SkThin is a large and complex organ that serves protective and regulatory functions and is responsible for communication between the external environment and the inner organism. To fulfill these functions, skin has evolved as an organ with a complex anatomy derived from both the ectoderm (epidermis) and mesoderm (dermis). The skin includes not only these two major compartments but also important appendages, including hair follicles, sweat and sebaceous glands, nerve endings, and blood vessels, all of which have intricate spatial arrangements that render s is an entry focused on extrusion bioprinting for skin applications. Bioprinting technologies have the ability to combine various human cell phenotypes, signaling proteins, extracellular matrix (ECM) components, and other scaffold-like biomaterials and are currently being exploited for the fabrication of human skin, broadly aiming to achieve two main goals. The first goal is to meet the urgent clinical demand for skin equivalents, which can range in complexity from advanced dressings for chronic wounds to biomimetic skin grafts to help restore the barrier function in complex ulcers, burns, or traumatic postsurgical wounds. The second important motivation for skin biofabrication of the full skin organ challengingis to create disease models for in vitro research and drug development.
- bioprinting
- skin
- ulcer
- hydrogel
- bioink
- technology readiness level
- biological therapy
- tissue engineering
- regenerative medicine
1. Introduction
Skin is a large and complex organ that serves protective and regulatory functions and is responsible for communication between the external environment and the inner organism. To fulfill these functions, skin has evolved as an organ with a complex anatomy derived from both the ectoderm (epidermis) and mesoderm (dermis). The skin includes not only these two major compartments but also important appendages, including hair follicles, sweat and sebaceous glands, nerve endings, and blood vessels [1], all of which have intricate spatial arrangements that render fabrication of the full skin organ challenging.
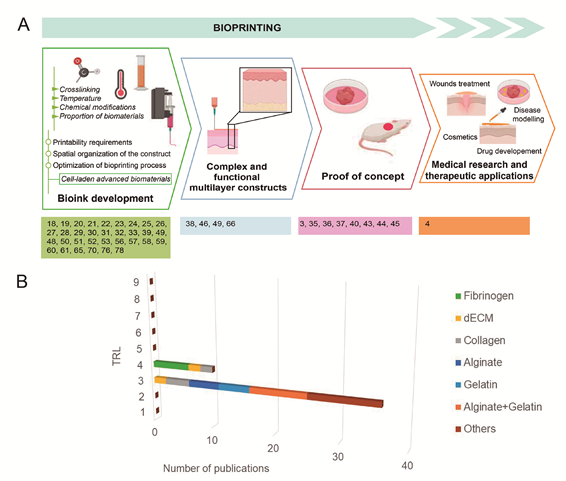
Among the different bioprinting technologies (i.e., inkjet, laser, extrusion, stereolithography, and microfluidics), extrusion has been identified as the most suitable for manufacturing soft tissue [6] (Figure 1). Thus, we performed a systematic review to estimate the possibilities of extrusion bioprinting for skin applications, describing the main cell phenotypes, signaling proteins, and bioinks (hydrogels) used in extrusion platforms. To understand the current limitations of this technology and how far we are from creating functional skin, we have roughly estimated the maturity of extrusion bioprinting for skin conditions by applying the technology readiness level (TRL) concept to the retrieved studies.
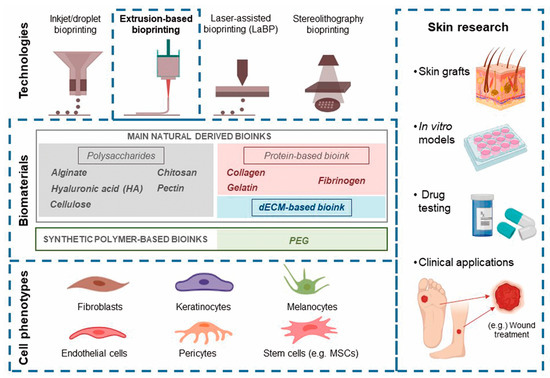
Figure 1. The main bioprinting technologies, printable biomaterials, and cell phenotypes used in skin bioprinting, as well as the main translational applications of this technology.
21. Skin Biology and Relevant Aspects for Bioprinting
The epidermis, the outermost layer of the skin (0.5–1.5 mm thick), is a thin stratified squamous epithelium that acts as a protective shield for the internal structures of the body, regulates hydration, and provides color to the skin. The complexity of skin originates not only from the myriad of cell phenotypes but also the spatial organization of both the cells and the ECM (i.e., its histology). Therefore, one of the main challenges of skin fabrication using bioprinting techniques is to not only deposit the components of the skin but also to the precisely reproduce a biomimetic tissue. For this, reproducing the architecture is critical; the biomanufactured substitute must present 4 or 5 layers in the epidermis (stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, stratum basale) and rete ridges. The latter are epithelial extensions that project to the underlying connective tissue (dermis). They are found flattened in scar tissue as well as in most of the biofabricated skin models, thereby reducing epidermal thickness and compromising its barrier function.
High cellularity is a hallmark of the epidermis. Keratinocytes, the most represented cell phenotype, are tightly packed by adherens junctions (cadherins), and produce keratin, which is a resistant and fibrous protein that serves as a barrier. The identification of keratin in the biofabricated skin confirms the epidermal formation, since keratin 10 is a marker of early epidermal differentiation as well as involucrin and filaggrin late differentiation markers [7]. In addition, connected to the keratinocytes and forming the epidermal–melanin unit (EMU), there are melanocytes (melanocyte-to-keratinocyte ratio, 1:36). These cells produce the photoprotective pigment melanin and distribute it to keratinocytes through the EMU, providing color to the skin and protection from UV light. Furthermore, there are less abundant tissue-resident dendritic cells called Langerhans cells, the first immunological defense for the body, and mechanoreceptor cells called Merkel cells, which enable the sensation of touch.
