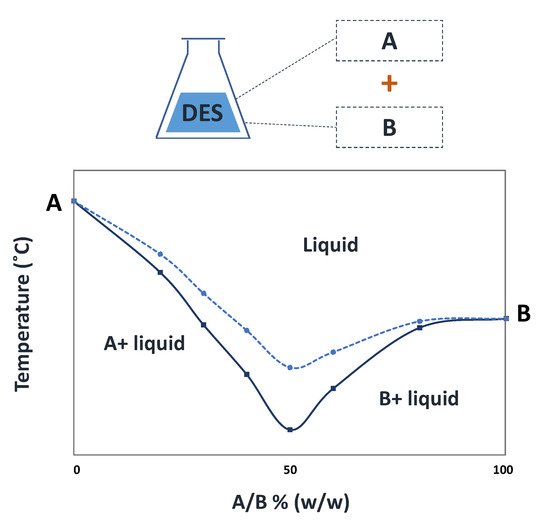
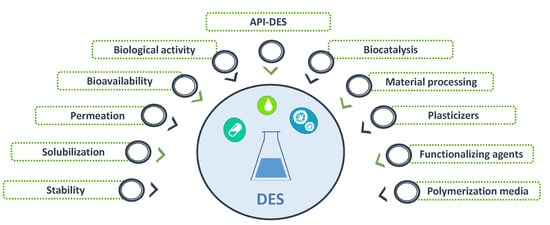
Deep eutectic solvents (DES) are eutectic mixtures that present a deviation from the ideal thermodynamic solid–liquid phase behavior, where a significant depression in the melting temperature occurs. If properly designed and chosen, DES may be liquid at room and the human body’s temperatures and display a biocompatible character, thus representing relevant options in the pharmaceutical field. Accordingly, DES have been studied as alternative solvents or in formulations of pharmaceuticals to improve their solubility and stability. Depending on the DES components, these mixtures might exhibit interesting biological activities compatible with several applications. The use of DES as functional agents or as novel liquid forms of active pharmaceutical ingredients (API-DES) with the goal of improving bioavailability, permeability and therapeutic efficacy of a given API stands as alternative strategies in the pharmaceutical field for drug delivery purposes.
- deep eutectic solvents
- active pharmaceutical ingredients
- solubility
- bioavailability
- therapeutic efficacy
1. Introduction
| DES | Molar Ratio | API Solubilized | Ref |
|---|
| [Ch]Cl–urea |
| 1:2 |
| Piroxicam |
2. DES Applications within the Pharmaceutical Field
2.1. DES in Biocatalysis to Produce APIs Precursors
| DES | Enzyme | API Precursor | Amount of DES (% v/v) | Yield (%) | Ref | |
|---|---|---|---|---|---|---|
| [Ch]Cl–urea | Candida rugosa lipase 1 | p-nitrophenol | 10 | 122.0 | [41] | |
| [Ch]Cl–glycerol | 103.0 | Aprepitant | [13] | |||
| [Ch]Cl–1,2-propanediol | ||||||
| [Ch]Cl–ethylene glycol | 90.0 | 1:2 | Aspirin | [15] | ||
| [Ch]Cl–urea–glycerol | 155.0 | [Ch]Cl–ethyleneglycol | 1:2 | |||
| [Ch]Cl–glycerol–ethylene glycol | Naproxen | [16] | ||||
| Betaine–glycerol–water | 1:2:1 | Indomethacin | [13] | |||
| 117.0 | ||||||
| [Ch]Cl–urea–thiourea | 120.0 | [Ch]Cl–malic acid | 1:1 | Celecoxib | [16] | |
| [Ch]Cl–formamide–thiourea | 83.0 | Camphor–menthol | 1:1 | Ibuprofen | [ | |
| [Ch]Cl–glycerol | Candida antarctica lipase B17] | |||||
| (R)-1-phenylethanol | 55 | 54.6 | [42] | [Ch]Cl–levulinic acid | 1:2 | Ketoprofen |
| [Ch]Cl–lactic acid | Lysinibacillus Fusiformis1 CGMCC1347 | [ | Vanillin | 15] | ||
| 20 | 98.9 | [43] | Parent API | API–DES | Molar ratio | Ref |
| [Ch]Cl–citric acid | 58.1 | Aspirin | [Ch]Cl–aspirin | 1:2 | [18] | |
| Ibuprofen | Ibuprofen–menthol | 1:3 | [19] | |||
| Ibuprofen | Ibuprofen–benzoic acid | 1:3 | [19] | |||
| [Ch]Cl–ethylene glycol | 110.7 | |||||
| [Ch]Cl–glycerol | 117.1 | Ibuprofen | Ibuprofen–phenylacetic acid | 1:2 | [19] | |
| Ibuprofen | ||||||
| [Ch]Cl–sorbitol | 125.6 | Ibuprofen–limonene | 1:8 | [20] | ||
| Ethambutol | Citric Acid–Ethambutol–water | 2:1:10 | [21] |
| [Ch]Cl–xylose |
| 129.5 |
| [Ch]Cl–glucose |
| 126.7 |
| [Ch]Cl–galactose |
| 129.9 |
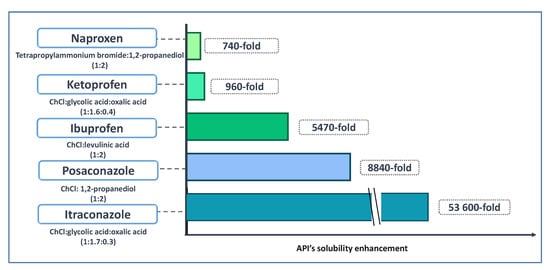
2.2. DES for APIs Solubilization
2.3. DES to Improve APIs Stability
| API | Reference Solvent | DES | Storage Conditions | Stability Improvement in DES Media | Ref |
|---|---|---|---|---|---|
| Chemical stability | |||||
| DES | Molar Ratio | Microorganism/Cell Line | Ref |
|---|
| Aspirin | Water |
