Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by MOHAMMED BERRADA and Version 2 by Dean Liu.
- MXene
- Drug Delivery
- Transdermal Drug Delivery
- Pharmacotherapy
- Oral Drug Delivery
1. Drug Delivery
Conventional cancer treatment methods, such as chemotherapy and photodynamic therapy (PDT) [1][2], can harm nonmalignant cells as well as malignant ones. The development of stimuli-responsive materials capable of identically sensing the comparatively lower pH of tumor cells [3] has the potential to greatly alleviate this problem [4]. Several studies have been conducted in recent years to establish an appropriate nanoplatform for drug carrier applications [5][6]. According to their intrinsic pH sensitivity and excellent photothermal conversion, MXenes provide the cumulative photothermal ablation and tailored medication release impact. Xing et al. reported cellulose and Ti3C2 MXene composite hydrogels [7], which when the anticancer agent is loaded doxorubicin, could accomplish faster release of the drug (DOX). When triggered by irradiation with an 808 nm light, light-induced enlargement of the pores inside the three-dimensional cellulose-based networks occurs. The scientists revealed that the combination of photothermal therapy (PTT) and extended adjuvant chemotherapy utilizing this nanoplatform was very efficient for simultaneous tumor elimination and tumor recurrence suppression, implying that it has the potential to grow into an efficient theranostics system in the future.
Ti3C2-based nanoplatforms for synergistic PTT, PDT and chemotherapy were developed by Liu et al. [8][9]. When irradiated with an 808 nm laser, the produced Ti3C2-based nanosheets showed an excellent extinction coefficient value of 28.6 Lg
1. Drug Delivery
Conventional cancer treatment methods, such as chemotherapy and photodynamic therapy (PDT) [25,83], can harm nonmalignant cells as well as malignant ones. The development of stimuli-responsive materials capable of identically sensing the comparatively lower pH of tumor cells [84] has the potential to greatly alleviate this problem [85]. Several studies have been conducted in recent years to establish an appropriate nanoplatform for drug carrier applications [86,87]. According to their intrinsic pH sensitivity and excellent photothermal conversion, MXenes provide the cumulative photothermal ablation and tailored medication release impact. Xing et al. reported cellulose and Ti3C2 MXene composite hydrogels [23], which when the anticancer agent is loaded doxorubicin, could accomplish faster release of the drug (DOX). When triggered by irradiation with an 808 nm light, light-induced enlargement of the pores inside the three-dimensional cellulose-based networks occurs. The scientists revealed that the combination of photothermal therapy (PTT) and extended adjuvant chemotherapy utilizing this nanoplatform was very efficient for simultaneous tumor elimination and tumor recurrence suppression, implying that it has the potential to grow into an efficient theranostics system in the future.
−1
·cm
−1, an amazing efficiency for photothermal conversion of around 58.3% and effective creation of singlet oxygen [9]. Doxorubicin (DOX), a chemotherapeutic drug, was placed in MXene with a hyaluronic acid coating applied to its surface (HA) to boost its biocompatibility. This also improved the selectivity towards malignant cells identified by the CD44 antigen, allowing for active targeting. In vitro and in vivo investigations have been performed, which uncovered that Ti3C2-DOX exhibits better biocompatibility, as well as tumor-specific accumulation behavior and drug-releasing ability in response to stimuli, and could be deployed in PTT/PDT/chemotherapy to destroy malignant cells and tumor tissues [10].
, an amazing efficiency for photothermal conversion of around 58.3% and effective creation of singlet oxygen [81]. Doxorubicin (DOX), a chemotherapeutic drug, was placed in MXene with a hyaluronic acid coating applied to its surface (HA) to boost its biocompatibility. This also improved the selectivity towards malignant cells identified by the CD44 antigen, allowing for active targeting. In vitro and in vivo investigations have been performed, which uncovered that Ti3C2-DOX exhibits better biocompatibility, as well as tumor-specific accumulation behavior and drug-releasing ability in response to stimuli, and could be deployed in PTT/PDT/chemotherapy to destroy malignant cells and tumor tissues [88].