Beneath the epidermis, separated by the basement layer, is the dermis. This is a thick layer of connective tissue composed primarily of two regions, the papillary and reticular dermis, with a low fibroblast density, arranged in a collagenous, anisotropic ECM. In order to assess the dermis growth in biofabricated substitutes, first, the presence of collagen I can be studied, which is an early produced dermis marker; later, fibrillin and elastin are deposited, and hence, they serve as late development markers [8]. Moreover, the presence of collagen IV is considered a mature skin hallmark [3]. The papillary region is tightly connected to the epidermis and provides structural support, cell nourishment, and waste removal. This connection can be pursued by bioprinting manufacturing, and it is commonly assessed by studying the presence of laminin, which is a basement layer protein that participates in the anchoring of the epidermal keratinocytes to the dermis. Blood vessels, nerves, and important appendage structures derived from invaginated epidermis, such as hair follicles and sweat and sebaceous glands, are found in the reticular dermis. Recently, stem cell niches within the skin have been discovered; these cells become multipotent and help in wound healing [9]. The complexity of skin originates not only from the myriad of cell phenotypes but also the spatial organization of both the cells and the ECM (i.e., its histology). Underneath the dermis is the hypodermis or subcutaneous tissue. It remains strongly connected to the dermis, since it contains sweat glands, hair follicle roots, nerves, and large blood and lymphatic vessels en route from the dermis. It is mainly composed of loose connective tissue, e.g., elastin and collagen fibers attached to the dermis, and fat accumulations, helping the skin to maintain the body temperature and acting as a cushion to protect the underlying structures [10].
Owing to its surface location, skin is continuously exposed to external threats, and it is especially sensitive to trauma and disease. Biological insights into the repair process have inspired the design of bioprinting approaches mimicking natural healing mechanisms. When an injury occurs, skin cells sense stressful environmental changes and try to restore skin homeostasis by initiating a dynamic stepwise process, which includes several overlapping biological processes: hemostasis, inflammation, angiogenesis, proliferation, epithelialization, and remodeling. Moreover, this process encompasses complex biochemical changes and crosstalk among multiple cell phenotypes [11]. Thus, the rationale for skin biofabrication should provide for adequate architecture and cellular diversity along with the complex molecular pool essential to fulfill skin functions.
In this context, bioprinting can also benefit from platelet-rich plasma (PRP) biotechnology, as it provides a unique pool of growth factors and cytokines that can enhance healing mechanisms [12][13]. In physiology, upon skin injury and vessel disruption, extravasated blood forms a clot filling the injured area. Activated platelets and leukocytes within this clot release growth factors and cytokines, establishing a cascade of molecular signals that drives tissue repair. Taking advantage of this mechanism, PRP-based therapies have been used to treat nonhealing wounds, with different degrees of success [14][15][16][17]. In fact, the platelet secretome contains more than 300 proteins, and among the crucial effectors of the repair function of PRP are platelet-derived growth factor (PDGF), Transforming growth factor (TGF), fibroblast growth factors (FGF), Epidermal Growth Factor (EGF), Hepatocyte Growth Factor (HGF), Connective tissue growth factor (CTGF), Vascular Endothelial Growth Factor (VEGF) [18]. Accordingly, the inclusion of PRP in bioink formulations can improve the efficacy of biofabricated skin equivalents.
32. Overview of Current Research on Extrusion-Based Skin Bioprinting
Following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we reviewed research articles published in the last five years to critically appraise advances concerning skin/dermal constructs manufactured through extrusion. We focused on research involving hydrogels (or their precursors) loaded with different cell phenotypes, adhering to the current bioink definition [19]. Thus, we excluded research regarding biomaterial inks not directly formulated with cells or the bioprinting of individual cells.
This field is in the early stage of development, and the bioprinting of personalized, mature skin constructs for implantation is far from being realized; certainly, most of this research is still focused on the improvement of printable biomaterials, mainly in terms of maintaining cell viability while retaining the integrity and resolution of the constructs. The main challenges include controlling the fluid properties of the biomaterial while preserving cell viability during extrusion through small-diameter nozzles and changing the polymer structure by postprinting crosslinking through ionic, covalent, or light entanglement to enable self-support and stability during the deposition of consecutive layers. Moreover, to become specialized bioinks for skin conditions, bioinks must deliver sensitive components, including living cells and signaling factors, in an aqueous environment with adequate pH and osmolarity to favor oxygen and nutrient diffusion, which are paramount for cell survival.
We first addressed printable cell phenotypes in hydrogel scaffolds. Next, we synthesized current research in extrusion bioprinting and classified research studies according to the two main extrusion trends: first, studies involving a biomimetic approach with natural hydrogels, i.e., fibrinogen, decellularized extracellular matrix (dECM), and collagen; second, studies focused on polymer bioink modification aiming to enhance the fabrication process.
In addition, we roughly estimated the maturity of extrusion bioprinting for skin conditions by applying TRL concepts to the retrieved studies.
32.1. Cells Applied in Skin Bioprinting
Skin bioinks (i.e., hydrogels) should mimic the properties of the ECM while preserving cell viability and activities during and after printing. One advantage of extrusion over other bioprinting modalities (i.e., inkjet printing) is that it allows the printing of hydrogels loaded with a high cell density. Commercial cell lines for keratinocytes, melanocytes, hair follicles, and endothelial and dermal fibroblasts are available. Alternatively, specific cell phenotypes can be isolated from skin biopsies. Conventional 2D cultures are commonly used to rapidly generate the millions of cells needed to bioprint tissue. In advanced biofabrication stages, aside from postprinting construct maturation, bioreactors can afford efficient expansion while meeting the tailored requirements for specific cell phenotypes. Various cell phenotypes have been explored to different degrees, including dermal fibroblasts, vascular cells (endothelial, pericytes and microvascular endothelial), keratinocytes, melanocytes, follicle dermal papilla cells, and various sources of stem cells.
The vast majority (61%) of constructs manufactured through extrusion lack complexity and include a single cell phenotype, which is mainly dermal fibroblasts [25][27][29][34][37][38][39][42][45][50][54][57][58][60][61][62]. Although these studies of a single cell phenotype play a role in furthering bioink research, they can only be considered the foundation for creating 3D-bioprinted skin constructs. Even though these methods can be applied to manufacture dermal constructs in an automated way, the resulting constructs differ little from hand-poured hydrogels seeded with fibroblasts. Unfortunately, these models fail to represent the entirety of the functions of the skin, which requires more complex systems integrating multiple cell phenotypes with complex molecular crosstalk.
