin-evening agents. Skin-whitening products containing highly toxic active ingredients (in particular mercury derivatives, hydroquinone and corticosteroids) are easily found on the market; the use of these depigmenting agents can be followed by a variety of adverse effects, with very serious and sometimes fatal complications, and is currently an emerging health concern in many countries.
1. Introduction
Melanins are polymorphous and multifunctional biopolymers that include eumelanin and pheomelanin; their synthesis in the skin, known as melanogenesis, occurs in melanocytes at the dermal-epidermal junction within intracellular organelles called melanosomes. Melanosomes are transferred via dendrites to the cytoplasm of surrounding keratinocytes, where they play a critical role in photoprotection. Melanogenesis is a complex process that comprises both enzymatic and chemical reactions and involves several enzymes: phenylalanine hydroxylase, tyrosinase (a glycosylated polyphenol oxidase containing copper), and tyrosine-related protein-1 (TRP-1) and TRP-2 (or dopamine tautomerase) [1]. Tyrosinase catalyses two distinct reactions in melanin biosynthesis: the hydroxylation of L-tyrosine to L-DOPA (L-3,4-dihydroxy-phenylalanine) and then the oxidation of L-DOPA to dopaquinone. Dopaquinone is highly reactive and, in the absence of thiol compounds, undergoes intramolecular cyclization, eventually leading to eumelanin; in the presence of thiols, such as cysteine or glutathione, it is converted to 5-S-cysteinyl DOPA or glutathionyl DOPA, eventually leading, through a complex series of reactions, to pheomelanin [1][2]. The pigments eumelanin (brown/black) and pheomelanin (red/yellow) both protect the skin from UV damage, although pheomelanin can also function as a powerful UV photosensitiser [3]. Melanins are polymorphous and multifunctional biopolymers that include eumelanin and pheomelanin; their synthesis in the skin, known as melanogenesis, occurs in melanocytes at the dermal-epidermal junction within intracellular organelles called melanosomes. Melanosomes are transferred via dendrites to the cytoplasm of surrounding keratinocytes, where they play a critical role in photoprotection. Melanogenesis is a complex process that comprises both enzymatic and chemical reactions and involves several enzymes: phenylalanine hydroxylase, tyrosinase (a glycosylated polyphenol oxidase containing copper), and tyrosine-related protein-1 (TRP-1) and TRP-2 (or dopamine tautomerase) [1]. Tyrosinase catalyses two distinct reactions in melanin biosynthesis: the hydroxylation of L-tyrosine to L-DOPA (L-3,4-dihydroxy-phenylalanine) and then the oxidation of L-DOPA to dopaquinone. Dopaquinone is highly reactive and, in the absence of thiol compounds, undergoes intramolecular cyclization, eventually leading to eumelanin; in the presence of thiols, such as cysteine or glutathione, it is converted to 5-S-cysteinyl DOPA or glutathionyl DOPA, eventually leading, through a complex series of reactions, to pheomelanin [1,2]. The pigments eumelanin (brown/black) and pheomelanin (red/yellow) both protect the skin from UV damage, although pheomelanin can also function as a powerful UV photosensitiser [3].
After UVB exposure, melanogenesis is induced by a variety of factors (cyclobutane pyrimidine dimers, alpha-melanocyte stimulating factor, stem cell factor, nitric oxide, adrenocorticotropic hormone or ACTH, endothelin-1) through different signaling pathways [1]; DNA repair also stimulates melanin production [4]. Melanin has a photoprotective effect by forming supranuclear caps in the cells of the human epidermis and thus reducing the formation of DNA photoproducts due to exposure to ultraviolet radiation; in addition, this pigment can chelate metal cations and exhibits antioxidant and radical-scavenging properties.
Skin lightening is defined as ‘the practice of using chemicals or any other product with depigmenting potential in an attempt to lighten skin tone or improve the complexion; these goals are achieved by decreasing the concentration of melanin to achieve a reduction in the physiological pigmentation of the skin’ [5]. Products used to achieve this purpose are known as depigmenting, skin-lightening, skin-bleaching, skin-brightening or skin-evening agents [6]. Several skin-lightening compounds have been developed and are now available for pharmaceutical and cosmetic purposes. Therapeutic indications for skin-lightening agents are generally aimed at the management of pigmentation disorders such as discolouration due to hormonal changes, melasma, age spots, senile/solar lentigo, post-inflammatory hyperpigmentation, and pigmented acne scars, as they reduce the hyperpigmentation of specific areas of the body and provide a more uniform skin colour. However, skin lightening is primarily a cosmetic procedure whose function is not only to lighten dark areas of the skin but also to achieve a generally lighter tone, particularly in countries where darker skin tones are prevalent and voluntary depigmentation meets the aesthetic criterion of a lighter skin [7].
Skin-lightening agents can operate through different mechanisms: the inhibition of tyrosinase transcription (e.g., tretinoin, retinol), the inhibition of tyrosinase (e.g., hydroquinone, azelaic acid, resveratrol), the acceleration of epidermal turnover (lactic acid, glycolic acid), the inhibition of melanosome transfer from melanocytes to surrounding keratinocytes, anti-inflammatory action and scavenging of free radicals, all of which have tyrosinase inhibition as their common goal [8][9]. Many effective depigmenting compounds available today can only be used as drugs (e.g., hydroquinone, retinoic acid) and should be restricted to use under dermatological supervision, while others are permitted in cosmetic products (e.g., alpha-hydroxy acids, arbutin, retinol, ascorbic acid). The development of new inhibitors of melanogenesis is of great importance in both the pharmaceutical and cosmetic industries. These inhibitors can originate from different sources, such as chemical synthesis and the screening of natural compounds and extracts [1]. In particular, the search for new whitening ingredients is driven by the demand for natural products, and traditional herbal derivatives are currently being investigated, as they are generally believed to be safer than synthetic compounds [5][9][10][11]. The different mechanisms of depigmenting agents legally authorised for medical and cosmetic purposes are summarised in Skin-lightening agents can operate through different mechanisms: the inhibition of tyrosinase transcription (e.g., tretinoin, retinol), the inhibition of tyrosinase (e.g., hydroquinone, azelaic acid, resveratrol), the acceleration of epidermal turnover (lactic acid, glycolic acid), the inhibition of melanosome transfer from melanocytes to surrounding keratinocytes, anti-inflammatory action and scavenging of free radicals, all of which have tyrosinase inhibition as their common goal [8,9]. Many effective depigmenting compounds available today can only be used as drugs (e.g., hydroquinone, retinoic acid) and should be restricted to use under dermatological supervision, while others are permitted in cosmetic products (e.g., alpha-hydroxy acids, arbutin, retinol, ascorbic acid). The development of new inhibitors of melanogenesis is of great importance in both the pharmaceutical and cosmetic industries. These inhibitors can originate from different sources, such as chemical synthesis and the screening of natural compounds and extracts [1]. In particular, the search for new whitening ingredients is driven by the demand for natural products, and traditional herbal derivatives are currently being investigated, as they are generally believed to be safer than synthetic compounds [5,9,10,11]. The different mechanisms of depigmenting agents legally authorised for medical and cosmetic purposes are summarised in Table 1
.
