1. Introduction
The remarkable developments in multimodal molecular imaging methods using various functional nanomaterials have led to the translation of many novel materials into the clinic. These nanomaterials are established to confront the crucial problems encountered by diagnostic imaging techniques
[1,2][1][2]. Theranostic imaging adopting nanomaterials presents significant improvements over the traditional approaches via increasing blood circulation times, enhanced diagnostic specificity, and organ-specific delivery
[3]. Theranostic imaging modalities improve the understanding of various biological processes via direct observation of available events in real-time. In recent years, growing interests in image-guided theranostics have elevated the researchers’ directions to study and understand the mechanistic aspects of multiple disease-related signaling to recognize and enable easy and early diagnosis
[4]. This further helped them to identify the complex neural networks in the brain process of cognitive therapies. Imaging techniques, such as MRI, fluorescence, bioluminescence, FRET (Förster (or Fluorescence) Resonance Energy Transfer), BRET (Bioluminescence resonance energy transfer), US (Ultra-sound), and PAI, provide necessary information about brain functions at anatomical, cellular, and molecular levels
[5,6][5][6]. Furthermore, the image-guided biological studies facilitate researchers to better understand the detailed biochemical processes involved in metabolic and functional physiological events (the macromolecular interaction in cells at healthy or diseased states) and translate them into pathologic functions associated with the disease to establish independent research directions
[7]. Abilities of engineered nanomaterials generated in theranostics ensure the dual capacity of therapeutic delivery
[8] and diagnosis to a preponderance of personalized medicine in clinical conditions to target different neurological diseases (
Figure 1). In spite of all these, the nanomaterials produce the imaging signals of delivered molecules which could carry the essential features of molecular imaging and which further translate the imaging signals towards image acquisition. Several nanostructured materials
[9] with magnetic properties
[10,11][10][11] are emerging due to their functional properties towards biomedical applications with reduced toxicity. However, these nanostructures could be regulated via external stimuli-based magnetic fields that could regulate a variety of tissue modulatory potentials in vivo
[12,13,14][12][13][14]. This functionalization could improve the target functions, biocompatibility, and surface area modifications in biological and clinical translations
[15,16,17][15][16][17]. One of the major criteria for any molecular imaging in brain theranostics would be the target-specificity of the delivery method with improved biocompatibility at the delivered site without much toxicity. The principal imaging associated factors, such as high sensitivity, non-invasive imaging detection systems, signal penetration without attenuation, and temporal and spatial resolution, are the significant factors that are critical for ensuring the clinical potential of the developed methods
[18]. These factors should be carefully included while making the functional nanoparticles for imaging applications of the brain. In addition, these nanoparticles should possess properties that enhance image quality, contrast, and targeting features during their modifications, which could play prominent roles in target-specific molecular screening
[19,20][19][20].