A highly elastic nanocomposite (NC) colloidal gel was manufactured in a factory using acrylamide’s in-place radical chemical action [32]. In particular, the authors used an exfoliated two-dimensional MXene nanosheet-based Ti3C2 as a crosslinking agent instead of traditional organic crosslinkers. NC hydrogolds presented enhanced mechanical properties with tensile strengths of 66.5 to 102.7 kPa, compressive strengths of 400.6 to 819.4 kPa and elongation of 2158.6% to 3047.5%. NC hydrogels exhibited good sustained-release performance, higher drug loading amounts (97.5–127.7 mg/g) and higher percentage releases (62.1–81.4%), greatly superior to those of the BIS/PAM hydrogel. The enhanced mechanical performances can be attributed to the honey-comb-like fine structure with uniform pores as well as more flexible polymer chains [32].
A highly elastic nanocomposite (NC) colloidal gel was manufactured in a factory using acrylamide’s in-place radical chemical action [11]. In particular, the authors used an exfoliated two-dimensional MXene nanosheet-based Ti3C2 as a crosslinking agent instead of traditional organic crosslinkers. NC hydrogolds presented enhanced mechanical properties with tensile strengths of 66.5 to 102.7 kPa, compressive strengths of 400.6 to 819.4 kPa and elongation of 2158.6% to 3047.5%. NC hydrogels exhibited good sustained-release performance, higher drug loading amounts (97.5–127.7 mg/g) and higher percentage releases (62.1–81.4%), greatly superior to those of the BIS/PAM hydrogel. The enhanced mechanical performances can be attributed to the honey-comb-like fine structure with uniform pores as well as more flexible polymer chains [11].
2. Sensors
MXenes are appealing for sensors due to their excellent conductivity and general-purpose surface chemistry. Sharma et al., however, reported the simple fabrication of a highly sensitive and robust capacitive pressure sensor for ultra-low-pressure measurement using MXene (Ti3C2Tx)/poly(vinylidene fluoride-trifluoroethylene) (PVDF-TrFE) nanofibrous composite scaffolds as a dielectric layer between biocompatible poly-(3,4 ethylenedioxythiophene) polystyrene. The sensor has a high sensitivity of 0.51 kPa
2. Sensors
MXenes are appealing for sensors due to their excellent conductivity and general-purpose surface chemistry. Sharma et al., however, reported the simple fabrication of a highly sensitive and robust capacitive pressure sensor for ultra-low-pressure measurement using MXene (Ti3C2Tx)/poly(vinylidene fluoride-trifluoroethylene) (PVDF-TrFE) nanofibrous composite scaffolds as a dielectric layer between biocompatible poly-(3,4 ethylenedioxythiophene) polystyrene. The sensor has a high sensitivity of 0.51 kPa
−1 and a low detection limit of 1.5 Pa [12]. Furthermore, it offers linear sensing over a large pressure range (0–400 kPa) as well as strong dependability over 10,000 cycles, even at extremely high pressure (>167 kPa). MXene loading improves the sensitivity of the nanofiber-based sensor by increasing the dielectric constant to 40 and decreasing the compressive modulus to 58% when compared to pristine PVDF-TrFE nanofiber scaffolds. The suggested sensor may be used to detect a patient’s health status by monitoring physiological data (pulse rate, breathing, muscle movements and eye twitching), and it is also a good candidate for a future generation human–machine interaction device [12].
and a low detection limit of 1.5 Pa [89]. Furthermore, it offers linear sensing over a large pressure range (0–400 kPa) as well as strong dependability over 10,000 cycles, even at extremely high pressure (>167 kPa). MXene loading improves the sensitivity of the nanofiber-based sensor by increasing the dielectric constant to 40 and decreasing the compressive modulus to 58% when compared to pristine PVDF-TrFE nanofiber scaffolds. The suggested sensor may be used to detect a patient’s health status by monitoring physiological data (pulse rate, breathing, muscle movements and eye twitching), and it is also a good candidate for a future generation human–machine interaction device [89].
As a result, the Au/MXene nanocomposite described in this paper might be used as an electrochemical transducer in electrochemical biosensors [90].
As a result, the Au/MXene nanocomposite described in this paper might be used as an electrochemical transducer in electrochemical biosensors [13].