Mechanisms of action of legally authorised skin-lightening agents.
|
| Skin Lightening Agents |
|
| Mechanism of Action |
|
|
| Hydroquinone, Azelaic acid, Ellagic acid, Kojic acid, Mequinol, Arbutin, Flavonoids, Resveratrol, N-acetyl glucosamine |
|
| Inhibition of tyrosinase activity [9] |
|
|
| Alpha-hydroxy acids, salicylic acid |
|
| Acceleration of epidermal turnover [9] |
|
|
| Retinoids |
|
| Inhibition of tyrosinase transcription, epidermal melanin dispersion [8] |
|
|
| Vitamin C, Vitamin E |
|
| Antioxidant action, acceleration of epidermal turnover [9] |
|
|
| Niacinamide, Soy proteins, Linoleic acid |
|
| Inhibition of melanosome transfer [8][9]
| [8,9]
|
2. Colourism and the Widespread Practices of Skin-Lightening
The belief that a fair complexion is a synonym for beauty and the widespread skin-lightening practices that result come from a complex interweaving of historical, cultural, social, psychological and economic factors.
Since ancient times, the canon of beauty has included a very fair complexion. During the 7th century in China, Empress We Zetian swallowed crushed pearls to obtain her fair complexion, a practice still popular among Chinese women. Greek women painted their faces with white lead (lead carbonate, very toxic) and Roman women adopted this practice using the same compound, which they called ‘cerussa’, as described by Pliny the Elder. Cleopatra (69–30 BC), queen of the Ptolemaic kingdom of Egypt, regularly bathed in acidic ass’s milk [12]. In different historical periods, there have been many reasons behind skin-lightening practices; they are linked to colonialism in countries such as Africa or India and slavery in America [12].
Dangerous skin-whitening practices are not limited to the past, and the search for means to obtain a white complexion has continued unabated in the following centuries; striking examples are the ingestion of arsenic waffles in Victorian times, the use of radiotherapy at the beginning of the 20th century, or even, very recently, the oral or intravenous administration of glutathione recommended in some African countries to pregnant women to lighten the skin of babies in the womb [12]. Although Coco Chanel made the tanned complexion fashionable in the 1920s, there are still several psychosocial, cultural and economic reasons why people lighten their skin, because light complexion is still perceived as a positive value in many countries and cultures. This prejudice has its origin in so-called colourism; although colourism and racism are often interconnected, they are two different phenomena. Racism is prejudice against people of a certain ethnic group or “race”, while colourism can be defined as “prejudice or discrimination against individuals with a dark skin tone, especially among people of the same ethnic or racial background”, as defined by the Oxford English Dictionary.
Consequently, colourism places a higher value on light-skinned people than on darker-skinned ones. The historical reasons for colourism vary from country to country. In the United States, they are deeply rooted in the enslavement of Africans, while in South Africa, India and Latin America they are a consequence of European colonisation; on the other hand, in East Asia, light complexions were historically idealised and linked to wealth and desirability [7].
Currently, skin lightening is a practice that is mainly used in non-white communities around the world, including Africa, Asia, the Middle East and America; these countries now represent markets where demand for skin lightening products is strong. As reported by leading market research report provider Million Insights [13], the global skin lightening products market size is expected to reach $13.7 billion by 2025, and the CAGR (Compound Annual Growth Rate) is expected to grow at a rate of 7.4% from 2019 to 2025. The Asia-Pacific region is the largest market for this product type, accounting for 54.3% of global revenue in 2018.
Skin whitening is mainly practised by women, but in some geographical regions, it is also becoming popular among men and young adults [14]. At the moment, the largest number of studies on the spread of such products focuses on Africa. Skin whitening practices have been popular among African countries since the 1950s; the main motivation behind this phenomenon is the perception of greater economic and social privileges associated with fair skin.
It is estimated that about 75% of women in Nigeria, 60% in Senegal, 50% in Mali and 30% in Ghana regularly use bleaching products [15].
Compared to other African countries, South Africa has a lower rate of skin lighteners; after banning the use of hydroquinone in cosmetics and over-the-counter medicines in 1990, it became the first country in the world to ban skin lighteners, followed by Rwanda, Côte d’Ivoire, Tanzania, Kenya and Ghana [15].
Such practices are also very popular in Asian countries such as India, China, Japan, Korea and Arabia. In these cultures, having a fair complexion is considered an important element of female beauty, partly based on traditional values and partly due to the influences of Western colonial heritage [16]. In India, the words ‘fair’ and ‘beautiful’ are synonymous, and fairness is a distinctive feature.