| Antibacterial activity | [Ch]Cl–1,2-propanediol | 14 h at 80 °C | Cleavage into salicylic and acetic acids is 8.2 times slower | |||||
| [Ch]Cl–urea | 1:2 | E. coli S. enteritidis S. aureus L. moncytogenes | [15] | |||||
| [ | 70 | ] | Imipenem | Water | Betaine–urea | 7-day storage at 25 °C | 7-fold stability improvement | [14] |
| [Ch]Cl–acetamide | Clavulanic acid | Water | 2.5-fold stability improvement | |||||
| [Ch]Cl–ethylene glycol | Light stability | |||||||
| 64 | ||||||||
| [Ch]Cl–glycerol | 5,10,15,20-tetrakis(4-hydroxyphenyl)-porphyrin (THPP) | Methanol | Citric acid–glucose | 3 to 5 h exposure to 765 W∙m−2 (310–800 nm) irradiation to an endpoint of 8 h corresponding to 1.2 × 106 lux∙h (400–800 nm) |
Lower rate of photodegradation | [60] | ||
| Curcumin | Methanol | [Ch]Cl–glycerol] | ||||||
| [Ch]Cl–triethylene glycol | 2 h exposure to sunlight | Preserved stability | ||||||
| [Ch]Cl–xylitol | 1:1 | [ | Thermal stability | |||||
| [Ch]Cl–D-sorbitol | Chondroitinase ABCI | Phosphate buffer |
||||||
| [Ch]Cl–xylose/water | [Ch]Cl–glycerol Betaine–glycerol |
15 days storage at −20 °C | Enzyme activity retention of 95% and 80%, respectively, vs loss of activity after 5 days in its absence | 1:1:1[68] | ||||
| Human interferon-α2 |
Phosphate buffer | [Ch]Cl–fructose | Short-term (2 h) and long-term (3 months) storage at 37 °C | Preservation of structural integrity and activity | [69] | |||
2.4. DES with Biological Activity
| [Ch]Cl–sucrose/water | |||
| 5:2 | |||
| [Ch]Cl–fructose/water | |||
| 5:2:5 | |||
| [Ch]Cl–glucose/water | |||
| [Ch]Cl–p-toluenesulfonic acid | 1:1 | ||
| [Ch]Cl–oxalic acid | 1:1 | ||
| [Ch]Cl–levulinic acid | 1:2 | ||
| [Ch]Cl–malonic acid | 1:1 | ||
| [Ch]Cl–malic acid | |||
| [Ch]Cl–citric acid | |||
| [Ch]Cl–tartaric acid | 2:1 | ||
| Caprylic acid–myristic acid | 3:1 | E. coli P. aeruginosa |
[73] |
| Caprylic acid–stearic acid | 4:1 | ||
| Caprylic acid–lauric acid | 2:1 | S. aureus MRSA |
|
| Antifungal activity | |||
| [Ch]Cl–zinc chloride | 1:2 | P. chrysosporium A. niger L. tigrinus C. cylindracea |
[74] |
| [Ch]Cl–malonic acid | 1:1 | ||
| [Ch]Cl–p-toluenesulfonic acid | 1:3 | ||
| [Ch]Cl–urea | 1:2 | ||
| [Ch]Cl–glycerol | 1:2 | ||
| [Ch]Cl–ethylene glycol | 1:3 | ||
| [Ch]Cl–diethylene glycol | 1:2 | ||
| [Ch]Cl–triethylene glycol | 1:3 | ||
| [Ch]Cl–fructose | 2:1 | ||
| [Ch]Cl–glucose | |||
| Anti-tumoral activity | |||
| [Ch]Cl–glycerol | 1:3 | Human prostate cancer (PC3); Human malignant melanoma (A375); Human colon adenocarcinoma (HT29); Human breast cancer (MCF-7) |
[75] |
| [Ch]Cl–ethylene glycol | |||
| [Ch]Cl–urea | |||
| [Ch]Cl–triethylene glycol | |||
| [Ch]Cl–fructose | 5:2 | Human cervical cancer (HelaS3); Human ovarian cancer (CaOV3); Mouse skin cancer (B16F10) |
[76] |
| [Ch]Cl–glucose | |||
| [Ch]Cl–sucrose | 4:1 | ||
| [Ch]Cl–glycerol | 1:2 | ||
| [Ch]Cl–malonic acid | 1:1 | ||
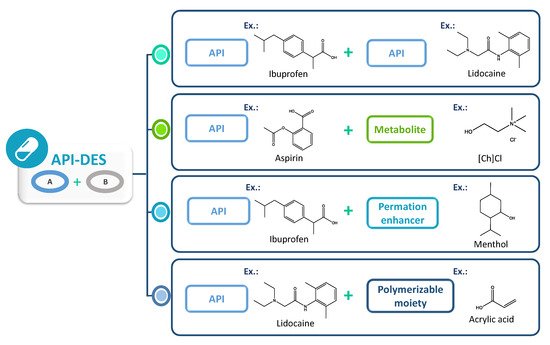
2.5. API-DES Formulations
2.6. DES in Drug Delivery Systems
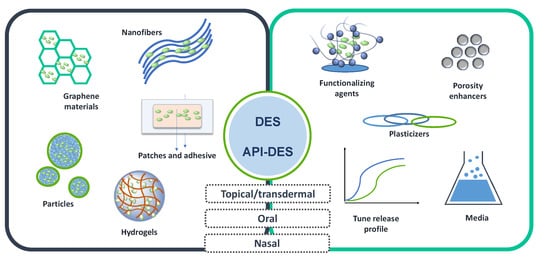
3. Conclusions and Future Perspectives
References
- Shekunov, B.Y.; York, P. Crystallization process in pharmaceutical technology and drug delivery design. J. Cryst. Growth 2000, 211, 122–136.Shekunov, B.Y.; York, P. Crystallization process in pharmaceutical technology and drug delivery design. J. Cryst. Growth 2000, 211, 122–136.