3. Photothermal Therapy (PTT)
Radiation and chemotherapy are currently quite often used as cancer treatment procedures. However, these approaches are not highly focused and can cause harm to normal tissues in addition to cancer cells, resulting in serious side effects. Improved selectivity thereby decreases the adverse effects by using light-controlled treatments such as PTT [14]. Photothermal agents are delivered into cancer tissues, where they convert the light energy to heat energy. Cancer cells, in general, have poor heat resistance, therefore when heat is generated, these cells are eliminated. Since visible light has a poor tissue permeation, near-infrared (NIR) radiations are often employed in PTT. There are two classifications of NIR based on the wavelength of radiation: (1) the first NIR bio-window, which has a wavelength range of 750 to 1000 nm, and (2) the second near-infrared bio-window, which has a wavelength range of 1000 to 1350 nm. According to studies, the second NIR bio-window has several benefits over the first one, such as a lower required laser penetration depth and a higher maximum allowable exposure (MPE). Consequently, its uses are restricted due to a scarcity of materials with effective NIR light absorption and photothermal conversion ability. MXene-based materials are shown to be more efficient in both bio-windows, which is a tremendous benefit. In addition, MXenes’ vast surface area provides anchoring sites and enables an effective build-up of toxins in tumor cells while undergoing cancer therapy.
Hussein et al. developed multiple Ti3C2Tx-based 2D plasmonic nanocomposites (Au/MXene and Au/Fe3O4/MXene) with comparable anti-cancer photothermal treatment (PTT) capabilities, but with lower in vivo toxicity than pure MXene. Morphological evaluation using XRD, SEM and TEM demonstrated that Au/MXene and Au/Fe3O4/MXene were effectively synthesized. In vitro, both novel composites demonstrated a significant dose-dependent PTT impact against MCF7 human breast cancer cells. In vivo acute toxicity experiments utilizing zebrafish embryos revealed that Au/MXene and Au/Fe3O4/MXene exhibited lower embryonic mortality (LC50 1000 g/mL) than pure MXene (LC50 = 257.46 g/mL) [15].
Szuplewska et al. [16] demonstrated the performance of the photothermal treatment approach employing “Ti2C-PEG” by assessing the exposed cells to progressively higher quantities of the tested substance under 808 nm laser irradiation for 2 min.
The effective death of malignant cells was observed after 24 h of incubation with 2D Ti2C and additional NIR laser irradiation. In comparison, non-malignant cells survived the PTT treatment with a viability of more than 70%, even at a concentration of 37.5 g/mL. The chemical composition of the surface and the “Ti2C-PEG” flakes’ relatively small planar dimension may result in strong MXene affinity for cell membranes, resulting in successful endocytosis into the cells and nanomaterial dispersion primarily inside the cellular cytoplasm. The ability of delaminated “Ti2C-PEG” to convert light to thermal energy results in an increase in intracellular temperature (up to 50 °C for MCF-7 cells treated with 62.5 g·mL
3. Photothermal Therapy (PTT)
Radiation and chemotherapy are currently quite often used as cancer treatment procedures. However, these approaches are not highly focused and can cause harm to normal tissues in addition to cancer cells, resulting in serious side effects. Improved selectivity thereby decreases the adverse effects by using light-controlled treatments such as PTT [5]. Photothermal agents are delivered into cancer tissues, where they convert the light energy to heat energy. Cancer cells, in general, have poor heat resistance, therefore when heat is generated, these cells are eliminated. Since visible light has a poor tissue permeation, near-infrared (NIR) radiations are often employed in PTT. There are two classifications of NIR based on the wavelength of radiation: (1) the first NIR bio-window, which has a wavelength range of 750 to 1000 nm, and (2) the second near-infrared bio-window, which has a wavelength range of 1000 to 1350 nm. According to studies, the second NIR bio-window has several benefits over the first one, such as a lower required laser penetration depth and a higher maximum allowable exposure (MPE). Consequently, its uses are restricted due to a scarcity of materials with effective NIR light absorption and photothermal conversion ability. MXene-based materials are shown to be more efficient in both bio-windows, which is a tremendous benefit. In addition, MXenes’ vast surface area provides anchoring sites and enables an effective build-up of toxins in tumor cells while undergoing cancer therapy.
Hussein et al. developed multiple Ti3C2Tx-based 2D plasmonic nanocomposites (Au/MXene and Au/Fe3O4/MXene) with comparable anti-cancer photothermal treatment (PTT) capabilities, but with lower in vivo toxicity than pure MXene. Morphological evaluation using XRD, SEM and TEM demonstrated that Au/MXene and Au/Fe3O4/MXene were effectively synthesized. In vitro, both novel composites demonstrated a significant dose-dependent PTT impact against MCF7 human breast cancer cells. In vivo acute toxicity experiments utilizing zebrafish embryos revealed that Au/MXene and Au/Fe3O4/MXene exhibited lower embryonic mortality (LC50 1000 g/mL) than pure MXene (LC50 = 257.46 g/mL) [91].