Fibroblasts are crucial for dermal formation and wound repair, as in the presence of appropriate stimuli, including but not limited to PDGF, IGF-I, and TGF-β1, they synthesize ECM-forming proteins and additional signaling factors. The latter are involved in both autocrine (i.e., TGF-b, connective tissue growth factor (CTGF), VEGF, PDGF-BB) and paracrine (i.e., ICAM-1, VCAM-1, IL-6, IL-8, IL-15, MMPs, CCL2, CCL7, TIMP-1) signaling; thus, they not only participate in fibroblast communication but also coordinate their activities with surrounding cells, i.e., immune cells, endothelial cells, and stem cells in niches [12][64].
However, poor advances in bioprinting blood and lymphatic vessels have limited the translational application of skin constructs. The vascular and lymphatic systems located in the dermis are essential for the proper distribution of oxygen and nutrients and removal of waste, respectively. In addition, they are involved in inflammatory skin conditions and wound healing. Despite their importance, only 7 of the 47 articles reviewed reported the blending of fibroblasts with endothelial cells or pericytes [3][4][7][20][26][30][43].
The primary function of the skin, i.e., serving as a barrier to pathogen invasion, requires a healthy epidermal layer made mainly of keratinocytes. Altered barrier function is involved in inflammatory skin conditions [4]. However, only two works employed melanocytes [20][31], which fabricate the photoprotective pigment melanin. The interplay between the two main cell phenotypes of the epidermis, i.e., keratinocytes and melanocytes, is crucial to form the EMU and distribute melanin to keratinocytes, supporting the protective function of the skin against light and heat.
Moreover, the interaction between fibroblasts and keratinocytes is required for the recovery of skin homeostasis. Indeed, keratinocytes instruct fibroblasts to produce several tissue-forming factors, i.e., keratinocyte growth factor (KGF), fibroblast growth factor (FGF), IL-6, GM-CSF, hepatocyte growth factor (HGF), IL-6, IL-19, and PDGF-BB [64][65]. Nonetheless, merely eleven of the reviewed articles introduced fibroblasts and keratinocytes together in their models [22][23][24][31][32][66], and more importantly, only five of these works have successfully created vascularized, full-thickness skin substitutes [3][4][7][20][26].
32.2. Stem Cell Sources
Despite the potential envisioned for mesenchymal stem cells (MSCs) and their secretome in tissue engineering [67], interest in combining bioprinting and stem cell research has grown recently. Different stem cell sources were used in 15 of the works, including bone marrow, adipose tissue, perinatal tissues (umbilical cord, Wharton’s jelly), and amniotic fluid.
The most commonly used stem cells are bone marrow-derived mesenchymal stem cells (BM-MSCs), as they were the first to be isolated [28][43][46][47][55][56][63]. They have a multilineage differentiation capacity and can differentiate into several cell types, including skin-like cells, i.e., fibroblasts [46], keratinocytes, endothelial cells, and pericytes [68]. Moreover, during physiological wound healing, circulating MSCs are recruited to the wound site and differentiate into skin cell phenotypes [68].
Adipose tissue-derived stem cells (ASCs) are a more advantageous type of MSC. Unlike BM-MSCs, ASCs can be easily isolated in large quantities from abundantly available human adipose tissue through a minimally invasive procedure. ASCs have also shown potential in wound healing. They can differentiate into keratinocytes, fibroblasts, and endothelial cells, as well as release a healing milieu of cytokines and growth factors that support angiogenesis, fibroblast migration, and fibronectin and collagen production [69]. Therefore, their use is considered promising in skin regeneration, but as they were discovered later, only five of the reviewed studies used ASCs [7][26][36][40][55], and these studies mainly assessed cell viability. Only Kim BS et al. [7][26] proved the in vivo wound-healing properties of the fabricated scaffolds, reinforcing the benefits of including stem cells in bioprinted grafts.
On the other hand, pluripotent stem cells have further advantages, as they can differentiate into any somatic cell type of the body. Among these, embryonic stem cells (ESCs) are derived from the inner cell mass of blastocysts [48][51], and induced pluripotent stem cells (iPSCs) [4][33][59] are derived from somatic cells that have been reprogrammed to induce pluripotency. However, safety concerns linger because of their teratogenic potential. In addition, in the case of ESCs, very few cells are obtained from each extraction, and there are ethical concerns due to their embryonic origin. Therefore, there are critical issues regarding the application of these cells for clinical purposes, and their implementation in human therapy is challenging.
Very recently, human amniotic epithelial cells (AECs) have emerged as a safer source of pluripotent stem cells. They can be easily isolated from the inner amniotic membrane of the placenta, without invasive procedures or associated ethical issues. AECs have shown promising results in wound healing [70][71] and become great candidates for skin tissue engineering. In this way, Liu P et al. [41] developed a scaffold containing AECs and a special type of MSCs derived from the umbilical cord, Wharton’s jelly-derived MSCs (WJMSCs). While AECs are more likely to differentiate into keratinocytes, WJMSCs differentiate into fibroblasts and endothelial cells. Thus, in this work, they explored the development of a meaningful multi-layered skin construct with epidermal (AECs) and dermal (WJMSCs) compartments.
Despite the extensive number of cell phenotypes used in the reviewed studies, no single work has employed lymphatic, nerve, or sweat gland cells. These are strikingly important phenotypes for the recovery of skin physiology and homeostasis after injury, so research in this field needs to keep evolving to precisely mimic human skin and ensure clinical applications.
43. Skin Bioprinting, from Bench to Society, Technology Readiness Pathway
As depicted in Figure 2, most of the reviewed works focus on the first steps of the development. In fact, proof of concept was only achieved in eight of the works, which were the biomimetic bioinks from studies shown in Table 1. This demonstrates the immaturity of the technology, seldom validated in experimental research in vivo; no developments have reached sufficient maturity to be applied in a clinically relevant environment.