The caste system is often blamed for creating a division based on skin colour in Indian society, with the light-skinned Brahmins occupying the apex of the caste pyramid and the darker-skinned Dalits (‘untouchables’) at the base [17]. Therefore, the sale of skin lighteners is still flourishing in India, and these cosmetics account for about half of all skincare products sold, with an approximate value of USD 500 million [18]. Asian skin is particularly prone to suffer from pigmentation disorders [8]; in a recent study by Porcheron et al. [19], Chinese women were asked to look at several images of Chinese faces before and after manipulation with graphic design software, and then to rate the age and attractiveness of the subjects depicted. The women who participated in the study perceived the faces as younger and more attractive after the manipulation of dark spots and dark circles than after the reduction of wrinkles and skin sagging [19].
Skin whitening is a global phenomenon; unfortunately, abuse of this practice is common. The availability and spread of formulations containing toxic, unapproved and/or illegal ingredients without a prescription is of particular concern. These substances, despite their apparent efficacy, are responsible for serious acute and chronic effects. The following paragraphs outline the skin-whitening compounds of greatest concern for human health. To find relevant sources of information, the author used well-known databases, including MEDLINE (PubMed), Science Direct and Google Scholar.
3. Toxic Ingredients in Skin Lightening Cosmetics
Mercury
The main sources of mercury exposure in the past were industries (such as felt hat factories) and the composition and subsequent intake of mercury-based medicines. The use of mercury-containing skin-lightening products is currently a major cause of chronic mercury poisoning in some areas of the world [20]; despite their limited effectiveness, toxicity and the fact that they are banned in many countries, these products are still available and widely used worldwide.
They can be found in a range of creams, milks, lotions, gels, oils and soaps. Mercury occurs in three forms: elemental (or metallic) mercury (Hg
2. Colourism and the Widespread Practices of Skin-Lightening
The belief that a fair complexion is a synonym for beauty and the widespread skin-lightening practices that result come from a complex interweaving of historical, cultural, social, psychological and economic factors.
Since ancient times, the canon of beauty has included a very fair complexion. During the 7th century in China, Empress We Zetian swallowed crushed pearls to obtain her fair complexion, a practice still popular among Chinese women. Greek women painted their faces with white lead (lead carbonate, very toxic) and Roman women adopted this practice using the same compound, which they called ‘cerussa’, as described by Pliny the Elder. Cleopatra (69–30 BC), queen of the Ptolemaic kingdom of Egypt, regularly bathed in acidic ass’s milk [12]. In different historical periods, there have been many reasons behind skin-lightening practices; they are linked to colonialism in countries such as Africa or India and slavery in America [12].
Dangerous skin-whitening practices are not limited to the past, and the search for means to obtain a white complexion has continued unabated in the following centuries; striking examples are the ingestion of arsenic waffles in Victorian times, the use of radiotherapy at the beginning of the 20th century, or even, very recently, the oral or intravenous administration of glutathione recommended in some African countries to pregnant women to lighten the skin of babies in the womb [12]. Although Coco Chanel made the tanned complexion fashionable in the 1920s, there are still several psychosocial, cultural and economic reasons why people lighten their skin, because light complexion is still perceived as a positive value in many countries and cultures. This prejudice has its origin in so-called colourism; although colourism and racism are often interconnected, they are two different phenomena. Racism is prejudice against people of a certain ethnic group or “race”, while colourism can be defined as “prejudice or discrimination against individuals with a dark skin tone, especially among people of the same ethnic or racial background”, as defined by the Oxford English Dictionary.
Consequently, colourism places a higher value on light-skinned people than on darker-skinned ones. The historical reasons for colourism vary from country to country. In the United States, they are deeply rooted in the enslavement of Africans, while in South Africa, India and Latin America they are a consequence of European colonisation; on the other hand, in East Asia, light complexions were historically idealised and linked to wealth and desirability [7].
Currently, skin lightening is a practice that is mainly used in non-white communities around the world, including Africa, Asia, the Middle East and America; these countries now represent markets where demand for skin lightening products is strong. As reported by leading market research report provider Million Insights [13], the global skin lightening products market size is expected to reach $13.7 billion by 2025, and the CAGR (Compound Annual Growth Rate) is expected to grow at a rate of 7.4% from 2019 to 2025. The Asia-Pacific region is the largest market for this product type, accounting for 54.3% of global revenue in 2018.
Skin whitening is mainly practised by women, but in some geographical regions, it is also becoming popular among men and young adults [14]. At the moment, the largest number of studies on the spread of such products focuses on Africa. Skin whitening practices have been popular among African countries since the 1950s; the main motivation behind this phenomenon is the perception of greater economic and social privileges associated with fair skin.
It is estimated that about 75% of women in Nigeria, 60% in Senegal, 50% in Mali and 30% in Ghana regularly use bleaching products [15].
Compared to other African countries, South Africa has a lower rate of skin lighteners; after banning the use of hydroquinone in cosmetics and over-the-counter medicines in 1990, it became the first country in the world to ban skin lighteners, followed by Rwanda, Côte d’Ivoire, Tanzania, Kenya and Ghana [15].
Such practices are also very popular in Asian countries such as India, China, Japan, Korea and Arabia. In these cultures, having a fair complexion is considered an important element of female beauty, partly based on traditional values and partly due to the influences of Western colonial heritage [16]. In India, the words ‘fair’ and ‘beautiful’ are synonymous, and fairness is a distinctive feature.
The caste system is often blamed for creating a division based on skin colour in Indian society, with the light-skinned Brahmins occupying the apex of the caste pyramid and the darker-skinned Dalits (‘untouchables’) at the base [17]. Therefore, the sale of skin lighteners is still flourishing in India, and these cosmetics account for about half of all skincare products sold, with an approximate value of USD 500 million [18]. Asian skin is particularly prone to suffer from pigmentation disorders [8]; in a recent study by Porcheron et al. [19], Chinese women were asked to look at several images of Chinese faces before and after manipulation with graphic design software, and then to rate the age and attractiveness of the subjects depicted. The women who participated in the study perceived the faces as younger and more attractive after the manipulation of dark spots and dark circles than after the reduction of wrinkles and skin sagging [19].