Szuplewska et al. [92] demonstrated the performance of the photothermal treatment approach employing “Ti2C-PEG” by assessing the exposed cells to progressively higher quantities of the tested substance under 808 nm laser irradiation for 2 min.
The effective death of malignant cells was observed after 24 h of incubation with 2D Ti2C and additional NIR laser irradiation. In comparison, non-malignant cells survived the PTT treatment with a viability of more than 70%, even at a concentration of 37.5 g/mL. The chemical composition of the surface and the “Ti2C-PEG” flakes’ relatively small planar dimension may result in strong MXene affinity for cell membranes, resulting in successful endocytosis into the cells and nanomaterial dispersion primarily inside the cellular cytoplasm. The ability of delaminated “Ti2C-PEG” to convert light to thermal energy results in an increase in intracellular temperature (up to 50 °C for MCF-7 cells treated with 62.5 g·mL
Lin et al. developed an Nb2C-PVP hybrid photothermal agent to achieve in vivo photothermal ablation of tumor xenografts in mice with great efficiency at frequencies matching both bio-windows. A digital caliper was used to measure the tumor volume in six groups of mice every two days. It was revealed that the tumor-bearing areas of the control mice remained large after 16 days of various treatments. Similarly, the tumor-containing regions of Nb2C-PVP + NIR-I and Nb2C-PVP+NIR-II animals were totally eliminated [5].
It was discussed by Huang et al. that although Ti3C2-SP nanosheets have a high PCE (30.6%) when used as photothermal agents, a recent study found that Ta4C3-SP nanosheets had a higher PCE (44.7%) when exposed to an 808 nm laser and have been used in PTT[8]. These biomaterial Ta4C3-SP nanosheets have a sheet-like shape, a lateral dimension of 100 nm and high light absorption over a wide wavelength range. After 5 min of laser irradiation (1.5 W cm1), the temperature may quickly rise to around 55 °C. Furthermore, the Ta4C3-SP nanosheets demonstrate good thermal stability even after five heating and cooling cycles. Without laser irradiation, cell cytotoxicity revealed that Ta4C3-SP nanosheets had no influence on the survival of 4T1 cells, even at concentrations as high as 400 µg·mL
Lin et al. developed an Nb2C-PVP hybrid photothermal agent to achieve in vivo photothermal ablation of tumor xenografts in mice with great efficiency at frequencies matching both bio-windows. A digital caliper was used to measure the tumor volume in six groups of mice every two days. It was revealed that the tumor-bearing areas of the control mice remained large after 16 days of various treatments. Similarly, the tumor-containing regions of Nb2C-PVP + NIR-I and Nb2C-PVP+NIR-II animals were totally eliminated [86].
It was discussed by Huang et al. that although Ti3C2-SP nanosheets have a high PCE (30.6%) when used as photothermal agents, a recent study found that Ta4C3-SP nanosheets had a higher PCE (44.7%) when exposed to an 808 nm laser and have been used in PTT[80]. These biomaterial Ta4C3-SP nanosheets have a sheet-like shape, a lateral dimension of 100 nm and high light absorption over a wide wavelength range. After 5 min of laser irradiation (1.5 W cm1), the temperature may quickly rise to around 55 °C. Furthermore, the Ta4C3-SP nanosheets demonstrate good thermal stability even after five heating and cooling cycles. Without laser irradiation, cell cytotoxicity revealed that Ta4C3-SP nanosheets had no influence on the survival of 4T1 cells, even at concentrations as high as 400 µg·mL
−1, indicating their complete biocompatibility. The photothermal performances are affected by laser power density, which was proven by confocal fluorescence imaging after various treatments. Ta4C3-SP nanosheets may immediately collect in tumors after intravenous (i.v.) or intra-tumoral (i.t.) treatment in a mouse model, and tumor temperatures swiftly climbed to 60 °C (i.v.) or 68 °C (i.t.) from 30 °C after 6 min of 808 nm laser irradiation. Ta4C3-SP nanosheets may accomplish excellent photothermal elimination of tumors following irradiation with an 808 nm laser.
