High-efficiency utilization of CO2 facilitates the reduction of CO2 concentration in the global atmosphere and hence the alleviation of the greenhouse effect. The catalytic hydrogenation of CO2 to produce value-added chemicals exhibits attractive prospects by potentially building energy recycling loops.
- carbon dioxide
- hydrogenation
- heterogeneous catalysis
- methanol synthesis
1. Cu-Based Catalysts
2. In2O3-Based Catalysts
In2O3 was initially reported to show excellent catalytic activity for methanol steam reforming with extremely high selectivity of CO2 relative to that of Cu/ZnO catalysts [36]. In 2012, Ge’s group published a theoretical paper predicting the methanol synthesis activity of In2O3 from CO2 hydrogenation [37]. In the following paper, oxygen vacancy (D4) was deemed to play a key role in the activation of CO2 and stabilizing the key intermediates involved (see Figure 5 for the structure of ideal and defective In2O3(110) surface) [38]. Since then, a number of works have been published reporting the superior activities and selectivities of pure In2O3 or In2O3-based catalysts [39][40][41][42][43][44][45][46][47][48][49]. A very early experimental test was performed on commercial In2O3 powders after simple calcination in air at 500 °C for 5 h [46]. The catalyst showed somewhat comparable activities with the reported Cu-based catalysts. This could be a promising result since only pure In2O3 was applied, not to mention the unknown specific surface area.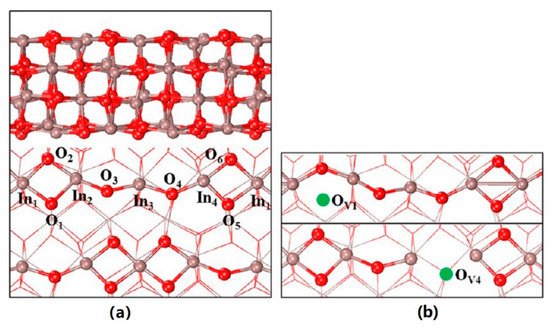
3. Nanoalloy Catalysts
Nanoalloy catalysts often exhibit unique electronic structures distinguished from either component [11][52][53][54]. As a result, the bonding properties of reactants, intermediates, or products can be tuned, which finally yields tunable activity and selectivity of the catalysts. Nanoalloy is formed either in the preparation process or under reaction conditions. The existence of nanoalloy can be validated by the finger-printed diffraction angle (2θ) in X-ray diffraction (XRD) [55], the alloy state of an element in X-ray photoelectron spectroscopy (XPS) [56], the specific vibrational mode of probe molecules binding to the alloy sites [57], or the lattice constant value shown in transmission electron microscopy (TEM) [58][59]. For a specific reaction, the turnover frequency (TOF) can be tuned by either the composition or the relative content [60]. Studt et al. compared the catalytic activities of three Ni-Ga catalysts via incipient wetness impregnation method and Cu/ZnO/Al2O3 catalyst via coprecipitation route as reference [7][60]. The better activity and methanol selectivity of the Ni5Ga3/SiO2 catalyst were attributed to the suppressed RWGS activity. While supported on mesoporous nitrogen-rich carbon, Ni5Ga3 was also found to have good activity for CO2 hydrogenation, though the activity is sensitive to the preparation method [59]. The author highlighted the freeze drying method that enables uniformly distributed metal nanoparticles with an average size of 2–5 nm and correlated the formation of NiGa alloy with the suitable local environment realized by the preparation method. Shi et al. [58] synthesized Cu-In intermetallic catalyst and investigated the effect of reduction temperature on the reaction activity. It was found that reduction temperature exerts a notable influence on the formation of alloy, the crystallite size, and the adsorption of gases. For instance, CuO was reduced to metallic Cu at 250 °C, and the Cu11In9 alloy appeared when the reduction temperature was above 300 °C. With higher reduction temperature, the crystallite size of Cu11In9 increases slightly, accompanied by an attenuated H2 adsorption ability. The CO2 adsorption ability varies notably with increasing the reduction temperature, and the maximum value was obtained with CuIn-300 (reduced at 300 °C), the trend of which agrees well with the STY of methanol tested at 240 °C and 3 MPa (for the same reason, the CO2 adsorption ability was thought to be the key factor for the design of Cu/In2O3 catalysts). Besides the temperature, reduction pressure also affects the formation of alloy. An X-ray absorption near edge structure (XANES) analysis on the reduction process of commercially available Cu/ZnO/Al2O3 catalyst at different pressure (1 mbar–10 bar) showed that Cu–Zn alloys were formed only under reduction pressure of 100 mbar or above [61]. The maximum reduction rate (simultaneous formation of copper (0)) is gradually shifted to low temperature by elevating the reduction pressure. The catalysts started to show methanol synthesis activity when the total pressure was above 1 bar, along with the increased formation of oxygen vacancies and other structural distortions in the ZnO phase. Note that, in this section, thwe researchers do not really attempt to emphasize the alloy formation in the activity modification for the metal supported on oxide since their interactions are not necessarily accompanied by the formation of nanoalloy. For Ni supported on In2O3, the authors emphasized the role of Ni–In alloy formation in determining its activity [41][54], while Hensen and coworkers [62] claimed the activity of Ni/In2O3 system stems from the synergy effect with no alloy formation evidenced. In any case, the formation of nanoalloy in modifying the electronic structure and hence the catalytic performance provides a novel idea for catalyst design.4. Other Catalysts