Figure 2. (A) Display of the reviewed publications according to the stage of development; (B) Distribution of analyzed bioinks for extrusion bioprinting according to technological development
While several companies focused on the bioprinter market business have reached TRL9 [90], the biofabrication of tissue equivalents for skin conditions is still in the early phase of laboratory research. In particular, research studies dealing with extrusion bioinks are at TRL3, while a few studies have progressed to TRL4 with testing of the bioprinted product in animal models (Figure 2). Indeed, no publications/clinical trials concerning the use of bioprinting for skin conditions in humans have been reported.[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92]Based on the original works identified in this review, technology transition to commercial products could be anticipated in the next future in the field of wound management. In order to meet the market and clinical demand, these bioprinted constructs should enable tissue repair and reconstruction of skin architecture in clinically relevant contexts, such as diabetic or vascular ulcers or burn wounds. To shorten the time to market, experimental research (TRL4-5) should generate data that are ready to be used in the certification of the bioprinted construct. A co-development interdisciplinary methodology should achieve constructs with high performance -cost ratio and, generate clinical data that meet regulatory issues associated with marketing authorization of the living constructs (i.e. Advanced Therapy Medicinal Products, ATMP).
54. Limitations and Future Directions
Extrusion bioprinting is highly interdisciplinary, as it involves the development of complex platforms requiring interdisciplinary knowledge, including knowledge of medical imaging, hardware and software to control multiple extruders (the printer), and advanced biomaterial development together with cell production, including a deep understanding of physiology and cellular biology. Moderate progress in extrusion bioprinting has led to a novel technology, which involves bioink extrusion in a yield stress fluid capable of supporting the extruded bioink (reviewed in [6]), joining competing requirements from the perspective of manufacturability (engineering) and biomimetics (life sciences).
In addition, commonly bioprinted constructs are not ready for in vivo applications and have to follow a maturation process, where architectural changes and remodeling are recognized as the fourth dimension of bioprinting [91]. Alternatively, remodeling can take place in the host tissue. In this context, the merger of robotics with bioprinting has evolved toward intraoperative bioprinting, spanning from engineering, cellular biology and biomaterials to medical sciences and surgery [92]. However, such far-reaching frontiers have created hype-type expectations because of the promising benefits achieved thus far.
Many intricate challenges need to be overcome before bioprinting technology achieves its full potential and transcends the accomplishments of tissue engineering. First, the so-called bioinks, i.e., cell-laden advanced biomaterials or natural polymers, have to be optimized to meet the requirements for printability, reproducibility and spatial organization of the construct; second, the living skin equivalent should be doped with a molecular pool of signaling proteins for the activation of healing mechanisms in a manner that can address the specific requirements of the skin as an organ and various medical conditions. The inclusion of cell signaling molecules in bioinks is often neglected, broadening the disparity between the in vitro and in vivo microenvironments. Thus, the confluence of the two perspectives, representing interdisciplinary inputs as reflected in bioink development, i.e., biomimicry and manufacturability, are required for further advancement toward the future translation of biofabrication.
Based on the original works identified in this review, technology transition to commercial products could be anticipated in the next future in the field of wound management. In order to meet the market and clinical demand, these bioprinted constructs should enable tissue repair and reconstruction of skin architecture in clinically relevant contexts, such as diabetic or vascular ulcers or burn wounds. To shorten the time to market, experimental research (TRL4-5) should generate data that are ready to be used in the certification of the bioprinted construct. A co-development interdisciplinary methodology should achieve constructs with high performance -cost ratio and, generate clinical data that meet regulatory issues associated with marketing authorization of the living constructs (i.e. Advanced Therapy Medicinal Products, ATMP).
Although we are still far from skin fabrication for regenerative medicine, the applications of bioprinted constructs also expand to the generation of in vitro models for drug discovery, which is technically easier with less regulatory constraints. These features help to speed TRL development and get earlier the market demand, while leveraging our accomplishments in biofabrication.
References
- Braza, M.E.; Fahrenkopf, M.P. Split-Thickness Skin Grafts. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2020.
- Andriotis, E.G.; Eleftheriadis, G.K.; Karavasili, C.; Fatouros, D.G. Development of Bio-Active Patches Based on Pectin for the Treatment of Ulcers and Wounds Using 3D-Bioprinting Technology. Pharmaceutics 2020, 12, 56, doi:10.3390/pharmaceutics12010056.
- Baltazar, T.; Merola, J.; Catarino, C.; Xie, C.B.; Kirkiles-Smith, N.C.; Lee, V.; Hotta, S.; Dai, G.; Xu, X.; Ferreira, F.C.; et al. Three Dimensional Bioprinting of a Vascularized and Perfusable Skin Graft Using Human Keratinocytes, Fibroblasts, Pericytes, and Endothelial Cells. Tissue Eng. Part A 2020, 26, 227–238, doi:10.1089/ten.tea.2019.0201.
- Liu, X.; Michael, S.; Bharti, K.; Ferrer, M.; Song, M.J. A biofabricated vascularized skin model of atopic dermatitis for preclinical studies. Biofabrication 2020, 12, 035002, doi:10.1088/1758-5090/ab76a1.
- Wei, Z.; Liu, X.; Ooka, M.; Zhang, L.; Song, M.J.; Huang, R.; Kleinstreuer, N.C.; Simeonov, A.; Xia, M.; Ferrer, M. Two-Dimensional Cellular and Three-Dimensional Bio-Printed Skin Models to Screen Topical-Use Compounds for Irritation Potential. Front. Bioeng. Biotechnol. 2020, 8, 109, doi:10.3389/fbioe.2020.00109.
- McCormack, A.; Highley, C.B.; Leslie, N.R.; Melchels, F.P.W. 3D Printing in Suspension Baths: Keeping the Promises of Bioprinting Afloat. Trends Biotechnol. 2020, 38, 584–593, doi:10.1016/j.tibtech.2019.12.020.
- Kim, B.S.; Gao, G.; Kim, J.Y.; Cho, D. 3D Cell Printing of Perfusable Vascularized Human Skin Equivalent Composed of Epidermis, Dermis, and Hypodermis for Better Structural Recapitulation of Native Skin. Adv. Healthc. Mater. 2019, 8, 1801019, doi:10.1002/adhm.201801019.
- Dussoyer, M.; Courtial, E.J.; Albouy, M.; Thepot, A.; Dos Santos, M.; Marquette, C.A. Mechanical properties of 3D bioprinted dermis: characterization and improvement; Science Repository OÜ, 2019;
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706, doi:10.1152/physrev.00067.2017.