, indicating their complete biocompatibility. The photothermal performances are affected by laser power density, which was proven by confocal fluorescence imaging after various treatments. Ta4C3-SP nanosheets may immediately collect in tumors after intravenous (i.v.) or intra-tumoral (i.t.) treatment in a mouse model, and tumor temperatures swiftly climbed to 60 °C (i.v.) or 68 °C (i.t.) from 30 °C after 6 min of 808 nm laser irradiation. Ta4C3-SP nanosheets may accomplish excellent photothermal elimination of tumors following irradiation with an 808 nm laser.
4. Bioimaging
4. Bioimaging
Bioimaging has sparked interest in early cancer detection because of the capacity to provide in-depth insights into biological activities as well as a wide variety of diagnostic markers [79]. Due to its user-friendliness and low-cost equipment, fluorescence microscopy has become one of the most common bioimaging modalities. Though MXenes have a relatively small luminosity in aqueous solutions, it has been discovered that attaching fluorescent molecules to their surface increases the brightness. The X-ray computed tomography (CT) scan is another significant bioimaging platform that facilitates the usage of MXene. Traditional CT contrast agents, such as iodine-containing compounds, had a poor blood circulation rate, the possibility for renal impairment and severe toxicity, necessitating the development of efficient and biocompatible materials. Tantalum-rich MXenes, such as Ta4C3, have been discovered to be suitable for use as CT imaging contrast agents due to their high biocompatibility, proper size and environmentally friendly manufacture [93]. MRI is another outstanding method, like CT, that may be utilized for bioimaging in patients. It is being used as a solution for people who have allergic responses to CT contrast agents. Materials containing gadolinium, which are extensively employed as MRI contrast agents, have been linked to an increased risk of kidney injury [8]. As an MRI contrast agent, the composites made by growing nanosized manganese oxide on MXenes have shown great promise [79].
Bioimaging has sparked interest in early cancer detection because of the capacity to provide in-depth insights into biological activities as well as a wide variety of diagnostic markers [17]. Due to its user-friendliness and low-cost equipment, fluorescence microscopy has become one of the most common bioimaging modalities. Though MXenes have a relatively small luminosity in aqueous solutions, it has been discovered that attaching fluorescent molecules to their surface increases the brightness. The X-ray computed tomography (CT) scan is another significant bioimaging platform that facilitates the usage of MXene. Traditional CT contrast agents, such as iodine-containing compounds, had a poor blood circulation rate, the possibility for renal impairment and severe toxicity, necessitating the development of efficient and biocompatible materials. Tantalum-rich MXenes, such as Ta4C3, have been discovered to be suitable for use as CT imaging contrast agents due to their high biocompatibility, proper size and environmentally friendly manufacture [18]. MRI is another outstanding method, like CT, that may be utilized for bioimaging in patients. It is being used as a solution for people who have allergic responses to CT contrast agents. Materials containing gadolinium, which are extensively employed as MRI contrast agents, have been linked to an increased risk of kidney injury [19]. As an MRI contrast agent, the composites made by growing nanosized manganese oxide on MXenes have shown great promise [17].
5. Bone Regeneration
According to recent research, MXene-based composites might be used in guided bone regeneration (GBR), which is often used in oral rehabilitation procedures such as dental surgical treatment and periodontal regeneration to protect healed bones from soft tissue interference [83].
Chen et al. created Ti3C2Tz-improved poly(lactic acid) (PLA) membranes by employing n-octyltriethoxysilane as an interfacial mediator (OTES) [94]. The addition of these Ti3C2Tx nanosheets increased the membranes’ biocompatibility, cell adhesion, osteogenic differentiation and proliferation. These nanocomposite membranes were strong and biocompatible, indicating that they might be used as the GBR membrane [94].
Zhang et al. [95] synthesized Ti3C2Tx MXene, wherein the flexible free-standing multilayered Ti3C2Tx MXene film could be readily peeled from the membrane filter after etching and drying. The MXene film measured around 50 mg and was roughly 40 m-thick, as characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM), affirming the multilayered MXene structure. The dried sample’s cross-section SEM picture revealed a distinctive multilayered stacked structure, and the surface appearance was rough and chaotic. Rough-surfaced materials are ideal for cell adhesion, proliferation and bone cell differentiation. Furthermore, Ti3C2Tx MXene’s thick multilayered stacking structure makes it an efficient obstacle for a GBR membrane to prevent fibroblast migration. The (Ti3C2Tx) XRD spectra demonstrated the elimination of Ti3AlC2 peaks and the presence of just one intense (002) peak. The XRD analysis confirmed earlier findings, revealing that the Ti3C2Tx MXene was effectively etched and produced. The water contact angle on MXene films was 39.47° (3.12°) according to a drop-shape investigation. Due to the functional groups on the Ti3C2Tx surface, the decreased water contact angles imply that the surface of MXene films is hydrophilic. According to several studies, this hydrophilicity increases cell adhesion and cell spreading. These features point to MXene’s potential for use in GBR treatment [95].