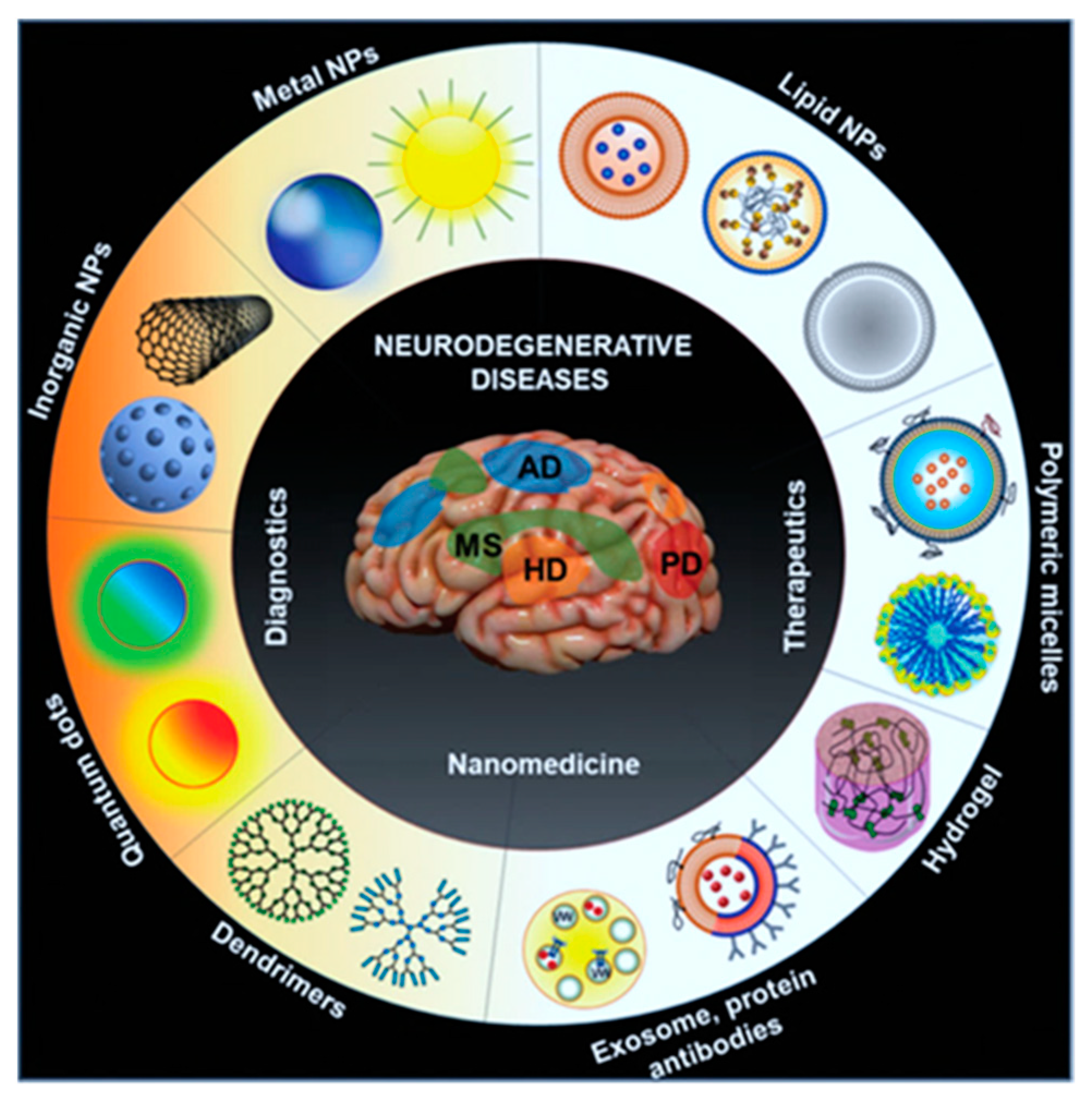
Figure 1. Scheme identifies the emerging different kinds of nanomaterial formulations attempted for the improved drug delivery approaches in neurological diseases
[8].
Brain with its complex neural network is difficult to study using various biochemical techniques. Non-invasive molecular imaging techniques with the potential to monitor dynamic physiological events in the brain could be an effective strategy for studying the complex processes of the nervous system
[21]. One of the significant breakthroughs in the longitudinal image monitoring of animals is molecular imaging, which allows researchers to observe biological events at physiological conditions without the need for sacrificing the animals
[22]. Gold nanoparticles with various surface modifications using imaging probes/candidates have allowed X-ray contrast imaging in disease diagnosis, including cancer. These innovative strategies might be applicable to detect tiny tumors in multiple organs, including the brain, for glioma, when they are a few-millimeter in size in vivo
[23,24][23][24]. In addition, the enzyme-modified functional gold nanoparticles systems were applied to make nanoparticles with the property of self-assembly and disassembly in vivo at the delivered target sites
[25,26,27][25][26][27]. Moreover, novel nanomaterials functionalized with fluorescent probes can be used for image-guided disease theranostics of the brain, while the coupled chemical agents functionalized at the surface of the nanoparticles could enhance the targeted delivery and therapy during theranostic applications
[28,29][28][29]. These contrast agents further help the researchers to get more detailed information via delivered functional nanomaterial systems to understand the biological systems and their interrelationship much better than the conventional methods
[30,31][30][31].