- Singhal, D.; Curatolo, W. Drug polymorphism and dosage form design: A practical perspective. Adv. Drug Deliv. Rev. 2004, 56, 335–347.Singhal, D.; Curatolo, W. Drug polymorphism and dosage form design: A practical perspective. Adv. Drug Deliv. Rev. 2004, 56, 335–347.
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 195727.Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 195727.
- Sekiguchi, K.; Obi, N. Studies on Absorption of Eutectic Mixture. I. A Comparison of the Behavior of Eutectic Mixture of Sulfathiazole and that of Ordinary Sulfathiazole in Man. Chem. Pharm. Bull. 1961, 9, 866–872.Sekiguchi, K.; Obi, N. Studies on Absorption of Eutectic Mixture. I. A Comparison of the Behavior of Eutectic Mixture of Sulfathiazole and that of Ordinary Sulfathiazole in Man. Chem. Pharm. Bull. 1961, 9, 866–872.
- El-Banna, H.M. Solid dispersion of pharmaceutical ternary systems I: Phase diagram of aspirin-acetaminophen-urea system. J. Pharm. Sci. 1978, 67, 1109–1111.El-Banna, H.M. Solid dispersion of pharmaceutical ternary systems I: Phase diagram of aspirin-acetaminophen-urea system. J. Pharm. Sci. 1978, 67, 1109–1111.
- Goud, N.R.; Suresh, K.; Sanphui, P.; Nangia, A. Fast dissolving eutectic compositions of curcumin. Int. J. Pharm. 2012, 439, 63–72.Goud, N.R.; Suresh, K.; Sanphui, P.; Nangia, A. Fast dissolving eutectic compositions of curcumin. Int. J. Pharm. 2012, 439, 63–72.
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 9, 70–71.Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 9, 70–71.
- Francisco, M.; Van Den Bruinhorst, A.; Kroon, M.C. Low-transition-temperature mixtures (LTTMs): A new generation of designer solvents. Angew. Chemie-Int. Ed. 2013, 52, 3074–3085.Francisco, M.; Van Den Bruinhorst, A.; Kroon, M.C. Low-transition-temperature mixtures (LTTMs): A new generation of designer solvents. Angew. Chemie-Int. Ed. 2013, 52, 3074–3085.
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.A.; Looi, C.Y. Applications of deep eutectic solvents in biotechnology and bioengineering—Promises and challenges. Biotechnol. Adv. 2017, 35, 105–134.Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.A.; Looi, C.Y. Applications of deep eutectic solvents in biotechnology and bioengineering—Promises and challenges. Biotechnol. Adv. 2017, 35, 105–134.
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solution Chem. 2019, 48, 962–982.Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solution Chem. 2019, 48, 962–982.
- Perna, F.M.; Vitale, P.; Capriati, V. Deep eutectic solvents and their applications as green solvents. Curr. Opin. Green Sustain. Chem. 2020, 21, 27–33.Perna, F.M.; Vitale, P.; Capriati, V. Deep eutectic solvents and their applications as green solvents. Curr. Opin. Green Sustain. Chem. 2020, 21, 27–33.
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082.Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082.
- Palmelund, H.; Andersson, M.P.; Asgreen, C.J.; Boyd, B.J.; Rantanen, J. Tailor-made solvents for pharmaceutical use? Experimental and computational approach for determining solubility in deep eutectic solvents. Int. J. Pharm. X 2019, 1, 100034.Palmelund, H.; Andersson, M.P.; Asgreen, C.J.; Boyd, B.J.; Rantanen, J. Tailor-made solvents for pharmaceutical use? Experimental and computational approach for determining solubility in deep eutectic solvents. Int. J. Pharm. X 2019, 1, 100034.
- Olivares, B.; Martínez, F.; Rivas, L.; Calde, C.; Munita, J.M.; Campodonico, P.R. A Natural Deep Eutectic Solvent Formulated to Stabilize β -Lactam Antibiotics. Sci. Rep. 2018, 8, 14900.Olivares, B.; Martínez, F.; Rivas, L.; Calde, C.; Munita, J.M.; Campodonico, P.R. A Natural Deep Eutectic Solvent Formulated to Stabilize β -Lactam Antibiotics. Sci. Rep. 2018, 8, 14900.