5. Bone Regeneration
According to recent research, MXene-based composites might be used in guided bone regeneration (GBR), which is often used in oral rehabilitation procedures such as dental surgical treatment and periodontal regeneration to protect healed bones from soft tissue interference [2].
Chen et al. created Ti3C2Tz-improved poly(lactic acid) (PLA) membranes by employing n-octyltriethoxysilane as an interfacial mediator (OTES) [20]. The addition of these Ti3C2Tx nanosheets increased the membranes’ biocompatibility, cell adhesion, osteogenic differentiation and proliferation. These nanocomposite membranes were strong and biocompatible, indicating that they might be used as the GBR membrane [20].
Zhang et al. [21] synthesized Ti3C2Tx MXene, wherein the flexible free-standing multilayered Ti3C2Tx MXene film could be readily peeled from the membrane filter after etching and drying. The MXene film measured around 50 mg and was roughly 40 m-thick, as characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM), affirming the multilayered MXene structure. The dried sample’s cross-section SEM picture revealed a distinctive multilayered stacked structure, and the surface appearance was rough and chaotic. Rough-surfaced materials are ideal for cell adhesion, proliferation and bone cell differentiation. Furthermore, Ti3C2Tx MXene’s thick multilayered stacking structure makes it an efficient obstacle for a GBR membrane to prevent fibroblast migration. The (Ti3C2Tx) XRD spectra demonstrated the elimination of Ti3AlC2 peaks and the presence of just one intense (002) peak. The XRD analysis confirmed earlier findings, revealing that the Ti3C2Tx MXene was effectively etched and produced. The water contact angle on MXene films was 39.47° (3.12°) according to a drop-shape investigation. Due to the functional groups on the Ti3C2Tx surface, the decreased water contact angles imply that the surface of MXene films is hydrophilic. According to several studies, this hydrophilicity increases cell adhesion and cell spreading. These features point to MXene’s potential for use in GBR treatment [21].
6. Antimicrobial Activity
Nanomaterials with two dimensions and polymers have been used and investigated for their antimicrobial utility [22][23]. Among them, MXene materials have demonstrated more antibacterial action than graphene oxide (GO), a well-studied antimicrobial agent, and Ti3C2 has demonstrated considerably stronger antibacterial activity against both E. coli and B. subtilis [24]. The precise role of MXenes’ antibacterial action is uncertain. The most plausible hypotheses are that: (1) the sharp edges of Ti3C2 MXenes allow for effective absorption on the surface of microorganisms, (2) the sharp edges of MXenes can damage the microbial membrane and (3) MXenes can react with biomolecules in the cytoplasm and cell wall, causing cell microstructure breakdown and thus bacterial species’ death [25].
Rasool et al. tested the antibacterial efficacy of three materials (Ti3AlC2 (MAX), as-produced ML-MXene and delaminated Ti3C2Tx nanosheets) against E. coli and B. subtilis to see how delamination affects MXenes’ antibacterial efficacy. The colony counting method was used to measure the bacterial growth inhibition.
6. Antimicrobial Activity
Nanomaterials with two dimensions and polymers have been used and investigated for their antimicrobial utility [96,97]. Among them, MXene materials have demonstrated more antibacterial action than graphene oxide (GO), a well-studied antimicrobial agent, and Ti3C2 has demonstrated considerably stronger antibacterial activity against both E. coli and B. subtilis [3]. The precise role of MXenes’ antibacterial action is uncertain. The most plausible hypotheses are that: (1) the sharp edges of Ti3C2 MXenes allow for effective absorption on the surface of microorganisms, (2) the sharp edges of MXenes can damage the microbial membrane and (3) MXenes can react with biomolecules in the cytoplasm and cell wall, causing cell microstructure breakdown and thus bacterial species’ death [98].
Rasool et al. tested the antibacterial efficacy of three materials (Ti3AlC2 (MAX), as-produced ML-MXene and delaminated Ti3C2Tx nanosheets) against E. coli and B. subtilis to see how delamination affects MXenes’ antibacterial efficacy. The colony counting method was used to measure the bacterial growth inhibition.