Skin whitening is a global phenomenon; unfortunately, abuse of this practice is common. The availability and spread of formulations containing toxic, unapproved and/or illegal ingredients without a prescription is of particular concern. These substances, despite their apparent efficacy, are responsible for serious acute and chronic effects. The following paragraphs outline the skin-whitening compounds of greatest concern for human health. To find relevant sources of information, the author used well-known databases, including MEDLINE (PubMed), Science Direct and Google Scholar.
Keywords used to search for articles included colourism (or colourism), skin lightening products, skin whitening products, mercury derivatives, hydroquinone, topical corticosteroids and clobetasol. This research was carried out in the second half of 2021, and most of the articles consulted to write this review have been published in the decade 2010–2020. Government and official bodies’ websites were also consulted to get information on legislative aspects.
3. Toxic Ingredients in Skin Lightening Cosmetics
Mercury
The main sources of mercury exposure in the past were industries (such as felt hat factories) and the composition and subsequent intake of mercury-based medicines. The use of mercury-containing skin-lightening products is currently a major cause of chronic mercury poisoning in some areas of the world [20]; despite their limited effectiveness, toxicity and the fact that they are banned in many countries, these products are still available and widely used worldwide.
They can be found in a range of creams, milks, lotions, gels, oils and soaps. Mercury occurs in three forms: elemental (or metallic) mercury (Hg
), inorganic mercury compounds (existing in two oxidative states: mercurous, Hg
++) and organic mercury compounds. Inorganic mercury (mercurous chloride or calomel, Hg2Cl2; ammoniated mercury, HgNH2Cl; mercuric iodide, HgI2; mercurous oxide, Hg2O; mercuric chloride, HgCl2) is the one commonly found in skin-lightening preparations [21], although methylmercury has recently been reported to be present in a skin-lightening product in the United States [22]. Mercury compounds suppress melanin production in skin melanocytes. Tyrosinase, a type III copper protein, plays a key role in the synthesis of this pigment because, as already mentioned, it catalyses the first two reactions of melanin production: the hydroxylation of the amino acid L-tyrosinase to DOPA and then the conversion of DOPA to dopaquinone. Mercurous and mercuric ions inhibit melanin synthesis by competing with copper in tyrosinase; in particular, they bind to the His residue of the catalytic centre of the enzyme. This inhibition is irreversible [23].
Humans are exposed to mercury through dermal absorption, inhalation of elemental mercury vapours (especially in cases of long-term exposure) and ingestion. Inorganic mercury salts contained in creams and other cosmetics are easily absorbed through the skin; they penetrate the epidermis and are also absorbed through sweat glands, sebaceous glands and hair follicles [24], through which Hg eventually accumulates in the hair of the scalp [25]. Abbas et al. [25] studied Hg exposure in students using cosmetics to lighten their skin and found that Hg concentrations in the hair had a statistically significant correlation with the Hg concentration in cosmetics. Dermal absorption depends on factors such as mercury concentration, frequency of product application, skin integrity, lipophilicity of the vehicle in the cosmetic product and the hydration of the stratum corneum; ingestion of mercury can occur after topical application around the mouth and hand-to-mouth contact [26].
Inorganic mercury compounds accumulate in the body’s organs, with the highest concentration found in the kidney, near the proximal tubule; urinary excretion is the main route of elimination [24]. Since inorganic mercury compounds are not lipophilic, they do not readily pass through the blood-placenta or the blood-brain barriers [24]; however, prolonged dermal exposure can lead to accumulation in the central nervous system and neurotoxicity [27]. Inorganic mercury salts are mainly excreted in urine and faeces; high levels of urinary mercury are frequently associated with the use of mercury-containing skin lighteners.
According to current information on exposure to cosmetics for commonly used products (1.22 g for a face cream and 4.42 g for a body lotion; [28]), a consumer using a cosmetic containing 10,000 ppm mercury could absorb up to 450 μg in a single application [29]. Increased and often elevated concentrations of mercury in urine have been found in people who used skin-lightening products and also in family members who were not using them [27][30][31]. The inhalation of elemental mercury vapours depends on the transformation of inorganic mercury compounds into elemental mercury, which can occur in the presence of low pH and UV radiation [32][33]. The following reaction shows the dissociation of calomel into mercuric chloride and elemental mercury, which evaporates into the air:
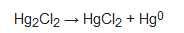 In vapour form, elemental mercury is lipid-soluble and highly diffusible through cell membranes; it is easily absorbed in the lungs, but also through the nose by the olfactory pathway [24], enters the bloodstream and rapidly spreads throughout the body, crossing the blood-brain barrier and accumulating in the central nervous system. Elemental mercury is also oxidised to mercuric form in tissue cells [24]. This reaction shows how the prolonged use of creams containing mercury salts can spread elemental mercury within the household via contaminated items (clothes, towels) or surfaces. Copan et al. [27] reported several cases of widespread household contamination in Mexico and California. Mercury vapour levels detected in bedrooms ranged from undetectable to 8 μg/m
In vapour form, elemental mercury is lipid-soluble and highly diffusible through cell membranes; it is easily absorbed in the lungs, but also through the nose by the olfactory pathway [24], enters the bloodstream and rapidly spreads throughout the body, crossing the blood-brain barrier and accumulating in the central nervous system. Elemental mercury is also oxidised to mercuric form in tissue cells [24]. This reaction shows how the prolonged use of creams containing mercury salts can spread elemental mercury within the household via contaminated items (clothes, towels) or surfaces. Copan et al. [27] reported several cases of widespread household contamination in Mexico and California. Mercury vapour levels detected in bedrooms ranged from undetectable to 8 μg/m ) and organic mercury compounds. Inorganic mercury (mercurous chloride or calomel, Hg2Cl2; ammoniated mercury, HgNH2Cl; mercuric iodide, HgI2; mercurous oxide, Hg2O; mercuric chloride, HgCl2) is the one commonly found in skin-lightening preparations [21], although methylmercury has recently been reported to be present in a skin-lightening product in the United States [22]. Mercury compounds suppress melanin production in skin melanocytes. Tyrosinase, a type III copper protein, plays a key role in the synthesis of this pigment because, as already mentioned, it catalyses the first two reactions of melanin production: the hydroxylation of the amino acid L-tyrosinase to DOPA and then the conversion of DOPA to dopaquinone. Mercurous and mercuric ions inhibit melanin synthesis by competing with copper in tyrosinase; in particular, they bind to the His residue of the catalytic centre of the enzyme. This inhibition is irreversible [23].