- Randall, M.J.; Jüngel, A.; Rimann, M.; Wuertz-Kozak, K. Advances in the Biofabrication of 3D Skin in vitro: Healthy and Pathological Models. Front. Bioeng. Biotechnol. 2018, 6, 154, doi:10.3389/fbioe.2018.00154.
- Takeo, M.; Lee, W.; Ito, M. Wound Healing and Skin Regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267–a023267, doi:10.1101/cshperspect.a023267.
- Andia, I.; Maffulli, N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat. Rev. Rheumatol. 2013, 9, 721–730, doi:10.1038/nrrheum.2013.141.
- Andia, I.; Maffulli, N. A contemporary view of platelet-rich plasma therapies: moving toward refined clinical protocols and precise indications. Regen. Med. 2018, 13, 717–728, doi:10.2217/rme-2018-0042.
- de Carvalho, C.K.L.; Fernandes, B.L.; de Souza, M.A. Autologous Matrix of Platelet-Rich Fibrin in Wound Care Settings: A Systematic Review of Randomized Clinical Trials. J. Funct. Biomater. 2020, 11, 31, doi:10.3390/jfb11020031.
- del Pino-Sedeño, T.; Trujillo-Martín, M.M.; Andia, I.; Aragón-Sánchez, J.; Herrera-Ramos, E.; Iruzubieta Barragán, F.J.; Serrano-Aguilar, P. Platelet-rich plasma for the treatment of diabetic foot ulcers: A meta-analysis: Platelet-rich plasma for diabetic foot ulcers. Wound Repair Regen. 2019, 27, 170–182, doi:10.1111/wrr.12690.
- San Sebastian, K.M.; Lobato, I.; Hernández, I.; Burgos-Alonso, N.; Gomez-Fernandez, M.C.; López, J.L.; Rodríguez, B.; March, A.G.; Grandes, G.; Andia, I. Efficacy and safety of autologous platelet rich plasma for the treatment of vascular ulcers in primary care: Phase III study. BMC Fam. Pract. 2014, 15, 211, doi:10.1186/s12875-014-0211-8.
- Perez-Zabala, E.; Basterretxea, A.; Larrazabal, A.; Perez-del-Pecho, K.; Rubio-Azpeitia, E.; Andia, I. Biological approach for the management of non-healing diabetic foot ulcers. J. Tissue Viability 2016, 25, 157–163, doi:10.1016/j.jtv.2016.03.003.
- Andia, I.; Abate, M. Platelet-rich plasma: underlying biology and clinical correlates. Regen. Med. 2013, 8, 645–658, doi:10.2217/rme.13.59.
- Groll, J.; Burdick, J.A.; Cho, D.-W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jüngst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication 2018, 11, 013001, doi:10.1088/1758-5090/aaec52.
- Pisani, S.; Dorati, R.; Scocozza, F.; Mariotti, C.; Chiesa, E.; Bruni, G.; Genta, I.; Auricchio, F.; Conti, M.; Conti, B. Preliminary investigation on a new natural based poly(gamma‐glutamic acid)/Chitosan bioink. J. Biomed. Mater. Res. B Appl. Biomater. 2020, jbm.b.34602, doi:10.1002/jbm.b.34602.
- Tigner, T.J.; Rajput, S.; Gaharwar, A.K.; Alge, D.L. Comparison of Photo Cross Linkable Gelatin Derivatives and Initiators for Three-Dimensional Extrusion Bioprinting. Biomacromolecules 2020, 21, 454–463, doi:10.1021/acs.biomac.9b01204.
- Zidarič, T.; Milojević, M.; Gradišnik, L.; Stana Kleinschek, K.; Maver, U.; Maver, T. Polysaccharide-Based Bioink Formulation for 3D Bioprinting of an In Vitro Model of the Human Dermis. Nanomaterials 2020, 10, 733, doi:10.3390/nano10040733.
- Compaan, A.M.; Song, K.; Huang, Y. Gellan Fluid Gel as a Versatile Support Bath Material for Fluid Extrusion Bioprinting. ACS Appl. Mater. Interfaces 2019, 11, 5714–5726, doi:10.1021/acsami.8b13792.
- Motealleh, A.; Dorri, P.; Schäfer, A.H.; Kehr, N.S. 3D bioprinting of triphasic nanocomposite hydrogels and scaffolds for cell adhesion and migration. Biofabrication 2019, 11, 035022, doi:10.1088/1758-5090/ab15ca.
- Osidak, E.O.; Karalkin, P.A.; Osidak, M.S.; Parfenov, V.A.; Sivogrivov, D.E.; Pereira, F.D.A.S.; Gryadunova, A.A.; Koudan, E.V.; Khesuani, Y.D.; Кasyanov, V.A.; et al. Viscoll collagen solution as a novel bioink for direct 3D bioprinting. J. Mater. Sci. Mater. Med. 2019, 30, 31, doi:10.1007/s10856-019-6233-y.
- Rutz, A.L.; Gargus, E.S.; Hyland, K.E.; Lewis, P.L.; Setty, A.; Burghardt, W.R.; Shah, R.N. Employing PEG crosslinkers to optimize cell viability in gel phase bioinks and tailor post printing mechanical properties. Acta Biomater. 2019, 99, 121–132, doi:10.1016/j.actbio.2019.09.007.
- Won, J.-Y.; Lee, M.-H.; Kim, M.-J.; Min, K.-H.; Ahn, G.; Han, J.-S.; Jin, S.; Yun, W.-S.; Shim, J.-H. A potential dermal substitute using decellularized dermis extracellular matrix derived bio-ink. Artif. Cells Nanomedicine Biotechnol. 2019, 47, 644–649, doi:10.1080/21691401.2019.1575842.
- Pereira, R.F.; Sousa, A.; Barrias, C.C.; Bártolo, P.J.; Granja, P.L. A single-component hydrogel bioink for bioprinting of bioengineered 3D constructs for dermal tissue engineering. Mater. Horiz. 2018, 5, 1100–1111, doi:10.1039/C8MH00525G.
- Wang, L.L.; Highley, C.B.; Yeh, Y.-C.; Galarraga, J.H.; Uman, S.; Burdick, J.A. Three-dimensional extrusion bioprinting of single- and double-network hydrogels containing dynamic covalent crosslinks: 3D EXTRUSION BIOPRINTING. J. Biomed. Mater. Res. A 2018, 106, 865–875, doi:10.1002/jbm.a.36323.