In view of the perspective, the blood–brain barrier (BBB), which interfaces between the central nervous system (CNS) and the vascular compartment, can block the delivery of administered nanoparticles circulating in the body to the brain’s extravascular cells. BBB is critical in protecting brain cells from the vascular contents to maintain homeostasis; in contrast, it represents an intense challenge in drug delivery applications. The permeability modulations of BBB can be graded by light-laser intensity
[32]. The permeability of BBB is entirely reversible and involves increased paracellular diffusion or opening at the delivered sites without leading to a significant disruption in the structure of neurovascular units of the brain. This strategy allows the delivery and entry of multiple therapeutic agents, such as immunoglobulins and viral gene therapy vectors, cargo-laden liposomes, functionalized nanomaterials, peptide conjugates, and nanomaterials with small molecules
[33]. The researchers anticipate this theranostic nanotechnology development might be helpful in tissue regions accessible to novel imaging applications and open novel venues in screening and therapeutic interventions in CNS diseases. Thus, the recent emergence of nanomaterial systems could be applied for disease or cellular pathway imaging at the molecular level by linking with theranostic imaging fields via providing potentials to grow molecular imaging field both in vitro and in vivo applications
[34,35,36][34][35][36]. Furthermore, various imaging methods, such as optical/magnetic resonance, have been achieved via developing shape-tuned nanomaterials, such as nanospheres, nanorods, nanocoils, and nanoclusters, to test under various physiological conditions
[9,14,37,38][9][14][37][38]. Moreover, the currently used nanoparticles for MRI imaging have broader magnetic properties, and most of them are paramagnetic or super-paramagnetic in nature
