Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Cheol-Hee Kim.
Rare diseases are those which affect a small number of people compared to the general population. However, many patients with a rare disease remain undiagnosed, and a large majority of rare diseases still have no form of viable treatment. Approximately 40% of rare diseases include neurologic and neurodevelopmental disorders. In order to understand the characteristics of rare neurological disorders and identify causative genes, various model organisms have been utilized extensively.
- rare diseases
- neurological rare diseases
- undiagnosed diseases
1. Introduction
Rare diseases affect a relatively small portion of people compared to other prevalent diseases; thus, characteristic issues arise, due to their rarity [1]. Although the definition of a rare disease varies slightly from country to country, the United States Congress defined a rare disease in the Orphan Drug Act of 1983 as a condition affecting fewer than 200,000 patients. The total number of Americans suffering from rare diseases is estimated at 25–30 million, while 30 million people are affected by rare disease across Europe [1].
Globally, there are about 8000 rare diseases, including genetic disorders, rare cancers, auto-immune disease, and infectious diseases, which are often serious, progressive, and chronic conditions [2]. Many rare diseases show a variety of signs starting at birth or childhood, including proximal spinal muscular atrophy, neurofibromatosis, chondrodysplasia osteogenesis imperfecta, and Rett syndrome. Rare diseases which appear during adulthood include those such as Huntington diseases, Crohn diseases, amyotrophic lateral sclerosis, and Charcot–Marie–Tooth diseases.
The exact cause of many rare diseases is still unknown, but a large majority of them (~80%) have a genetic cause, including a direct single gene, multifactorial, or chromosome changes [3]. In some cases, genetic changes causing disease are passed from one generation to the next. In other cases, they randomly occur in a person who is the first in a family to receive diagnosis [3]. Many patients with rare conditions undergo a “diagnostic odyssey”, trying various clinical approaches and comprehensive biochemical and genetic tests in hopes of an accurate assessment; however, often, they must wait years for a definitive diagnosis. National and international researchers have made progress in learning how to diagnose, clinically treat, and even prevent many rare diseases (Table 1). Unfortunately, 30% of child patients, which make up 50% of rare-disease patients, die before the age of 5 [3], while approximately 40% of known rare diseases include neurologic and neurodevelopmental disorders.
Table 1. List of rare disease research program or network.
| Program/Network Name | Goals | Homepage Address |
|---|---|---|
| Genetic and Rare Diseases Information Center |
Providing the general public with the latest information on various rare diseases in an easy-to-understand manner. | https://rarediseases.info.nih.gov/diseases |
| International rare disease research consortium |
Contributing to the development of new treatments for rare diseases and methods to uncover the genetic causes of rare diseases. | https://irdirc.org/ |
| National Organization for Rare Disorders |
Raising awareness of rare diseases and improve access to treatment and medical services for patients and their families. | https://rarediseases.org |
| Orphanet (global network) |
Providing international reference knowledge base for rare diseases and orphan drugs. | https://www.orpha.net/ |
| Providing the scientific datasets (research, clinical trials, drugs, etc.) related to rare diseases and orphan drugs. | http://www.orphadata.org/ | |
| Rare Diseases Clinical Research Network |
Providing support for clinical studies and facilitating collaboration, study enrollment, and data sharing. | https://ncats.nih.gov/rdcrn |
| Undiagnosed Diseases Network |
Accelerating identification of genetic causes of rare diseases by validating candidate genes, using model organisms. | https://undiagnosed.hms.harvard.edu/research |
2. Model Organisms for Rare Disease Research
In vitro approaches that use mainly cell or tissue culture can help predict clinical outcomes [4] but are limited in mimicking rare human diseases. The selection of an appropriate model organism is critical for preclinical research. Several important factors of consideration include species similarity to humans (i.e., the closer the phylogeny, the more similar the genetic composition, anatomy, and physiology), genetic homogeneity, a priori knowledge, cost, availability, translatability of results, ease of operation, ethical implications, etc. [5].
Over the past few years, clustered regularly interspaced repeat (CRISPR)/CRISPR-associated protein 9 (Cas9) system (CRISPR/Cas9) genome editing technology has transformed the field and has greatly expanded the repertoire of cell/animal systems available for rare disease modeling [6]. The use of this genome editing technology with genetic model organisms has enhanced ourthe understanding of human rare diseases. For example, mouse and zebrafish models, at first glance, appear completely unrelated to humans; however, on the genetic and physiological level, they respectively share about 85% and 71% of the same genes and possess major organ systems in common, such as the central nervous system, circulatory system, digestive system, etc. To study the functional consequences of the hundreds of rare variants discovered by genome sequencing, researchers have developed the use of specific model organisms, including roundworms (C. elegans), fruit flies (Drosophila melanogaster), and zebrafish (Danio rerio).
2.1. Caenorhabditis elegans (C. elegans)
The significance of non-mammalian model organisms has been recognized for quite some time [1,6][1][6]. The Nobel Prize in Physiology or Medicine has been awarded to researchers for their discoveries in apoptosis (2022) and RNA interference using worms (2006).