- Ahn, G.; Min, K.-H.; Kim, C.; Lee, J.-S.; Kang, D.; Won, J.-Y.; Cho, D.-W.; Kim, J.-Y.; Jin, S.; Yun, W.-S.; et al. Precise stacking of decellularized extracellular matrix based 3D cell-laden constructs by a 3D cell printing system equipped with heating modules. Sci. Rep. 2017, 7, 8624, doi:10.1038/s41598-017-09201-5.
- Li, H.; Tan, Y.J.; Leong, K.F.; Li, L. 3D Bioprinting of Highly Thixotropic Alginate/Methylcellulose Hydrogel with Strong Interface Bonding. ACS Appl. Mater. Interfaces 2017, 9, 20086–20097, doi:10.1021/acsami.7b04216.
- Ouyang, L.; Highley, C.B.; Sun, W.; Burdick, J.A. A Generalizable Strategy for the 3D Bioprinting of Hydrogels from Nonviscous Photo-crosslinkable Inks. Adv. Mater. 2017, 29, 1604983, doi:10.1002/adma.201604983.
- Shi, P.; Laude, A.; Yeong, W.Y. Investigation of cell viability and morphology in 3D bio-printed alginate constructs with tunable stiffness: 3D BIO-PRINTED ALGINATE CONSTRUCTS WITH TUNABLE STIFFNESS. J. Biomed. Mater. Res. A 2017, 105, 1009–1018, doi:10.1002/jbm.a.35971.
- Dubbin, K.; Hori, Y.; Lewis, K.K.; Heilshorn, S.C. Dual-Stage Crosslinking of a Gel-Phase Bioink Improves Cell Viability and Homogeneity for 3D Bioprinting. Adv. Healthc. Mater. 2016, 5, 2488–2492, doi:10.1002/adhm.201600636.
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 2016, 6, 29977, doi:10.1038/srep29977.
- Stunova, A.; Vistejnova, L. Dermal fibroblasts—A heterogeneous population with regulatory function in wound healing. Cytokine Growth Factor Rev. 2018, 39, 137–150, doi:10.1016/j.cytogfr.2018.01.003.
- Jorgensen, A.M.; Varkey, M.; Gorkun, A.; Clouse, C.; Xu, L.; Chou, Z.; Murphy, S.V.; Molnar, J.; Lee, S.J.; Yoo, J.J.; et al. Bioprinted Skin Recapitulates Normal Collagen Remodeling in Full-Thickness Wounds. Tissue Eng. Part A 2020, 26, 512–526, doi:10.1089/ten.tea.2019.0319.
- Kim, B.S.; Kwon, Y.W.; Kong, J.-S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.-B.; Lee, H.; Kim, J.H.; Cho, D.-W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53, doi:10.1016/j.biomaterials.2018.03.040.
- Attalla, R.; Puersten, E.; Jain, N.; Selvaganapathy, P.R. 3D bioprinting of heterogeneous bi- and tri-layered hollow channels within gel scaffolds using scalable multi-axial microfluidic extrusion nozzle. Biofabrication 2018, 11, 015012, doi:10.1088/1758-5090/aaf7c7.
- Rutz, A.L.; Hyland, K.E.; Jakus, A.E.; Burghardt, W.R.; Shah, R.N. A Multimaterial Bioink Method for 3D Printing Tunable, Cell-Compatible Hydrogels. Adv. Mater. 2015, 27, 1607–1614, doi:10.1002/adma.201405076.
- Shi, Y.; Xing, T.L.; Zhang, H.B.; Yin, R.X.; Yang, S.M.; Wei, J.; Zhang, W.J. Tyrosinase-doped bioink for 3D bioprinting of living skin constructs. Biomed. Mater. 2018, 13, 035008, doi:10.1088/1748-605X/aaa5b6.
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte–Fibroblast Interactions in Wound Healing. J. Invest. Dermatol. 2007, 127, 998–1008, doi:10.1038/sj.jid.5700786.
- Albanna, M.; Binder, K.W.; Murphy, S.V.; Kim, J.; Qasem, S.A.; Zhao, W.; Tan, J.; El-Amin, I.B.; Dice, D.D.; Marco, J.; et al. In Situ Bioprinting of Autologous Skin Cells Accelerates Wound Healing of Extensive Excisional Full-Thickness Wounds. Sci. Rep. 2019, 9, 1856, doi:10.1038/s41598-018-38366-w.
- Hakimi, N.; Cheng, R.; Leng, L.; Sotoudehfar, M.; Ba, P.Q.; Bakhtyar, N.; Amini-Nik, S.; Jeschke, M.G.; Günther, A. Handheld skin printer: in situ formation of planar biomaterials and tissues. Lab. Chip 2018, 18, 1440–1451, doi:10.1039/C7LC01236E.
- Cubo, N.; Garcia, M.; del Cañizo, J.F.; Velasco, D.; Jorcano, J.L. 3D bioprinting of functional human skin: production and in vivo analysis. Biofabrication 2016, 9, 015006, doi:10.1088/1758-5090/9/1/015006.
- Seol, Y.-J.; Lee, H.; Copus, J.S.; Kang, H.-W.; Cho, D.-W.; Atala, A.; Lee, S.J.; Yoo, J.J. 3D bioprinted biomask for facial skin reconstruction. Bioprinting 2018, 10, e00028, doi:10.1016/j.bprint.2018.e00028.
- Kim, B.S.; Lee, J.-S.; Gao, G.; Cho, D.-W. Direct 3D cell-printing of human skin with functional transwell system. Biofabrication 2017, 9, 025034, doi:10.1088/1758-5090/aa71c8.
- Harrell, C.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467, doi:10.3390/cells8050467.
- Kajave, N.S.; Schmitt, T.; Nguyen, T.-U.; Kishore, V. Dual crosslinking strategy to generate mechanically viable cell-laden printable constructs using methacrylated collagen bioinks. Mater. Sci. Eng. C 2020, 107, 110290, doi:10.1016/j.msec.2019.110290.