Humans are exposed to mercury through dermal absorption, inhalation of elemental mercury vapours (especially in cases of long-term exposure) and ingestion. Inorganic mercury salts contained in creams and other cosmetics are easily absorbed through the skin; they penetrate the epidermis and are also absorbed through sweat glands, sebaceous glands and hair follicles [24], through which Hg eventually accumulates in the hair of the scalp [25]. Abbas et al. [25] studied Hg exposure in students using cosmetics to lighten their skin and found that Hg concentrations in the hair had a statistically significant correlation with the Hg concentration in cosmetics. Dermal absorption depends on factors such as mercury concentration, frequency of product application, skin integrity, lipophilicity of the vehicle in the cosmetic product and the hydration of the stratum corneum; ingestion of mercury can occur after topical application around the mouth and hand-to-mouth contact [26].
Inorganic mercury compounds accumulate in the body’s organs, with the highest concentration found in the kidney, near the proximal tubule; urinary excretion is the main route of elimination [24]. Since inorganic mercury compounds are not lipophilic, they do not readily pass through the blood-placenta or the blood-brain barriers [24]; however, prolonged dermal exposure can lead to accumulation in the central nervous system and neurotoxicity [27]. Inorganic mercury salts are mainly excreted in urine and faeces; high levels of urinary mercury are frequently associated with the use of mercury-containing skin lighteners.
According to current information on exposure to cosmetics for commonly used products (1.22 g for a face cream and 4.42 g for a body lotion; [28]), a consumer using a cosmetic containing 10,000 ppm mercury could absorb up to 450 μg in a single application [29]. Increased and often elevated concentrations of mercury in urine have been found in people who used skin-lightening products and also in family members who were not using them [27,30,31]. The inhalation of elemental mercury vapours depends on the transformation of inorganic mercury compounds into elemental mercury, which can occur in the presence of low pH and UV radiation [32,33]. The following reaction shows the dissociation of calomel into mercuric chloride and elemental mercury, which evaporates into the air:
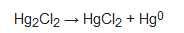
In vapour form, elemental mercury is lipid-soluble and highly diffusible through cell membranes; it is easily absorbed in the lungs, but also through the nose by the olfactory pathway [24], enters the bloodstream and rapidly spreads throughout the body, crossing the blood-brain barrier and accumulating in the central nervous system. Elemental mercury is also oxidised to mercuric form in tissue cells [24]. This reaction shows how the prolonged use of creams containing mercury salts can spread elemental mercury within the household via contaminated items (clothes, towels) or surfaces. Copan et al. [27] reported several cases of widespread household contamination in Mexico and California. Mercury vapour levels detected in bedrooms ranged from undetectable to 8 μg/m ; these cosmetics may therefore contaminate people living in the same house, notably children and the elderly.
It is worth mentioning that, according to health-based risk levels for elemental mercury in the air set by regulatory agencies (e.g., ATSDR, Agency for Toxic Substances and Disease Register), 1 μg/m
is the indoor air level below which staying in an environment is considered safe and no remediation is required, while 10 μg/m
is the level above which evacuation of residents is recommended; at levels ranging from 1 to 10 μg/m
evacuation is not required but remediation is recommended [27].
It is worth mentioning that, according to health-based risk levels for elemental mercury in air set by regulatory agencies (e.g., ATSDR, Agency for Toxic Substances and Disease Register), 1 μg/m is the indoor air level below which staying in an environment is considered safe and no remediation is required, while 10 μg/m
is the level above which evacuation of residents is recommended; at levels ranging from 1 to 10 μg/m
3, evacuation is not required but remediation is recommended [27].
The toxic effects of topically applied mercury-containing products have been documented since the beginning of the 20th century. Acute or chronic exposure can result in dermal, gastrointestinal, neurological and renal toxicity. Organic and metallic mercury, which is more lipophilic, is more typically associated with neurological damage, while inorganic mercury more often causes kidney damage. Dermatological effects include allergic contact dermatitis, redness, erythroderma, nail discolouration, purpura and paradoxical hyperpigmentation [34].
Gastrointestinal symptoms include a metallic taste in the mouth, gingivostomatitis, nausea and hypersalivation. Neuropsychiatric signs and symptoms are the most significant indicators of mercury poisoning due to the use of skin brighteners; the most frequent are tremor, muscle weakness, peripheral neuropathy, depression, psychosis, anxiety, dizziness, headache and vision loss [35]. Regarding renal damage, acute exposure to mercury usually causes tubular injury (acute tubular necrosis), while chronic exposure is more frequently responsible for glomerular injury [34]. A common clinical sign in exposed infants and children is hypertension [27]. Since mercury crosses the placenta, the use of skin lighteners containing mercury during pregnancy may lead to pre- and post-natal intoxication, leading to serious harmful effects on babies [35][36].
Several studies have evaluated mercury levels in popular skin-whitening products, demonstrating that these cosmetics are an important exposure pathway to Hg and a threat to human health. For example, Peregrino et al. [37] analysed 16 locally produced Mexican skin-whitening creams using cold vapour atomic absorption spectrometry (CV-AAS). Mercury was detected in six of the 16 samples, in amounts ranging from 878 to 36,000 ppm. In a 2014 investigation, Hamann et al. [29] investigated 549 US skin-lightening cosmetics, both online and in shops, using X-ray fluorescence spectrometry. Of the products tested, 6% contained mercury above 1000 ppm and 45% contained mercury above 10,000 ppm. Copan et al. [27] reported that mercury levels of artisanal lightening creams from Mexico containing calomel ranged from 28,000 to 210,000 ppm. More recently, Ricketts et al. [22] used X-ray fluorescence and cold vapour atomic absorption spectroscopy to assess mercury concentrations in 60 popular lightening cosmetics on the Jamaican market. Mercury concentrations ranged from 0.05 ppm to 17,547 ppm; six contained mercury above 1 ppm, the maximum allowable limit set by the US Food and Drug Administration (FDA), and three were characterised by a particularly high mercury presence (17,547, 465.73 and 422.04 ppm respectively). Creams contained more mercury than soaps and lotions. On the other hand, a survey of 62 lightening creams and soaps on the Ghanaian market revealed that mercury levels in all samples tested ranged from <0.001 to 0.327 ± 0.062 μg/g and were therefore below FDA limits [38].