C. elegans is an unsegmented pseudocoelomate, lacking circulatory and respiratory systems, whose body segments are serially repeated one after the other [7]. C. elegans was used primarily for neuronal development research in 1963 and has since seen extensive use as a model organism for research into neural and molecular mechanisms of learning, memory, mating behavior, chemotaxis, thermotaxis, and mechano-transduction [8]. In addition, the C. elegans model has provided insights into finer mechanistic details of human health, including inter-cellular signaling pathways (e.g., Notch signaling), intra-cellular pathways (e.g., autophagy), molecular machines (e.g., spliceosome), and multi-cellular processes (e.g., basement membrane biology) [9].
In 2019, the first C. elegans multicellular organism underwent whole-genome sequencing and is the only organism to have completed its connectome (the “wiring diagram” of neurons) [10,11,12][10][11][12]. There are 20,512 protein-coding genes [13], and 83% of the worm proteome is found to have human homologous genes [14]. Only 11% or less contain roundworm-specific genes. These findings provide the basis by which C. elegans has served as a suitable model organism for human gene functional research [15,16,17,18][15][16][17][18]. In the case of rare disease modeling, C. elegans provides an ideal system to study human diseases.
2.2. Fruit Fly (Drosophila melanogaster)
Drosophila melanogaster is a species of fly in the Drosophila family (taxonomic order Diptera). For the past century, the Drosophila melanogaster has been used as a model organism to understand the fundamentals of genetics, developmental biology, immunity, and neuroscience [19,20][19][20]. Five Nobel Prizes have been awarded to fruit fly scientists for their work with the animal in 2017. Drosophila melanogaster has emerged as an important model system for dissecting and understanding the molecular mechanisms underlying rare human diseases, due to its rapid life cycle, relatively simple genetics (only four pairs of chromosomes), and large number of offspring per generation. This rise is owed in part to the first genome-wide survey of ~1000 genes registered in the Online Mendelian Inheritance in Man (OMIM), which found that 75% of disease-causing genes in humans are conserved in Drosophila [21]. Of the approximately 4000 human disease-related genes currently displayed in OMIM, ~85% have homologues in Drosophila. Considering that ~65% of protein-coding genes are conserved between humans and flies, the data suggest that genes conserved between these species are more likely to implicate genetic disease in humans [22,23][22][23]. In addition to being used as a tool for dissecting rare disease mechanisms and exploring potential therapeutic avenues, flies have emerged as a key tool in explaining variants of uncertain significance found in patients [24,25][24][25].
2.3. Zebrafish (Danio rerio)
The zebrafish (Danio rerio) is a small freshwater fish, 3-to-4 cm long, with a lifespan of about 2 years. Zebrafish have become increasingly important in scientific research since the 1960s, due to several distinct advantages over other vertebrate models.
Because of the simplicity of their natural habitat, lab maintenance of a zebrafish colony is much easier than simulating the conditions necessary for mammals. Therefore, zebrafish can be grown in a cost-effective manner. Their short generation time of 3 months helps accelerate experimental progress [26], and ex utero development facilitates the observation and rapid experimental manipulation of embryos. In addition, zebrafish have large clutch sizes, ranging from 200 to 300 embryos per adult mating pair, which ensures a robust stock of animals for research work. Due to these features, combined with the relatively small size of the embryo/larva/adult, zebrafish are well-suited for high-throughput screening of potential neuroactive compounds.
The zebrafish possesses many characteristics that make it an invaluable model to study human diseases [27]; however, one of its unique advantages is the unparalleled optical clarity of the embryo, which allows for the visualization of individual genes (fluorescently stained or labeled) throughout development, using non-invasive imaging techniques [28,29,30,31][28][29][30][31] (Figure 1A). This transparency of the embryo also facilitates genetic manipulation, such that gene function can readily be studied by the injection of synthetic mRNA or plasmid DNA into early stage zebrafish embryos generating transgenic zebrafish lines or altering gene function through genome editing techniques, such as the inclusion of zinc finger nucleases (ZFNs), transcription activation-like effector nucleases (TALENs), and the clustered regularly interspaced repeat (CRISPR)/CRISPR-associated protein 9 (Cas9) system [32,33,34][32][33][34]. Furthermore, the zebrafish genome has been sequenced, and 71% of human genes and 82% of human disease-related genes have orthologs in zebrafish [35]. Both ZFNs and TALENs require the creation of customized protein compositions for each target site, and these systems are not suitable for large-scale applications. However, the CRISPR/Cas9 system relies on target-site recognition by a custom guide RNA (gRNA) molecule and requires only one oligonucleotide to be designed for each target site. The success of CRISPR-mediated transgenic transformation in zebrafish can largely be attributed to frame-shift-generating null alleles via non-homologous extremity joint (NHEJ)-mediated CRISPR-induced DNA break repair [35].