- Liu, P.; Shen, H.; Zhi, Y.; Si, J.; Shi, J.; Guo, L.; Shen, S.G. 3D bioprinting and in vitro study of bilayered membranous construct with human cells-laden alginate/gelatin composite hydrogels. Colloids Surf. B Biointerfaces 2019, 181, 1026–1034, doi:10.1016/j.colsurfb.2019.06.069.
- Montheil, T.; Maumus, M.; Valot, L.; Lebrun, A.; Martinez, J.; Amblard, M.; Noël, D.; Mehdi, A.; Subra, G. Inorganic Sol–Gel Polymerization for Hydrogel Bioprinting. ACS Omega 2020, 5, 2640–2647, doi:10.1021/acsomega.9b03100.
- Xin, S.; Chimene, D.; Garza, J.E.; Gaharwar, A.K.; Alge, D.L. Clickable PEG hydrogel microspheres as building blocks for 3D bioprinting. Biomater. Sci. 2019, 7, 1179–1187, doi:10.1039/C8BM01286E.
- Giuseppe, M.D.; Law, N.; Webb, B.; A. Macrae, R.; Liew, L.J.; Sercombe, T.B.; Dilley, R.J.; Doyle, B.J. Mechanical behaviour of alginate-gelatin hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 79, 150–157, doi:10.1016/j.jmbbm.2017.12.018.
- Law, N.; Doney, B.; Glover, H.; Qin, Y.; Aman, Z.M.; Sercombe, T.B.; Liew, L.J.; Dilley, R.J.; Doyle, B.J. Characterisation of hyaluronic acid methylcellulose hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 77, 389–399, doi:10.1016/j.jmbbm.2017.09.031.
- Sasaki, M.; Abe, R.; Fujita, Y.; Ando, S.; Inokuma, D.; Shimizu, H. Mesenchymal Stem Cells Are Recruited into Wounded Skin and Contribute to Wound Repair by Transdifferentiation into Multiple Skin Cell Type. J. Immunol. 2008, 180, 2581–2587, doi:10.4049/jimmunol.180.4.2581.
- Chu, D.-T.; Nguyen Thi Phuong, T.; Tien, N.L.B.; Tran, D.K.; Minh, L.B.; Thanh, V.V.; Gia Anh, P.; Pham, V.H.; Thi Nga, V. Adipose Tissue Stem Cells for Therapy: An Update on the Progress of Isolation, Culture, Storage, and Clinical Application. J. Clin. Med. 2019, 8, 917, doi:10.3390/jcm8070917.
- Mendes, B.B.; Gómez-Florit, M.; Hamilton, A.G.; Detamore, M.S.; Domingues, R.M.A.; Reis, R.L.; Gomes, M.E. Human platelet lysate-based nanocomposite bioink for bioprinting hierarchical fibrillar structures. Biofabrication 2019, 12, 015012, doi:10.1088/1758-5090/ab33e8.
- Pepelanova, I.; Kruppa, K.; Scheper, T.; Lavrentieva, A. Gelatin-Methacryloyl (GelMA) Hydrogels with Defined Degree of Functionalization as a Versatile Toolkit for 3D Cell Culture and Extrusion Bioprinting. Bioengineering 2018, 5, 55, doi:10.3390/bioengineering5030055.
- Raddatz, L.; Lavrentieva, A.; Pepelanova, I.; Bahnemann, J.; Geier, D.; Becker, T.; Scheper, T.; Beutel, S. Development and Application of an Additively Manufactured Calcium Chloride Nebulizer for Alginate 3D-Bioprinting Purposes. J. Funct. Biomater. 2018, 9, 63, doi:10.3390/jfb9040063.
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020, doi:10.1088/1758-5090/8/3/035020.
- Li, Z.; Huang, S.; Liu, Y.; Yao, B.; Hu, T.; Shi, H.; Xie, J.; Fu, X. Tuning Alginate-Gelatin Bioink Properties by Varying Solvent and Their Impact on Stem Cell Behavior. Sci. Rep. 2018, 8, 8020, doi:10.1038/s41598-018-26407-3.
- Li, Y.; Jiang, X.; Li, L.; Chen, Z.-N.; Gao, G.; Yao, R.; Sun, W. 3D printing human induced pluripotent stem cells with novel hydroxypropyl chitin bioink: scalable expansion and uniform aggregation. Biofabrication 2018, 10, 044101, doi:10.1088/1758-5090/aacfc3.
- 3D Bioprinting: Principles and Protocols; Crook, J.M., Ed.; Methods in Molecular Biology; Springer US: New York, NY, 2020; Vol. 2140; ISBN 978-1-07-160519-6.
- Pu, L.; Meng, M.; Wu, J.; Zhang, J.; Hou, Z.; Gao, H.; Xu, H.; Liu, B.; Tang, W.; Jiang, L.; et al. Compared to the amniotic membrane, Wharton’s jelly may be a more suitable source of mesenchymal stem cells for cardiovascular tissue engineering and clinical regeneration. Stem Cell Res. Ther. 2017, 8, 72, doi:10.1186/s13287-017-0501-x.
- Skardal, A.; Mack, D.; Kapetanovic, E.; Atala, A.; Jackson, J.D.; Yoo, J.; Soker, S. Bioprinted Amniotic Fluid-Derived Stem Cells Accelerate Healing of Large Skin Wounds. STEM CELLS Transl. Med. 2012, 1, 792–802, doi:10.5966/sctm.2012-0088.
- Liu, W.; Heinrich, M.A.; Zhou, Y.; Akpek, A.; Hu, N.; Liu, X.; Guan, X.; Zhong, Z.; Jin, X.; Khademhosseini, A.; et al. Extrusion Bioprinting of Shear‐Thinning Gelatin Methacryloyl Bioinks. Adv. Healthc. Mater. 2017, 6, 1601451, doi:10.1002/adhm.201601451.
- Derr, K.; Zou, J.; Luo, K.; Song, M.J.; Sittampalam, G.S.; Zhou, C.; Michael, S.; Ferrer, M.; Derr, P. Fully Three-Dimensional Bioprinted Skin Equivalent Constructs with Validated Morphology and Barrier Function. Tissue Eng. Part C Methods 2019, 25, 334–343, doi:10.1089/ten.tec.2018.0318.
- Anitua, E.; Sánchez, M.; Orive, G.; Andia, I. Delivering growth factors for therapeutics. Trends Pharmacol. Sci. 2008, 29, 37–41, doi:10.1016/j.tips.2007.10.010.