MoS2 and MoS2-based materials have been widely used as lubricants [63], transistors [64], heterogeneous catalysts [65][66][67], and gas sensors [68]. Due to the special lattice structure, MoS2 is easily peeled into thin layers or even a single atomic layer [69]. The electronic structure of two-dimensional MoS2 is sensitive to the status of surface vacancies. The sulfur (S) vacancies especially located at edges or in-plane, exhibit completely different catalytic activities [70][71][72]. The edge S vacancies were thought to catalyze the CO2 hydrogenation to methane, while the in-plane S vacancies were proven to be ideal active sites for CO2 hydrogenation to methanol [70]. Over the in-plane sulfur vacancy-rich MoS2 nanosheets, a methanol selectivity of 94.3% at a CO2 conversion of 12.5% was achieved at 180 °C, and the catalyst was stable for over 3000 h without any deactivation. The findings enlightened the potential role of the in-plane vacancies in catalysis, and further modification of the vacancies-controllable MoS2 material or two-dimensional material is meaningful. Due to the unique structural characteristics, MOF materials were featured with highly ordered and tunable porous structures, high surface area, flexible organic linkers, and metal centers [73][74][75]. Accordingly, MOFs can be a template for porous material synthesis [30], supporting materials for nanocatalysts [75][76], or act as catalysts individually by introducing active metal centers as nodes or located on the MOF linkers [77][78]. Although many MOFs suffer from high temperature and moisture conditions, some selected MOF-based catalysts, including UiO-bpy [75], MOF-74 [76], UiO-67 [78], and UiO-66 [79], were reported to have good thermal stability even under moisture condition, of which further tuning of the catalytic properties is likely foreseen. Another promising catalyst catalog for CO2 hydrogenation to methanol is solid solution catalysts [80][81][82][83]. Wang et al. have prepared a series of ZnO-ZrO2 solid solution catalysts through the coprecipitation method [81]. Both methanol selectivity and CO2 conversion reach maximum over the catalyst when Zn/(Zn + Zr) ratio is around 13%. Methanol selectivity of 86% to 91% with CO2 conversion of 10% was achieved under the condition of 5.0 MPa, 24,000 mL·g−1h−1 and 320 °C. The catalyst was proved to show long-term thermal and chemical stabilities against sintering and poisoning by, e.g., SO2 or H2S. Density functional theory (DFT) simulation results suggested that Zn and Zr provide the adsorption sites for H2 and CO2, respectively. Therefore, a solid solution catalyst takes advantage of both components to achieve the synergetic effect. Such synergetic effect was also observed in other solid solution catalysts such as MaZrOx (Ma = Cd, Ga) [82]. Another interesting aspect of the ZnO-ZrO2 solid solution catalyst is that its activity was reported to be sensitive to the preparation method (the microstructure) rather than the ZnO/ZrO2 ratio. The 20% ZnO-ZrO2 catalyst prepared by the evaporation-induced self-assembly process exhibited better methanol synthesis activity than that of the coprecipitation method [80]. The enhanced activity of the former was ascribed to its larger specific surface area related to the mesoporous structure and more active sites for CO2 and H2 adsorption (which are possibly correlated with the larger surface area).5. Mechanistic Understanding
As shown in the above text, large numbers of catalysts have been reported on their superior activities for CO2 hydrogenation to methanol. They may be synthesized with different methods, from different sorts and proportions of raw materials, treated with different parameters, or activated under different reaction conditions. Consequently, the resulted catalysts are featured with varied compositions (at surface region), microscopic morphologies, particle sizes, surface area, bonding properties, etc., finally leading to unique activity, selectivity, and stability. One of the practically noticeable consequences is the gradually improved STY and methanol selectivity in the newest publications. Parallel to the improvement in catalyst development, a comprehensive understanding of the structure–activity relationship has made a lot of progress based on systematic kinetic analysis, surface science study, operando techniques, theoretical simulations, etc., although one must note that some reported mechanisms might be case-dependent and not expanded to other systems.
An active site is one of the most important concepts in the study of catalysis [84][85][86]. It functions in multiway, providing adsorption sites for reactants, diffusion routes for adsorbate, suitable microscopic geometric matching for the reaction, appropriate bonding to intermediates, etc. Therefore, an active site is generally not a single point, a special micro or macrostructure, or a certain element with a specified chemical state, but the combination of a series of microscopic locations with distinctive functions and special elemental, electronic and geometric matching for the certain specific catalytic reaction [86]. It is an arduous task to build up a panorama of an active site, not to mention the difficulties superimposed by the structural complexity of nano-sized particles and numerous interferential issues. An alternative and feasible way is to simplify the catalyst system, e.g., to perform reactions on structurally well-defined model catalysts, such as single crystals, polycrystalline, and thin films [87][88][89]. TIn the researchersis section, we summarize the works about active sites for catalytic hydrogenation of CO2 to CH3OH on both modeled single crystals and realistic powder catalysts.