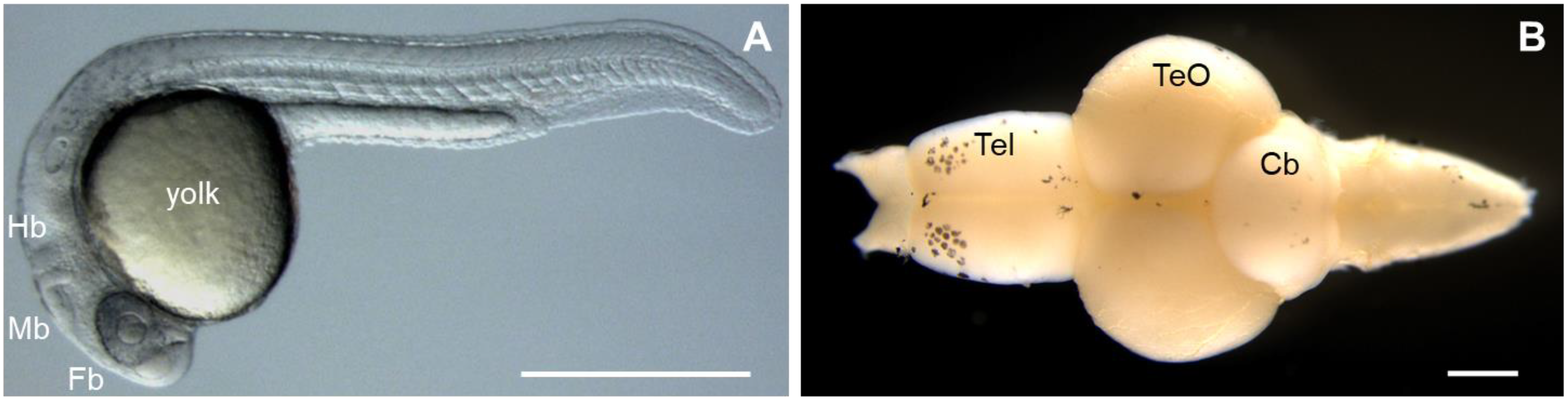
Figure 1. Major features of the zebrafish (Danio rerio). (A) Zebrafish embryo at day 1 of development. Fb, forebrain; Mb, midbrain; Hb, hindbrain. (B) Dissection of brain from an adult zebrafish. Tel, telencephalon; TeO, optic tectum; Cb, cerebellum. Rostral part of the brain is pointing to the left. Scale bars: 500 µm.
Interestingly, similarities between the zebrafish and human nervous system (anatomy and physiological signaling) have been reported [33]. The zebrafish brain is composed of the forebrain (telencephalon), midbrain (optic tectum), and hindbrain (cerebellum) (Figure 1B); and many cells, including astrocytes, oligodendrocytes, microglia, cerebellar Purkinje cells, myelin, and motor neurons, are also similar to human cells. Further studies of spinal nerve patterning, neural differentiation, and vertebrate network development in adult zebrafish revealed similarities to higher vertebrates [33]. Due to these characteristics, zebrafish are widely used to validate candidate disease genes and to elucidate the molecular mechanisms and pathophysiology of neurological disease. The continued increase in the use of zebrafish in biomedical research publications reflects their expanding popularity [33] (Table 2).
Table 2. Zebrafish modeling for rare neurological diseases.
| Gene Name | Related Disease | Zebrafish Phenotype | Publication |
|---|---|---|---|
| sod1 | Amyotrophic Lateral Sclerosis | Motor neuron loss Muscle atrophy |
[36] |
| fus | Shortened motor neuron length Decreased neuromuscular junction Impaired motor behavior Decreased life span Increase of the smallest tau transcripts |
[37] | |
| tardbp | Axonopathy of the motor neurons Premature of axonal branch |
[38] | |
| c9orf72 | Impaired motor behavior Cognitive impairment Muscle atrophy Motor neuron loss |
[39] | |
| fam50a | Armfield XLID syndrome | Abnormal neurogenesis Abnormal craniofacial patterning |
[40] |
| dyrk1a | Down Syndrome and Autism | Decreased brain size Increased anxiolytic behavior Impaired social interaction/cohesion |
[41] |
| wdr11 | Idiopathic Hypogonadotropic Hypogonadism Kallmann Syndrome |
Delayed puberty Impaired sense of smell |
[42] |
| eftud2 | Mandibulofacial Dysostosis, Guion–Almeida Type | Decreased brain size Small eyes Curved body Early embryonic lethality |
[43] |
| zc4h2 | Miles–Carpenter Syndrome | Abnormal swimming Increased twitching Motor hyperactivity Eye movement deficits Pectoral fin contractures |
[44] |
| phf21a | Potocki–Shaffer Syndrome | Abnormal head and jaw size Change of head and face shape |
[45] |
| eif4a3 | Richieri–Costa–Pereira Syndrome | Underdevelopment of craniofacial cartilage and bone structures | [46] |
| eif2b5 | Vanishing White Matter Disease | Early embryonic lethality Loss of oligodendrocyte precursor cells Impaired motor behavior |
[47] |
| eif2b3 | Defected myelin gene expression Defected glial cell differentiation |
[48] | |
| sam2 | 12q14.1 Deletion Syndrome | Increased of fear, anxiety-related behaviors, and autism | [49] |