- Quílez, C.; de Aranda Izuzquiza, G.; García, M.; López, V.; Montero, A.; Valencia, L.; Velasco, D. Bioprinting for Skin. In 3D Bioprinting: Principles and Protocols; Crook, J.M., Ed.; Methods in Molecular Biology; Springer US: New York, NY, 2020; pp. 217–228 ISBN 978-1-07-160520-2.
- Choudhury, D.; Tun, H.W.; Wang, T.; Naing, M.W. Organ-Derived Decellularized Extracellular Matrix: A Game Changer for Bioink Manufacturing? Trends Biotechnol. 2018, 36, 787–805, doi:10.1016/j.tibtech.2018.03.003.
- Wu, Z.; Su, X.; Xu, Y.; Kong, B.; Sun, W.; Mi, S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci. Rep. 2016, 6, 24474, doi:10.1038/srep24474.
- Moreno-Arotzena, O.; Meier, J.; del Amo, C.; García-Aznar, J. Characterization of Fibrin and Collagen Gels for Engineering Wound Healing Models. Materials 2015, 8, 1636–1651, doi:10.3390/ma8041636.
- Del Amo, C.; Borau, C.; Movilla, N.; Asín, J.; García-Aznar, J.M. Quantifying 3D chemotaxis in microfluidic-based chips with step gradients of collagen hydrogel concentrations. Integr. Biol. 2017, 9, 339–349, doi:10.1039/c7ib00022g.
- Del Amo, C.; Perez-Valle, A.; Perez-Zabala, E.; Perez-del-Pecho, K.; Larrazabal, A.; Basterretxea, A.; Bully, P.; Andia, I. Wound Dressing Selection Is Critical to Enhance Platelet-Rich Fibrin Activities in Wound Care. Int. J. Mol. Sci. 2020, 21, 624, doi:10.3390/ijms21020624.
- Unagolla, J.M.; Jayasuriya, A.C. Enhanced cell functions on graphene oxide incorporated 3D printed polycaprolactone scaffolds. Mater. Sci. Eng. C 2019, 102, 1–11, doi:10.1016/j.msec.2019.04.026.
- Panwar, A.; Tan, L. Current Status of Bioinks for Micro-Extrusion-Based 3D Bioprinting. Molecules 2016, 21, 685, doi:10.3390/molecules21060685.
- Ooi, H.W.; Mota, C.; ten Cate, A.T.; Calore, A.; Moroni, L.; Baker, M.B. Thiol–Ene Alginate Hydrogels as Versatile Bioinks for Bioprinting. Biomacromolecules 2018, 19, 3390–3400, doi:10.1021/acs.biomac.8b00696.
- Lee, H.J.; Kim, Y.B.; Ahn, S.H.; Lee, J.-S.; Jang, C.H.; Yoon, H.; Chun, W.; Kim, G.H. A New Approach for Fabricating Collagen/ECM-Based Bioinks Using Preosteoblasts and Human Adipose Stem Cells. Adv. Healthc. Mater. 2015, 4, 1359–1368, doi:10.1002/adhm.201500193.
- Bociaga, D.; Bartniak, M.; Grabarczyk, J.; Przybyszewska, K. Sodium Alginate/Gelatine Hydrogels for Direct Bioprinting—The Effect of Composition Selection and Applied Solvents on the Bioink Properties. Materials 2019, 12, 2669, doi:10.3390/ma12172669.
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: an overview. Biomater. Sci. 2018, 6, 915–946, doi:10.1039/C7BM00765E.
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271, doi:10.1016/j.biomaterials.2015.08.045.
- Alves, L.; Medronho, B.; Antunes, F.E.; Fernández-García, M.P.; Ventura, J.; Araújo, J.P.; Romano, A.; Lindman, B. Unusual extraction and characterization of nanocrystalline cellulose from cellulose derivatives. J. Mol. Liq. 2015, 210, 106–112, doi:10.1016/j.molliq.2014.12.010.
- Turley, E.A.; Noble, P.W.; Bourguignon, L.Y.W. Signaling Properties of Hyaluronan Receptors. J. Biol. Chem. 2002, 277, 4589–4592, doi:10.1074/jbc.R100038200.
- Baier, C.; Baader, S.L.; Jankowski, J.; Gieselmann, V.; Schilling, K.; Rauch, U.; Kappler, J. Hyaluronan is organized into fiber-like structures along migratory pathways in the developing mouse cerebellum. Matrix Biol. 2007, 26, 348–358, doi:10.1016/j.matbio.2007.02.002.
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343, doi:10.1016/j.biomaterials.2015.10.076.
- Aya, K.L.; Stern, R. Hyaluronan in wound healing: Rediscovering a major player: Hyaluronan in wound healing. Wound Repair Regen. 2014, 22, 579–593, doi:10.1111/wrr.12214.
- Wu, C.; Wang, B.; Zhang, C.; Wysk, R.A.; Chen, Y.-W. Bioprinting: an assessment based on manufacturing readiness levels. Crit. Rev. Biotechnol. 2017, 37, 333–354, doi:10.3109/07388551.2016.1163321.
- MedicalCountermeasures.gov Available online: https://www.medicalcountermeasures.gov/trl/integrated-trls/ (accessed on Aug 31, 2020).
- Naveau, A.; Smirani, R.; Catros, S.; de Oliveira, H.; Fricain, J.-C.; Devillard, R. A Bibliometric Study to Assess Bioprinting Evolution. Appl. Sci. 2017, 7, 1331, doi:10.3390/app7121331.
- Costa, P.F. Translating Biofabrication to the Market. Trends Biotechnol. 2019, 37, 1032–1036, doi:10.1016/j.tibtech.2019.04.013.
- Yang, Q.; Gao, B.; Xu, F. Recent Advances in 4D Bioprinting. Biotechnol. J. 2020, 15, 1900086, doi:10.1002/biot.201900086.
- Wu, Y.; Ravnic, D.J.; Ozbolat, I.T. Intraoperative Bioprinting: Repairing Tissues and Organs in a Surgical Setting. Trends Biotechnol. 2020, 38, 594–605, doi:10.1016/j.tibtech.2020.01.004.