In conclusion, despite several restrictive regulations, skin-whitening preparations containing mercury continue to be available on the global market, and without geographical limitations; the public continues to have access to these products even in countries with stronger market surveillance, as they can be purchased online, in ethnic markets, from abroad and even from flea markets. Furthermore, the results of these investigations indicate that the mercury content of skin-whitening cosmetics is sometimes extremely high and poses a serious health threat, putting consumers and their families at risk of poisoning.
Mercury is sometimes mentioned on the packaging and its concentration is occasionally indicated, but in many cases, it is not even included among the ingredients, so consumers are not necessarily well informed by what is on the label. For these reasons, doctors should be aware that mercury-containing skin-lightening cosmetics, although illegal, are still available on the market, and that symptoms (dermatitis, weakness, myalgias, change of taste, paresthesias) of unclear cause could be related to mercury poisoning, even when patients claim not to use them [29].
4. Hydroquinone
Hydroquinone is a water-soluble phenolic compound in the form of colourless or white crystals; it is a ubiquitous molecule and is found in tea, coffee, beer, berries, propolis and some mushrooms. A natural derivative of hydroquinone is α-arbutin, a glycoside in whose structure the hydroquinone is bound to a D-glucose molecule. Arbutin had skin-lightening properties due to the inactivation of tyrosinase, and possesses antioxidant properties that can contribute to its depigmenting action; Although there is the possibility that a small amount of hydroquinone can be produced by cutaneous microorganisms or UV radiations when arbutin is applied to the skin, this molecule has intrinsic depigmenting properties, that are not dependent on the release of hydroquinone. Hydroquinone’s use for depigmenting purposes became widespread in the following decades and is currently utilised in managing various hyperpigmentation disorders such as melasma, chloasma, freckles, age spots and post-inflammatory hyperpigmentation caused by acne or trauma. Although hydroquinone has been the dermatological gold standard for skin lightening for over 50 years, in recent times regulatory agencies in Japan, Europe and the United States have questioned its safety. Considering the risks to human health associated with its application, hydroquinone has been banned from cosmetics in Europe since 2001 and its use in skin-lightening cosmetic formulations is currently illegal; cosmetics containing hydroquinone have also been banned in several other countries, such as the United Kingdom, Australia and Japan.
Hydroquinone inhibits the activity of the tyrosinase enzyme by blocking the oxidation of tyrosine to DOPA and the subsequent oxidation of DOPA, causing the cessation of melanin synthesis; hydroquinone treatment decreases the number of melanised melanosomes and leads to the formation of abnormally melanised melanosomes, which eventually die [39]. Recently, Aspengren et al. [40] demonstrated that tyrosinase is not the only cellular target of hydroquinone, since this compound severely affects certain cytoskeletal structures (microtubules, actin filaments) in cultured melanophores of Xenopus laevis in a dose-dependent manner. Depigmentation by hydroquinone is reversible; after its suspension, melanin synthesis resumes [40]. The use of skin-lightening products containing hydroquinone can cause short- and medium-term effects, both acute and chronic. The most common acute complication is irritant contact dermatitis [34]. The most common complication induced by prolonged exposure to hydroquinone is exogenous ochronosis, a localised hyperpigmentation of the skin with asymptomatic blue-black and grey-brown macules with no systemic manifestations, histologically characterised by banana-shaped ochre deposits and irregularly shaped collagen bundles in the dermis [41][42]. Although the aetiology of this hyperpigmentation is unknown, it has been suggested that hydroquinone may inhibit homogentisic acid oxidase in the dermis, resulting in local accumulation of homogentisic acid which then polymerises to form ochronotic pigment [43]. The carcinogenicity and genotoxicity of hydroquinone are so well established in rodents that an association between long-term use of this compound as a skin brightener and squamous cell carcinoma in humans has been suggested [44][45][46]. However, regulatory authorities have concluded that there is insufficient data to classify hydroquinone as a carcinogen. For example, the American Conference of Governmental Industrial Hygienists classified hydroquinone as ‘A3’, which means that it is recognised as carcinogenic in animals but the relevance for humans is unknown [43]. Similarly, the International Agency for Research on Cancer (IARC) has classified hydroquinone in Group 3 (not classifiable as a human carcinogen); hydroquinone is not classifiable as a human carcinogen, based on limited evidence in experimental animals and inadequate evidence in humans. In the EU, hydroquinone is classified as Carc. Cat 2 H351 (suspected of causing cancer) and Muta. Cat 2 (suspected of causing genetic defects) according to Regulation (EC) No 1272/2008 (CLP Regulation), Annex VI1 [47].