- Lu, C.; Cao, J.; Wang, N.; Su, E. Significantly improving the solubility of non-steroidal anti-inflammatory drugs in deep eutectic solvents for potential non-aqueous liquid administration. R. Soc. Chem. 2013, 7, 955–959.Lu, C.; Cao, J.; Wang, N.; Su, E. Significantly improving the solubility of non-steroidal anti-inflammatory drugs in deep eutectic solvents for potential non-aqueous liquid administration. R. Soc. Chem. 2013, 7, 955–959.
- Mokhtarpour, M.; Shekaari, H.; Zafarani-moattar, M.T.; Golgoun, S. Solubility and solvation behavior of some drugs in choline based deep eutectic solvents at different temperatures. J. Mol. Liq. 2019, 297, 111799.Mokhtarpour, M.; Shekaari, H.; Zafarani-moattar, M.T.; Golgoun, S. Solubility and solvation behavior of some drugs in choline based deep eutectic solvents at different temperatures. J. Mol. Liq. 2019, 297, 111799.
- Phaechamud, T.; Tuntarawongsa, S.; Charoensuksai, P. Evaporation Behavior and Characterization of Eutectic Solvent and Ibuprofen Eutectic Solution. AAPS PharmSciTech 2016, 17, 1213–1220.Phaechamud, T.; Tuntarawongsa, S.; Charoensuksai, P. Evaporation Behavior and Characterization of Eutectic Solvent and Ibuprofen Eutectic Solution. AAPS PharmSciTech 2016, 17, 1213–1220.
- Abbott, A.P.; Ahmed, E.I.; Prasad, K.; Qader, I.B.; Ryder, K.S. Liquid pharmaceuticals formulation by eutectic formation. Fluid Phase Equilib. 2017, 448, 2–8.Abbott, A.P.; Ahmed, E.I.; Prasad, K.; Qader, I.B.; Ryder, K.S. Liquid pharmaceuticals formulation by eutectic formation. Fluid Phase Equilib. 2017, 448, 2–8.
- Duarte, A.R.C.; Ferreira, A.S.D.; Barreiros, S.; Cabrita, E.; Reis, R.L.; Paiva, A. A comparison between pure active pharmaceutical ingredients and therapeutic deep eutectic solvents: Solubility and permeability studies. Eur. J. Pharm. Biopharm. 2017, 114, 296–304.Duarte, A.R.C.; Ferreira, A.S.D.; Barreiros, S.; Cabrita, E.; Reis, R.L.; Paiva, A. A comparison between pure active pharmaceutical ingredients and therapeutic deep eutectic solvents: Solubility and permeability studies. Eur. J. Pharm. Biopharm. 2017, 114, 296–304.
- Pereira, C.V.; Silva, J.M.; Rodrigues, L.; Reis, R.L.; Paiva, A.; Duarte, A.R.C.; Matias, A. Unveil the Anticancer Potential of Limomene Based Therapeutic Deep Eutectic Solvents. Sci. Rep. 2019, 9, 14926.Pereira, C.V.; Silva, J.M.; Rodrigues, L.; Reis, R.L.; Paiva, A.; Duarte, A.R.C.; Matias, A. Unveil the Anticancer Potential of Limomene Based Therapeutic Deep Eutectic Solvents. Sci. Rep. 2019, 9, 14926.
- Santos, F.; Leitão, M.I.P.S.; Duarte, A.R.C. Properties of therapeutic deep eutectic solvents of L-arginine and ethambutol for tuberculosis treatment. Molecules 2019, 24, 55.Santos, F.; Leitão, M.I.P.S.; Duarte, A.R.C. Properties of therapeutic deep eutectic solvents of L-arginine and ethambutol for tuberculosis treatment. Molecules 2019, 24, 55.
- Aroso, I.M.; Craveiro, R.; Rocha, Â.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Design of controlled release systems for THEDES—Therapeutic deep eutectic solvents, using supercritical fluid technology. Int. J. Pharm. 2015, 492, 73–79.Aroso, I.M.; Craveiro, R.; Rocha, Â.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Design of controlled release systems for THEDES—Therapeutic deep eutectic solvents, using supercritical fluid technology. Int. J. Pharm. 2015, 492, 73–79.
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 7.Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 7.
- Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic liquids as a potential tool for drug delivery systems. Med. Chem. Commun. 2016, 7, 1881–1897.Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic liquids as a potential tool for drug delivery systems. Med. Chem. Commun. 2016, 7, 1881–1897.
- Gad, S.C. Pharmaceutical Manufacturing Handbook: Production and Processes; John Wiley Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 314–316.Gad, S.C. Pharmaceutical Manufacturing Handbook: Production and Processes; John Wiley Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 314–316.
- Mota-Morales, J.D.; Gutiérrez, M.C.; Ferrer, M.L.; Sanchez, I.C.; Elizalde-Peña, E.A.; Pojman, J.A.; Monte, F.D.; Luna-Bárcenas, G. Deep eutectic solvents as both active fillers and monomers for frontal polymerization. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1767–1773.Mota-Morales, J.D.; Gutiérrez, M.C.; Ferrer, M.L.; Sanchez, I.C.; Elizalde-Peña, E.A.; Pojman, J.A.; Monte, F.D.; Luna-Bárcenas, G. Deep eutectic solvents as both active fillers and monomers for frontal polymerization. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1767–1773.