[39,40][39][40]. In addition, the other oxidized forms of metal nanomaterials (i.e., MnO and SPIONs, etc.) carry the MR-imaging (
Figure 2) features have been applied in brain theranostics
[41,42,43][41][42][43]. There has been the emergence of gold nanoparticles, nanorods, carbon nanoparticles or nanotubes, and graphene oxide nanomaterials, which are being translated to photoacoustic and other functional imaging modalities in neurological disorders, including brain cancers.
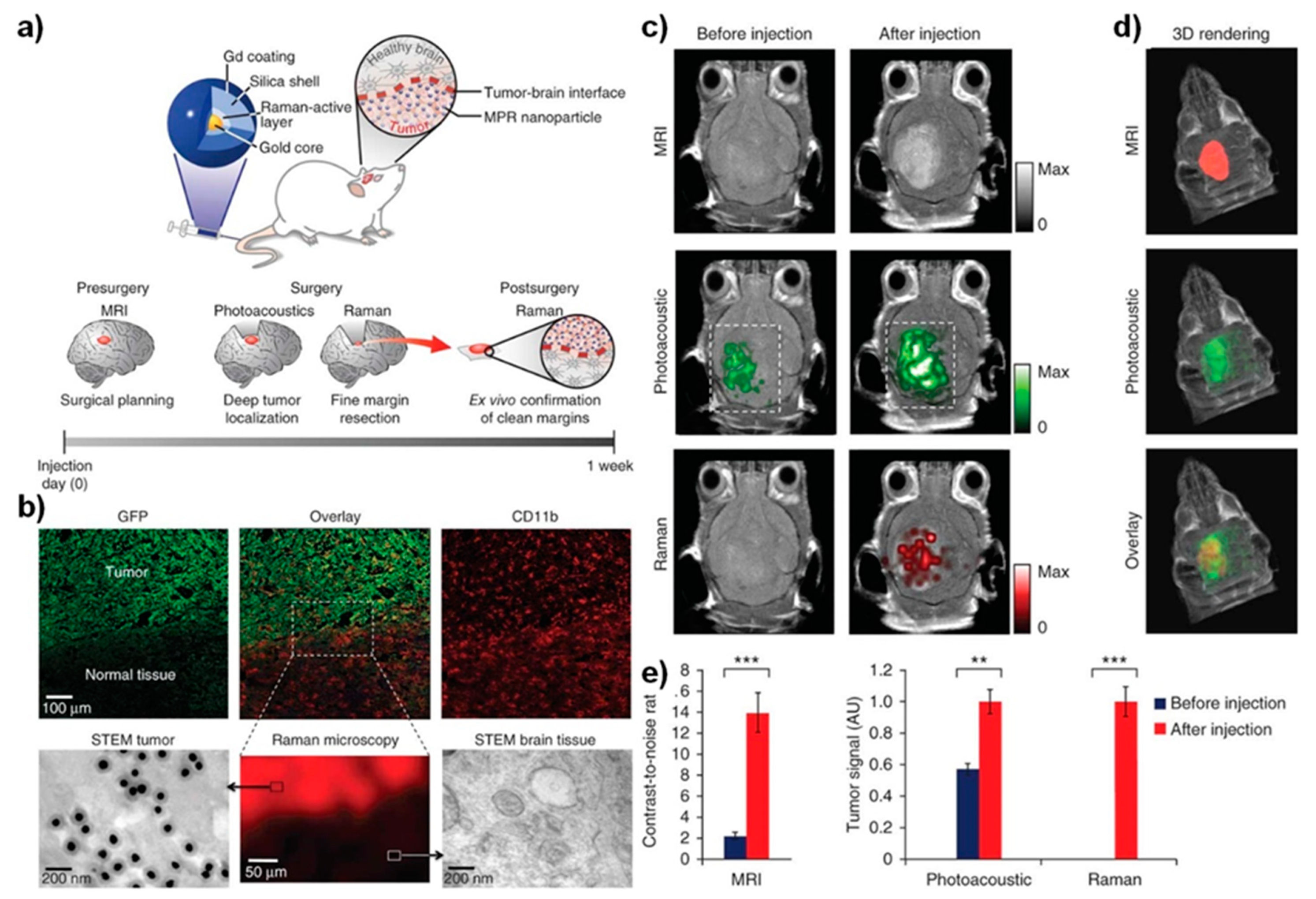
Figure 2. Triple-modality nanoparticle delivery and imaging concept to the brain tumor model. (
a) Delivery of nanoparticles circulates in the bloodstream; they diffuse through the disrupted blood–brain barrier and are then sequestered and retained by the tumor; upon employing photoacoustic imaging, the high resolution, and deep tissue penetration guide tumor resection intraoperatively in the surgical room. Following imaging strategies of the brain specimen can subsequently be examined as an imaging probe ex vivo to validate clear tumor margins. (
b) Immunohistochemistry of the tissue sections from the margin of the brain tumor stained for glial cells under confocal laser scanning microscopy. Scanning transmission electron microscope (STEM) images validated the presence of delivered nanoparticles in the brain tissue, whereas no such nanoparticles were seen in the healthy brain tissue. (
c) Two-dimensional axial MRI, Photoacoustic, and Raman images; (
d) three-dimensional (3D) rendering of magnetic resonance images with the tumor segmented overlay of the three-dimensional photoacoustic images. (
e) Corresponding quantitative signals of the nanoparticles from images shown in (
c,
d). Shown data represents mean ± S.E.M; ***
p < 0.001, **
p < 0.01
[43].