5. Corticosteroids
Topical corticosteroids are among the most widely prescribed drugs in clinical dermatology; they are used in the management of a wide range of medical conditions, due to their anti-inflammatory, antimitotic and immunomodulatory actions [48]. Abuse of topical corticosteroids, with the aim of achieving lighter skin, is a widespread practice in many countries, such as India and sub-Saharan African states, especially among women [6][49][50][51]. The misuse of these products is facilitated by their availability as cheap over-the-counter (OTC) drugs [51]. They are used as skin brighteners due to their potent depigmenting action and anti-inflammatory effects; clobetasol propionate, betamethasone dipropionate and fluocinonide (fluocinolone acetonide) are the most commonly used agents [12]. In order to exert their lightening action, corticosteroids are applied at high concentrations and over a large area of the body for prolonged periods (from a few months to a few years); long-term use and the conditions of application favour their dermal absorption [49]. The cosmetic use of corticosteroids is associated with a wide range of side effects, both dermatological and systemic. The mechanisms of corticosteroid-induced skin depigmentation are not yet fully understood. Several explanations have been proposed, including a direct cytotoxic effect, vasoconstriction, mechanical effects of oedema or an alteration in the regulation of melanogenesis [52]. Their lightening effect is initially thought to be mediated by local vasoconstriction, which gives the impression of an immediate reduction in skin pigmentation [20]; finally, corticosteroids lighten the skin by inhibiting pro-opiomelanocortin (POMC), a protein synthesised in the anterior pituitary that produces, by proteolytic cleavage, several biologically active peptides, including α-melanocyte-stimulating hormone (α-MSH), which regulates melanocyte function [34][49] Skin complications include acne vulgaris, allergic contact dermatitis, skin atrophy, hypertrichosis and telangiectasias. In addition, topical corticosteroids predispose to skin infections, such as dermatophytosis, folliculitis, erysipelas, scabies and viral warts [34]. Systemic adverse effects due to chronic corticosteroid use include Cushing’s syndrome, diabetes mellitus, immunosuppression, hypertension, and suppression of the hypothalamic-pituitary-adrenal axis with adrenal suppression, the latter being the most alarming complication, as it can lead to death [34].
, evacuation is not required but remediation is recommended [27].
The toxic effects of topically applied mercury-containing products have been documented since the beginning of the 20th century. Acute or chronic exposure can result in dermal, gastrointestinal, neurological and renal toxicity. Organic and metallic mercury, which is more lipophilic, is more typically associated with neurological damage, while inorganic mercury more often causes kidney damage. Dermatological effects include allergic contact dermatitis, redness, erythroderma, nail discolouration, purpura and paradoxical hyperpigmentation [34].
Gastrointestinal symptoms include a metallic taste in the mouth, gingivostomatitis, nausea and hypersalivation. Neuropsychiatric signs and symptoms are the most significant indicators of mercury poisoning due to the use of skin brighteners; the most frequent are tremor, muscle weakness, peripheral neuropathy, depression, psychosis, anxiety, dizziness, headache and vision loss [35]. Regarding renal damage, acute exposure to mercury usually causes tubular injury (acute tubular necrosis), while chronic exposure is more frequently responsible for glomerular injury [34]. A common clinical sign in exposed infants and children is hypertension [27]. Since mercury crosses the placenta, the use of skin lighteners containing mercury during pregnancy may lead to pre- and post-natal intoxication, leading to serious harmful effects on babies [35,36].
Several studies have evaluated mercury levels in popular skin-whitening products, demonstrating that these cosmetics are an important exposure pathway to Hg and a threat to human health. For example, Peregrino et al. [37] analysed 16 locally produced Mexican skin-whitening creams using cold vapour atomic absorption spectrometry (CV-AAS). Mercury was detected in six of the 16 samples, in amounts ranging from 878 to 36,000 ppm. In a 2014 investigation, Hamann et al. [29] investigated 549 US skin-lightening cosmetics, both online and in shops, using X-ray fluorescence spectrometry. Of the products tested, 6% contained mercury above 1000 ppm and 45% contained mercury above 10,000 ppm. Copan et al. [27] reported that mercury levels of artisanal lightening creams from Mexico containing calomel ranged from 28,000 to 210,000 ppm. More recently, Ricketts et al. [22] used X-ray fluorescence and cold vapour atomic absorption spectroscopy to assess mercury concentrations in 60 popular lightening cosmetics on the Jamaican market. Mercury concentrations ranged from 0.05 ppm to 17,547 ppm; six contained mercury above 1 ppm, the maximum allowable limit set by the US Food and Drug Administration (FDA), and three were characterised by a particularly high mercury presence (17,547, 465.73 and 422.04 ppm respectively). Creams contained more mercury than soaps and lotions. On the other hand, a survey of 62 lightening creams and soaps on the Ghanaian market revealed that mercury levels in all samples tested ranged from <0.001 to 0.327 ± 0.062 μg/g and were therefore below FDA limits [38].
In conclusion, despite several restrictive regulations, skin-whitening preparations containing mercury continue to be available on the global market, and without geographical limitations; the public continues to have access to these products even in countries with stronger market surveillance, as they can be purchased online, in ethnic markets, from abroad and even from flea markets. Furthermore, the results of these investigations indicate that the mercury content of skin-whitening cosmetics is sometimes extremely high and poses a serious health threat, putting consumers and their families at risk of poisoning.
Mercury is sometimes mentioned on the packaging and its concentration is occasionally indicated, but in many cases, it is not even included among the ingredients, so consumers are not necessarily well informed by what is on the label. For these reasons, doctors should be aware that mercury-containing skin-lightening cosmetics, although illegal, are still available on the market, and that symptoms (dermatitis, weakness, myalgias, change of taste, paresthesias) of unclear cause could be related to mercury poisoning, even when patients claim not to use them [29].
Hydroquinone
Hydroquinone is a water-soluble phenolic compound in the form of colourless or white crystals; it is a ubiquitous molecule and is found in tea, coffee, beer, berries, propolis and some mushrooms. A natural derivative of hydroquinone is α-arbutin, a glycoside in whose structure the hydroquinone is bound to a D-glucose molecule. Arbutin had skin-lightening properties due to the inactivation of tyrosinase, and possesses antioxidant properties that can contribute to its depigmenting action; Although there is the possibility that a small amount of hydroquinone can be produced by cutaneous microorganisms or UV radiations when arbutin is applied to the skin, this molecule has intrinsic depigmenting properties, that are not dependent on the release of hydroquinone. Hydroquinone’s use for depigmenting purposes became widespread in the following decades and is currently utilised in managing various hyperpigmentation disorders such as melasma, chloasma, freckles, age spots and post-inflammatory hyperpigmentation caused by acne or trauma. Although hydroquinone has been the dermatological gold standard for skin lightening for over 50 years, in recent times regulatory agencies in Japan, Europe and the United States have questioned its safety. Considering the risks to human health associated with its application, hydroquinone has been banned from cosmetics in Europe since 2001 and its use in skin-lightening cosmetic formulations is currently illegal; cosmetics containing hydroquinone have also been banned in several other countries, such as the United Kingdom, Australia and Japan.