- Lynam, J.G.; Kumar, N.; Wong, M.J. Deep eutectic solvents’ ability to solubilize lignin, cellulose, and hemicellulose; thermal stability; and density. Bioresour. Technol. 2017, 238, 684–689.Lynam, J.G.; Kumar, N.; Wong, M.J. Deep eutectic solvents’ ability to solubilize lignin, cellulose, and hemicellulose; thermal stability; and density. Bioresour. Technol. 2017, 238, 684–689.
- Almeida, C.M.R.; Magalhães, J.M.C.S.; Souza, H.K.S.; Gonçalves, M.P. The role of choline chloride-based deep eutectic solvent and curcumin on chitosan films properties. Food Hydrocoll. 2018, 81, 456–466.Almeida, C.M.R.; Magalhães, J.M.C.S.; Souza, H.K.S.; Gonçalves, M.P. The role of choline chloride-based deep eutectic solvent and curcumin on chitosan films properties. Food Hydrocoll. 2018, 81, 456–466.
- Pedro, S.N.; Freire, M.G.; Freire, C.S.R.; Silvestre, A.J.D.; Pedro, S.N.; Freire, M.G.; Freire, C.S.R.; Silvestre, A.J.D. Deep eutectic solvents comprising active pharmaceutical ingredients in the development of drug delivery systems development of drug delivery systems. Expert Opin. Drug Deliv. 2019, 16, 497–506.Pedro, S.N.; Freire, M.G.; Freire, C.S.R.; Silvestre, A.J.D.; Pedro, S.N.; Freire, M.G.; Freire, C.S.R.; Silvestre, A.J.D. Deep eutectic solvents comprising active pharmaceutical ingredients in the development of drug delivery systems development of drug delivery systems. Expert Opin. Drug Deliv. 2019, 16, 497–506.
- Emami, S.; Shayanfar, A. Deep eutectic solvents for pharmaceutical formulation and drug delivery applications. Pharm. Dev. Technol. 2020, 25, 779–796.Emami, S.; Shayanfar, A. Deep eutectic solvents for pharmaceutical formulation and drug delivery applications. Pharm. Dev. Technol. 2020, 25, 779–796.
- Zainal-Abidin, M.H.; Hayyan, M.; Ngoh, G.C.; Wong, W.F.; Looi, C.Y. Emerging frontiers of deep eutectic solvents in drug discovery and drug delivery systems. J. Control. Release 2019, 316, 168–195.Zainal-Abidin, M.H.; Hayyan, M.; Ngoh, G.C.; Wong, W.F.; Looi, C.Y. Emerging frontiers of deep eutectic solvents in drug discovery and drug delivery systems. J. Control. Release 2019, 316, 168–195.
- Nguyen, C.H.; Augis, L.; Fourmentin, S.; Barratt, G.; Legrand, F.X. Deep Eutectic Solvents for Innovative Pharmaceutical Formulations. In Deep Eutectic Solvents for Medicine, Gas Solubilization and Extraction of Natural Substances; Fourmentin, S., Costa Gomes, M., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2021.Nguyen, C.H.; Augis, L.; Fourmentin, S.; Barratt, G.; Legrand, F.X. Deep Eutectic Solvents for Innovative Pharmaceutical Formulations. In Deep Eutectic Solvents for Medicine, Gas Solubilization and Extraction of Natural Substances; Fourmentin, S., Costa Gomes, M., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2021.
- Gutiérrez, A.; Atilhan, M.; Aparicio, S. A theoretical study on lidocaine solubility in deep eutectic solvents. Phys. Chem. Chem. Phys. 2018, 20, 27464–27473.Gutiérrez, A.; Atilhan, M.; Aparicio, S. A theoretical study on lidocaine solubility in deep eutectic solvents. Phys. Chem. Chem. Phys. 2018, 20, 27464–27473.
- Abranches, D.O.; Larriba, M.; Silva, L.P.; Melle-Franco, M.; Palomar, J.F.; Pinho, S.P.; Coutinho, J.A.P. Using COSMO-RS to design choline chloride pharmaceutical eutectic solvents. Fluid Phase Equilib. 2019, 497, 71–78.Abranches, D.O.; Larriba, M.; Silva, L.P.; Melle-Franco, M.; Palomar, J.F.; Pinho, S.P.; Coutinho, J.A.P. Using COSMO-RS to design choline chloride pharmaceutical eutectic solvents. Fluid Phase Equilib. 2019, 497, 71–78.
- Abdelraheem, E.M.M.; Busch, H.; Hanefeld, U.; Tonin, F. Biocatalysis explained: From pharmaceutical to bulk chemical production. React. Chem. Eng. 2019, 4, 1878–1894.
- Klibanov, A.M. Improving enzymes by using them in organic solvents. Nature 2001, 409, 241–246.
- Fragnelli, M.C.; Hoyos, P.; Romano, D.; Gandolfi, R.; Alcántara, A.R.; Molinari, F. Enantioselective reduction and deracemisation using the non-conventional yeast Pichia glucozyma in water/organic solvent biphasic systems: Preparation of (S)-1,2-diaryl-2-hydroxyethanones (benzoins). Tetrahedron 2012, 68, 523–528.
- Lindberg, D.; de la Fuente Revenga, M.; Widersten, M. Deep eutectic solvents (DESs) are viable cosolvents for enzyme-catalyzed epoxide hydrolysis. J. Biotechnol. 2010, 147, 169–171.
- Yadav, N.; Bhakuni, K.; Bisht, M.; Bahadur, I.; Venkatesu, P. Expanding the Potential Role of Deep Eutectic Solvents toward Facilitating the Structural and Thermal Stability of α-Chymotrypsin. ACS Sustain. Chem. Eng. 2020, 8, 10151–10160.