To further envisage the importance of nanomaterials in brain theranostics, functional nanomaterials with the widespread application have been developed with significant roles in the field of emerging nano-theranostics of molecular imaging. Nanotechnology is an engineering discipline that comprises the characterization and application of nanoscale (1–999 nm) materials in a single structural dimension for disease diagnosis applications. Most of the engineered nanomaterials are designed to display various functional features, such as high sensitivity, selectivity, and tunable properties, that are absent, or their properties vary from other bulk materials
[44,45][44][45]. In addition, these nanoscale materials can be modified to possess novel features, such as optical, magnetic, structural, and electronic properties, which are not originally present in the bulked materials. These utilizations of nanomaterials are attracting their applications in various aspects of drug delivery, molecular diagnosis, theranostics, and improved cancer therapies, including brain cancer or other neurological disorders, such as Alzheimer’s and Parkinson diseases. However, before the researchers apply these nanomaterials for brain theranostics of nanotechnology in novel drug delivery and imaging, there must be several aspects that should be considered for better outcomes, which include improved biocompatibility, less or no toxicity, non-agglomeration in the body upon injection, longer half-life, and in vivo stability and improved target specificity with enhanced imaging qualities at the target sites
[46]. As shown in
Table 1, several forms of nanomaterials have been engineered and applied for the brain targeting functions with improved therapeutic outcomes. In addition to that, Zhang et.al. developed an engineered nanomaterial platform to act as disease contrast agents in brain imaging. These materials showed less toxicity and a prolonged circulation time upon delivery
[47]. In addition, the potentialities of nanomaterials associated toxicities could be escaped via certain structural modifications in the shape or properties, whereas the nanomaterials controlled to show slow and sustained release at the delivery site of the brain upon linked using targeting receptors/ligands involved to suppress the associated toxicities in vitro and in vivo
[48,49,50][48][49][50]. Recently, Sukumar et.al. developed a multifunctional nanosystem using gold iron oxide nanoparticles (GIONs) conjugated with receptor targeting peptides with less toxicity for targeting glioblastoma via intranasal administration to overcome BBB