Hydroquinone inhibits the activity of the tyrosinase enzyme by blocking the oxidation of tyrosine to DOPA and the subsequent oxidation of DOPA, causing the cessation of melanin synthesis; hydroquinone treatment decreases the number of melanised melanosomes and leads to the formation of abnormally melanised melanosomes, which eventually die [42]. Recently, Aspengren et al. [43] demonstrated that tyrosinase is not the only cellular target of hydroquinone, since this compound severely affects certain cytoskeletal structures (microtubules, actin filaments) in cultured melanophores of Xenopus laevis in a dose-dependent manner. Depigmentation by hydroquinone is reversible; after its suspension, melanin synthesis resumes [43]. The use of skin-lightening products containing hydroquinone can cause short- and medium-term effects, both acute and chronic. The most common acute complication is irritant contact dermatitis [34]. The most common complication induced by prolonged exposure to hydroquinone is exogenous ochronosis, a localised hyperpigmentation of the skin with asymptomatic blue-black and grey-brown macules with no systemic manifestations, histologically characterised by banana-shaped ochre deposits and irregularly shaped collagen bundles in the dermis [48,49]. Although the aetiology of this hyperpigmentation is unknown, it has been suggested that hydroquinone may inhibit homogentisic acid oxidase in the dermis, resulting in local accumulation of homogentisic acid which then polymerises to form ochronotic pigment [47]. The carcinogenicity and genotoxicity of hydroquinone are so well established in rodents that an association between long-term use of this compound as a skin brightener and squamous cell carcinoma in humans has been suggested [51,52,53]. However, regulatory authorities have concluded that there is insufficient data to classify hydroquinone as a carcinogen. For example, the American Conference of Governmental Industrial Hygienists classified hydroquinone as ‘A3’, which means that it is recognised as carcinogenic in animals but the relevance for humans is unknown [47]. Similarly, the International Agency for Research on Cancer (IARC) has classified hydroquinone in Group 3 (not classifiable as a human carcinogen); hydroquinone is not classifiable as a human carcinogen, based on limited evidence in experimental animals and inadequate evidence in humans. In the EU, hydroquinone is classified as Carc. Cat 2 H351 (suspected of causing cancer) and Muta. Cat 2 (suspected of causing genetic defects) according to Regulation (EC) No 1272/2008 (CLP Regulation), Annex VI1 [54].
Corticosteroids
Topical corticosteroids are among the most widely prescribed drugs in clinical dermatology; they are used in the management of a wide range of medical conditions, due to their anti-inflammatory, antimitotic and immunomodulatory actions [56]. Abuse of topical corticosteroids, with the aim of achieving lighter skin, is a widespread practice in many countries, such as India and sub-Saharan African states, especially among women [6,50,57,58]. The misuse of these products is facilitated by their availability as cheap over-the-counter (OTC) drugs [58]. They are used as skin brighteners due to their potent depigmenting action and anti-inflammatory effects; clobetasol propionate, betamethasone dipropionate and fluocinonide (fluocinolone acetonide) are the most commonly used agents [12]. In order to exert their lightening action, corticosteroids are applied at high concentrations and over a large area of the body for prolonged periods (from a few months to a few years); long-term use and the conditions of application favour their dermal absorption [50]. The cosmetic use of corticosteroids is associated with a wide range of side effects, both dermatological and systemic. The mechanisms of corticosteroid-induced skin depigmentation are not yet fully understood. Several explanations have been proposed, including a direct cytotoxic effect, vasoconstriction, mechanical effects of oedema or an alteration in the regulation of melanogenesis [59]. Their lightening effect is initially thought to be mediated by local vasoconstriction, which gives the impression of an immediate reduction in skin pigmentation [20]; finally, corticosteroids lighten the skin by inhibiting pro-opiomelanocortin (POMC), a protein synthesised in the anterior pituitary that produces, by proteolytic cleavage, several biologically active peptides, including α-melanocyte-stimulating hormone (α-MSH), which regulates melanocyte function [34,50] Skin complications include acne vulgaris, allergic contact dermatitis, skin atrophy, hypertrichosis and telangiectasias. In addition, topical corticosteroids predispose to skin infections, such as dermatophytosis, folliculitis, erysipelas, scabies and viral warts [34]. Systemic adverse effects due to chronic corticosteroid use include Cushing’s syndrome, diabetes mellitus, immunosuppression, hypertension, and suppression of the hypothalamic-pituitary-adrenal axis with adrenal suppression, the latter being the most alarming complication, as it can lead to death [34].
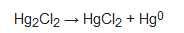 In vapour form, elemental mercury is lipid-soluble and highly diffusible through cell membranes; it is easily absorbed in the lungs, but also through the nose by the olfactory pathway [24], enters the bloodstream and rapidly spreads throughout the body, crossing the blood-brain barrier and accumulating in the central nervous system. Elemental mercury is also oxidised to mercuric form in tissue cells [24]. This reaction shows how the prolonged use of creams containing mercury salts can spread elemental mercury within the household via contaminated items (clothes, towels) or surfaces. Copan et al. [27] reported several cases of widespread household contamination in Mexico and California. Mercury vapour levels detected in bedrooms ranged from undetectable to 8 μg/m
In vapour form, elemental mercury is lipid-soluble and highly diffusible through cell membranes; it is easily absorbed in the lungs, but also through the nose by the olfactory pathway [24], enters the bloodstream and rapidly spreads throughout the body, crossing the blood-brain barrier and accumulating in the central nervous system. Elemental mercury is also oxidised to mercuric form in tissue cells [24]. This reaction shows how the prolonged use of creams containing mercury salts can spread elemental mercury within the household via contaminated items (clothes, towels) or surfaces. Copan et al. [27] reported several cases of widespread household contamination in Mexico and California. Mercury vapour levels detected in bedrooms ranged from undetectable to 8 μg/m