- Maugeri, Z.; Leitner, W.; Domínguez De María, P. Chymotrypsin-catalyzed peptide synthesis in deep eutectic solvents. Eur. J. Org. Chem. 2013, 2013, 4223–4228.
- Kim, S.H.; Park, S.; Yu, H.; Kim, J.H.; Kim, H.J.; Yang, Y.H.; Kim, Y.H.; Kim, K.J.; Kan, E.; Lee, S.H. Effect of deep eutectic solvent mixtures on lipase activity and stability. J. Mol. Catal. B Enzym. 2016, 128, 65–72.
- Panić, M.; Radović, M.; Maros, I.; Jurinjak Tušek, A.; Cvjetko Bubalo, M.; Radojčić Redovniković, I. Development of environmentally friendly lipase-catalysed kinetic resolution of (R,S)-1-phenylethyl acetate using aqueous natural deep eutectic solvents. Process Biochem. 2021, 102, 1–9.
- Yang, T.X.; Zhao, L.Q.; Wang, J.; Song, G.L.; Liu, H.M.; Cheng, H.; Yang, Z. Improving Whole-Cell Biocatalysis by Addition of Deep Eutectic Solvents and Natural Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2017, 5, 5713–5722.
- Villiers, M.M.D. Pharmaceutical solvents and solubilizing agents. In A Practical Guide to Contemporary Pharmacy Practice; Thompson, J.E., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009; Chapter 15; pp. 190–202.
- Nerurkar, J.; Beach, J.W.; Park, M.O.; Jun, H.W. Solubility of (±)-ibuprofen and S (+)-ibuprofen in the presence of cosolvents and cyclodextrins. Pharm. Dev. Technol. 2005, 10, 413–421.
- Li, Z.; Lee, P.I. Investigation on drug solubility enhancement using deep eutectic solvents and their derivatives. Int. J. Pharm. 2016, 505, 283–288.
- Berton, P.; Di Bona, K.R.; Yancey, D.; Rizvi, S.A.A.; Gray, M.; Gurau, G.; Shamshina, J.L.; Rasco, J.F.; Rogers, R.D. Transdermal Bioavailability in Rats of Lidocaine in the Forms of Ionic Liquids, Salts, and Deep Eutectic. ACS Med. Chem. Lett. 2017, 8, 498–503.
- Sut, S.; Faggian, M.; Baldan, V.; Poloniato, G.; Castagliuolo, I.; Grabnar, I.; Perissutti, B.; Brun, P.; Maggi, F.; Voinovich, D.; et al. Natural Deep Eutectic Solvents (NADES) to Enhance Berberine Absorption: An In Vivo Pharmacokinetic Study. Molecules 2017, 22, 1921.
- Faggian, M.; Sut, S.; Perissutti, B.; Baldan, V.; Grabnar, I.; Dall’Acqua, S. Natural Deep Eutectic Solvents (NADES) as a tool for bioavailability improvement: Pharmacokinetics of rutin dissolved in proline/glycine after oral administration in rats: Possible application in nutraceuticals. Molecules 2016, 21, 1531.
- Liu, Y.; Zhang, Y.; Chen, S.; Friesen, J.B.; Nikoli, D. The influence of natural deep eutectic solvents on bioactive natural products: Studying interactions between a hydrogel model and Schisandra chinensis metabolites. Fitoterapia 2018, 127, 212–219.
- Jeli, T.; Cysewski, P. Application of a computational model of natural deep eutectic solvents utilizing the COSMO-RS approach for screening of solvents with high solubility of rutin. J. Mol. Model. 2018, 24, 180.
- Jangir, A.K.; Lad, B.; Dani, U. In vitro toxicity assessment and enhanced drug solubility pro fi le of green deep eutectic solvent derivatives (DESDs) combined with theoretical. RSC Adv. 2020, 10, 24063–24072.
- Gutiérrez, A.; Aparicio, S.; Atilhan, M. Design of arginine-based therapeutic deep eutectic solvents as drug solubilization vehicles for active pharmaceutical ingredients. Phys. Chem. Chem. Phys. 2019, 21, 10621–10634.
- Gutiérrez, A.; Atilhan, M.; Aparicio, S. Theoretical Study on Deep Eutectic Solvents as Vehicles for the Delivery of Anesthetics. J. Phys. Chem. B 2020, 124, 1794–1805.
- Briscoe, C.J.; Hage, D.S. Factors affecting the stability of drugs and drug metabolites in biological matrices. Bioanalysis 2009, 1, 205–220.
- Ferrit, M.; Valle, C.; Martínez, F. The influence of the structural characteristics of the substrate and the medium on the stability of triflusal and acetylsalicylic acid in micellar systems. J. Mol. Liq. 2008, 142, 64–71.
- Ladeira, B.M.F.; Dias, C.J.; Gomes, A.T.P.C.; Tom, A.C.; Neves, M.G.P.M.S.; Moura, N.M.M.; Almeida, A.; Faustino, M.A.F. Cationic Pyrrolidine/Pyrroline-Substituted Porphyrins as Efficient Photosensitizers against E. coli. Molecules 2021, 26, 464.
- Karam, P.; El, N.; Saad, H. Enhancing porphyrin photostability when locked in metal–organic frameworks. Dalt. Trans. 2018, 47, 15765–15771.