Figure 1
A shows pictures of agar plates onto which control and bacterial cells were re-cultivated after being exposed to the same concentration of 100 µg/mL of nanomaterial for 4 h. The percentage growth inhibition of both bacterial strains exposed to the materials under research is depicted in
Figure 1
B. For E. coli and B. subtilis, MAX dispersion only inhibited growth by 14.39% ± 1.43% and 18.34% ± 1.59%, respectively. The antibacterial activity of the ML-Ti3C2Tx dispersion was somewhat greater than that of MAX, with E. coli and B. subtilis growth suppression of 30.55% ± 2.56% and 33.60% ± 2.89%, respectively (
Figure 1B). Therefore, when cells are exposed to a colloidal solution of delaminated Ti3C2Tx MXene, the vitality of E. coli and B. subtilis cells rises to 97.70% ± 2.87% and 97.04% ± 2.91%, respectively, indicating substantially higher inhibition [24]. The antibacterial activity of the three materials against both bacterial strains differed significantly, where delaminated Ti3C2Tx MXene, for instance, had substantially stronger antibacterial activity than MAX and MLTi3C2Tx MXene and could be explored in future experiments.
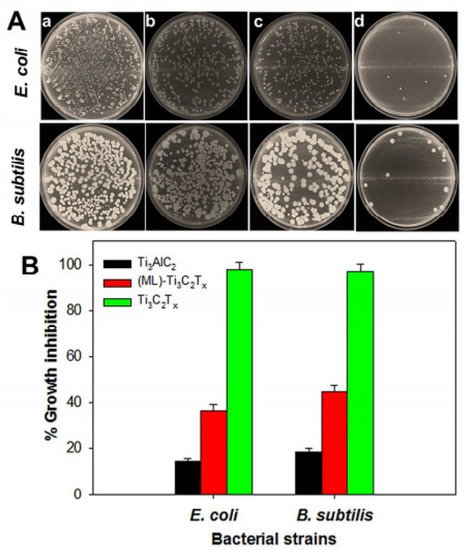
B). Therefore, when cells are exposed to a colloidal solution of delaminated Ti3C2Tx MXene, the vitality of E. coli and B. subtilis cells rises to 97.70% ± 2.87% and 97.04% ± 2.91%, respectively, indicating substantially higher inhibition [3]. The antibacterial activity of the three materials against both bacterial strains differed significantly, where delaminated Ti3C2Tx MXene, for instance, had substantially stronger antibacterial activity than MAX and MLTi3C2Tx MXene and could be explored in future experiments.

Figure 1.
(
A
) Photographs of agar plates onto which E. coli (
top panel
) and B. subtilis (
bottom panel
) bacterial cells were re-cultivated after treatment for 4 h with a control (
a
), and 100 µg/mL of Ti3AlC2 (
b
), ML-Ti3C2Tx (
c
) and delaminated Ti3C2Tx (
d
). (
B) Percentage of growth inhibition of bacterial cells treated with 100 µg/mL of Ti3AlC2, ML-Ti3C2Tx and delaminated Ti3C2Tx [24].
In another investigation [26], the same research group created micrometer-thick MXene (Ti3C2Tx)-based membranes with antibacterial characteristics. When fresh MXene (Ti3C2Tx)-based membranes were compared to a control polyvinylidene fluoride (PVDF) membrane, the antibacterial rate was 67% against E. coli and 73% against B. subtilis. Remarkably, the aged MXene (Ti3C2Tx)-based membranes could limit bacterial growth by more than 99%. According to the flow cytometry results, 70% of both bacteria were killed after 24 h of exposure to the membranes[24].
) Percentage of growth inhibition of bacterial cells treated with 100 µg/mL of Ti3AlC2, ML-Ti3C2Tx and delaminated Ti3C2Tx [3].
In another investigation [99], the same research group created micrometer-thick MXene (Ti3C2Tx)-based membranes with antibacterial characteristics. When fresh MXene (Ti3C2Tx)-based membranes were compared to a control polyvinylidene fluoride (PVDF) membrane, the antibacterial rate was 67% against E. coli and 73% against B. subtilis. Remarkably, the aged MXene (Ti3C2Tx)-based membranes could limit bacterial growth by more than 99%. According to the flow cytometry results, 70% of both bacteria were killed after 24 h of exposure to the membranes[3].
This entry is adapted from 10.3390/ma15051666