- Ferreira, J.; Menezes, P.F.; Kurachi, C.; Sibata, C.; Allison, R.R.; Bagnato, V.S. Photostability of different chlorine photosensitizers. Laser Phys. Lett. 2008, 5, 156–161.
- Opsvik, K.; Bruzell, E.; Hjorth, H. Improved antibacterial phototoxicity of a neutral porphyrin in natural deep eutectic solvents. J. Photochem. Photobiol. B Biol. 2015, 148, 188–196.
- Kroh, L.W. Caramelisation in food and beverages. Food Chem. 1994, 51, 373–379.
- Alejandra, D.; Méndez, C.; Gutierres, E.; Dionisio, E.J.; Afonso, M.; Buzalaf, R.; Oliveira, R.C.; Aparecida, M.; Moreira, A.; Cruvinel, T. Curcumin-mediated Antimicrobial Photodynamic Therapy reduces the viability and vitality of infected dentin caries microcosms. Photodiagnosis Photodyn. Ther. 2018, 24, 102–108.
- Luthria, R.R.K. and D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930.
- Jeliński, T.; Przybyłek, M.; Cysewski, P. Natural Deep Eutectic Solvents as Agents for Improving Solubility, Stability and Delivery of Curcumin. Pharm. Res. 2019, 36, 116.
- Leader, B.; Baca, Q.J.; Golan, D.E. Protein therapeutics: A summary and pharmacological classification. Nat. Rev. Drug Discov. 2008, 7, 21–39.
- Ammann, C. Stability Studies Needed to Define the Handling and Transport Conditions of Sensitive Pharmaceutical or Biotechnological Products. APPS PharmaSciTech 2011, 12, 1264–1275.
- Wolfson, L.J.; Gasse, F.; Lee-martin, S.; Lydon, P.; Magan, A.; Tibouti, A.; Johns, B.; Hutubessy, R.; Okwo-bele, J. Estimating the costs of achieving the WHO—UNICEF Global Immunization Vision and Strategy, 2006–2015. Bull. World Health Organ. 2015, 86, 27–39.
- Daneshjou, S.; Khodaverdian, S.; Dabirmanesh, B.; Rahimi, F.; Daneshjoo, S.; Ghazi, F.; Khajeh, K. Improvement of chondroitinases ABCI stability in natural deep eutectic solvents. J. Mol. Liq. 2017, 227, 21–25.
- Min, A.; Lee, S.; Lee, K.; Nam, M.W.; Jeong, M.; Lee, J.E.; Kim, N.W.; Yin, Y.; Yeon, S. Natural deep eutectic solvents as a storage medium for human interferon-α2: A green and improved strategy for room-temperature biologics. J. Ind. Eng. Chem. 2018, 65, 343–348.
- Zhao, B.Y.; Xu, P.; Yang, F.X.; Wu, H.; Zong, M.H.; Lou, W.Y. Biocompatible Deep Eutectic Solvents Based on Choline Chloride: Characterization and Application to the Extraction of Rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755.
- Rado, K.; Iva, Č.; Pani, M.; Markov, K.; Bubalo, M.C.; Frece, J. Antimicrobial, cytotoxic and antioxidative evaluation of natural deep eutectic solvents. Environ. Sci. Pollut. Res. 2018, 25, 14188–14196.
- Torregrosa-Crespo, J.; Marset, X.; Guillena, G.; Ramón, D.J.; María Martínez-Espinosa, R. New guidelines for testing “Deep eutectic solvents” toxicity and their effects on the environment and living beings. Sci. Total Environ. 2020, 704, 135382.
- Silva, J.M.; Silva, E.; Reis, R.L.; Rita, A.; Duarte, C. A closer look in the antimicrobial properties of deep eutectic solvents based on fatty acids. Sustain. Chem. Pharm. 2019, 14, 100192.
- Juneidi, I.; Hayyan, M.; Mohd Ali, O. Toxicity profile of choline chloride-based deep eutectic solvents for fungi and Cyprinus carpio fish. Environ. Sci. Pollut. Res. 2016, 23, 7648–7659.
- Hayyan, M.; Looi, C.Y.; Hayyan, A.; Wong, W.F. In Vitro and In Vivo Toxicity Profiling of Ammonium-Based Deep Eutectic Solvents. PLoS ONE 2015, 10, e0117934.
- Hayyan, M.; Mbous, Y.P.; Looi, C.Y.; Wong, W.F.; Hayyan, A.; Salleh, Z.; Mohd-Ali, O. Natural deep eutectic solvents: Cytotoxic profile. Springerplus 2016, 5, 913.
- Mbous, Y.P.; Hayyan, M.; Wong, W.F.; Looi, C.Y.; Hashim, M.A. Unraveling the cytotoxicity and metabolic pathways of binary natural deep eutectic solvent systems. Nat. Publ. Gr. 2017, 7, 41257.
- Carson, L.; Chau, P.K.W.; Earle, M.J.; Gilea, M.A.; Gilmore, B.F.; Gorman, S.P.; Mccann, T.; Seddon, K.R. Antibiofilm activities of 1-alkyl-3-methylimidazolium chloride ionic liquids. Green Chem. 2009, 44, 492–497.
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: Application to flavonoid extraction from Flos sophorae. Green Chem. 2015, 17, 1718–1727.
- Rado, K.; Curko, N.; Bubalo, M.C.; Kova, K.; Radoj, I. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT-Food Sci. Technol. 2016, 73, 45–51.
- Aroso, I.M.; Silva, J.C.; Mano, F.; Ferreira, A.S.D.; Dionísio, M.; Sá-Nogueira, I.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Dissolution enhancement of active pharmaceutical ingredients by therapeutic deep eutectic systems. Eur. J. Pharm. Biopharm. 2016, 98, 57–66.
- Silva, J.M.M.; Reis, R.L.; Paiva, A.; Rita, A.; Duarte, C. Design of functional therapeutic deep eutectic solvents based on choline chloride and ascorbic acid. ACS Sustain. Chem. Eng. 2018, 6, 10355–10363.
- Stott, P.W.; Williams, A.C.; Barry, B.W. Transdermal delivery from eutectic systems: Enhanced permeation of a model drug, ibuprofen. J. Control. Release 1998, 50, 297–308.
- Mano, F.; Martins, M.; Sá-Nogueira, I.; Barreiros, S.; Borges, J.P.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Production of Electrospun Fast-Dissolving Drug Delivery Systems with Therapeutic Eutectic Systems Encapsulated in Gelatin. AAPS PharmSciTech 2017, 18, 2579–2585.
- Gala, U.; Chuong, M.C.; Varanasi, R.; Chauhan, H. Characterization and Comparison of Lidocaine-Tetracaine and Lidocaine-Camphor Eutectic Mixtures Based on Their Crystallization and Hydrogen-Bonding Abilities. AAPS PharmSciTech 2015, 16, 528–536.
- Lazerges, M.; Espeau, P.; Crauste-Manciet, S.S.; Brossard, D.; Corvis, Y.; Agnely, F.; Huang, N. Topical Emulsions Based on Mixtures of Local Eutectic Anaesthetics and Fatty Acids as Analgesics, Antalgics, or as Sexual Retardants. U.S. Patent 20150105426A1, 6 April 2015.
- Wolbert, F.; Brandenbusch, C.; Sadowski, G. Selecting Excipients Forming Therapeutic Deep Eutectic Systems—A Mechanistic Approach. Mol. Pharm. 2019, 16, 3091–3099.
- Martins, M.A.R.; Silva, L.P.; Jorge, P.S.; Abranches, D.O.; Pinho, S.P.; Coutinho, J.A.P. The role of ionic vs. non-ionic excipients in APIs-based eutectic systems. Eur. J. Pharm. Sci. 2021, 156, 105583.
- Liu, C.; Qu, X.; Song, L.; Shang, R.; Wan, X.; Fang, L. Investigation on the effect of deep eutectic formation on drug-polymer miscibility and skin permeability of rotigotine drug-in-adhesive patch. Int. J. Pharm. 2019, 574, 118852.
- Li, Y.; Wu, X.; Zhu, Q.; Chen, Z.; Lu, Y.; Qi, J.; Wu, W. Improving the hypoglycemic effect of insulin via the nasal administration of deep eutectic solvents. Int. J. Pharm. 2019, 569, 118584.
- Zhang, Q.; Lin, Z.; Zhang, W.; Huang, T.; Jiang, J.; Ren, Y.; Zhang, R.; Li, W.; Zhang, X.; Tu, Q. Fabrication of green poly(vinyl alcohol) nanofibers using natural deep eutectic solvent for fast- dissolving drug delivery. RSC Adv. 2021, 11, 1012–1021.
- Roda, A.; Santos, F.; Matias, A.A.; Paiva, A.; Rita, A.; Duarte, C. Design and processing of drug delivery formulations of therapeutic deep eutectic systems for tuberculosis. J. Supercrit. Fluids 2020, 161, 104826.
- Serrano, M.C.; Gutiérrez, M.C.; Jiménez, R.; Ferrer, M.L.; Monte, F. Del Synthesis of novel lidocaine-releasing poly(diol-co-citrate) elastomers by using deep eutectic solvents. Chem. Commun. 2012, 48, 579–581.
- Al-akayleh, F.; Hani, H.; Ali, M.; Ghareeb, M.M.; Al-remawi, M. Therapeutic deep eutectic system of capric acid and menthol: Characterization and pharmaceutical application. J. Drug Deliv. Sci. Technol. 2019, 53, 101159.
- Sousa, A.M.M.; Souza, H.K.S.; Uknalis, J.; Liu, S.C.; Gonçalves, M.P.; Liu, L.S. Improving agar electrospinnability with choline-based deep eutectic solvents. Int. J. Biol. Macromol. 2015, 80, 139–148.
- Sharma, M.; Mukesh, C.; Mondal, D.; Prasad, K. Dissolution of α-chitin in deep eutectic solvents. RSC Adv. 2013, 3, 18149.
- Mokhtarpour, M.; Shekaari, H.; Shayanfar, A. Design and characterization of ascorbic acid based therapeutic deep eutectic solvent as a new ion-gel for delivery of sunitinib malate. J. Drug Deliv. Sci. Technol. 2020, 56, 101512.
- Hamdi, M.; Abidin, Z.; Hayyan, M.; Cheng, G.; Won, N.; Wong, F.; Yeng, C. Potentiating the anti-cancer profile of tamoxifen-Loaded graphene using deep eutectic solvents as functionalizing agents. Appl. Nanosci. 2020, 10, 293–304.
- Zainal-abidin, M.H.; Hayyan, M.; Ngoh, G.C.; Wong, W.F. Doxorubicin Loading on Functional Graphene as a Promising Nanocarrier Using Ternary Deep Eutectic Solvent Systems. ACS Omega 2019, 5, 1656–1668.