[51]. This smart construct delivered the small therapeutic microRNAs that altered gene expression while facilitating contrast CT and MR-imaging of the glioma cells. The delivered microRNA further sensitized the tumor cells to the delivered Temozolomide (TMZ) anticancer agent in vivo (
Figure 3). Similarly, Zhou et.al. developed a non-toxic nanoimaging material that specifically measured angiogenesis in glioblastoma
[52]. Thus, the emerging nanomaterials platforms to brain theranostics would emphasize this field upon using their distinctive characteristics, further supporting them for biomedical imaging modalities, including drug delivery and therapeutics
[53].
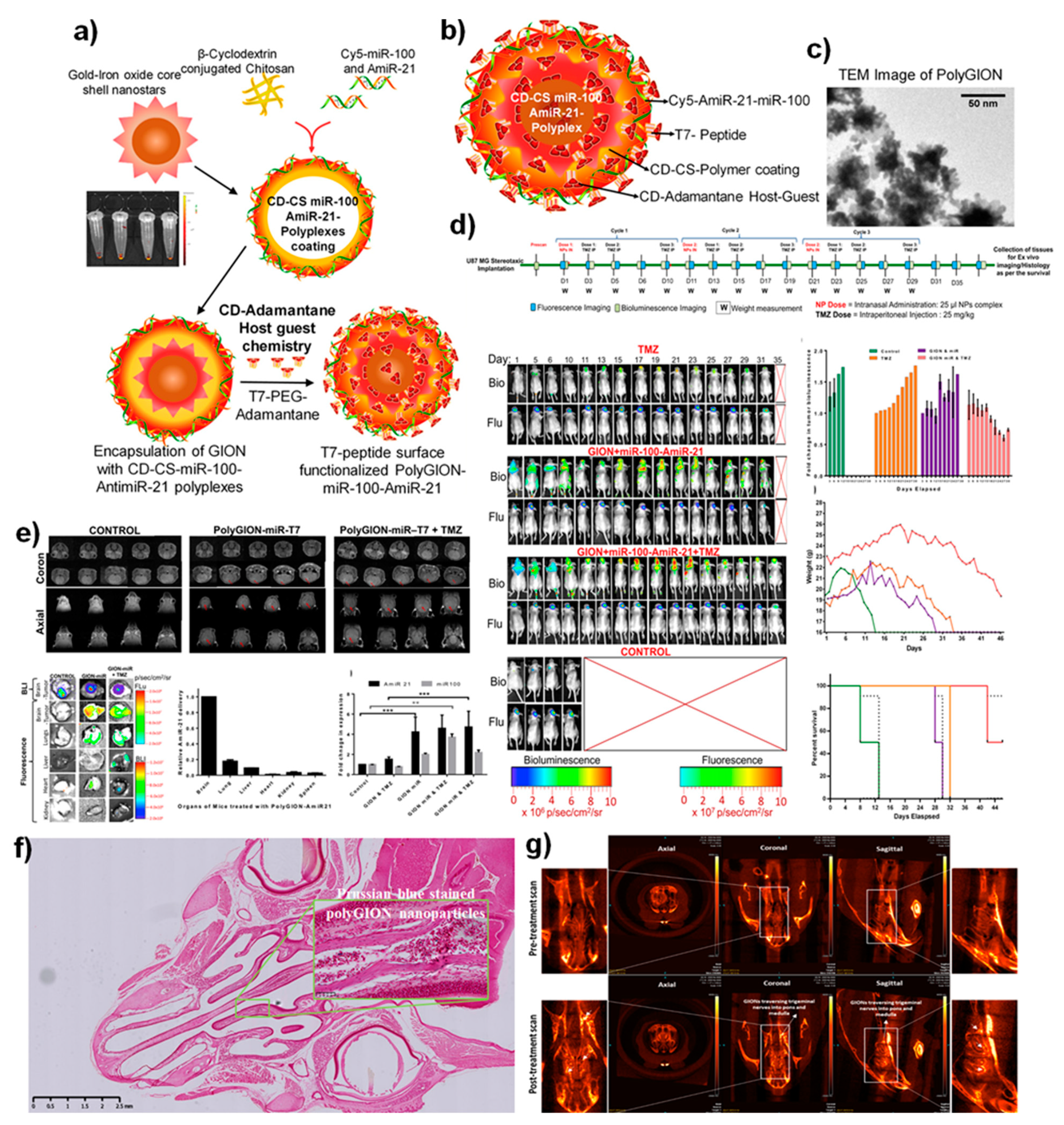
Figure 3. (
a) Schematic illustration of the synthesis of Poly-gold-iron oxide nanoparticles (polyGIONs) system and in vitro fluorescence images of Cy5 labeled miR-100 and antimiR-21 loaded cyclodextrin-chitosan (CD-CS) hybrid polymer complexes. (
b) Schematic of the as-prepared polyGION nanoparticle structure and the associated compositions. (
c) TEM micrograph of GIONs. (
d) In vivo treatment flow chart of the therapeutic design and imaging timelines; fluorescence (Cy5-miRNA loaded nanoparticles) and bioluminescence (FLuc-EGFP expressing glioblastoma model); quantitative measurements for the tumor bioluminescence measured concerning treatment duration; mice body weight profiles over the treatment duration and their survival curve indicates the intranasally delivered nanoparticles towards the theranostic efficacy. (
e) 3T MRI scanning (coronal and axial) of the polyGIONs-miRNAs treated mice brain imaging; biodistribution; ex vivo fluorescence imaging, and qRT-PCR of antimiR-21 and miR-100 expression levels
[51]. (
f) H&E-stained histological image shows the nasal epithelium, followed by iron-specific Prussian blue staining (inset figure) to trace the accumulation of polyGION nanoparticles in mice intranasal cavities. (
g) microCT imaging of mice head scan shows the non-treated (control) and T7-polyGION-CD-CS NPs administered in vivo. Corresponding microCT scan images depict the migration of IN administered T7-polyGION-CD-CS NPs nanoparticles movements through the olfactory nerve pathway into the olfactory bulb and passing into trigeminal nerve pathway, thereby entering the pons and medulla of the mice brain. Shown data represents mean ± S.E.M; ***
p < 0.001, **
p < 0.01
[51].
Table 1. A list of functionalized theranostic nanoformulations developed to deliver therapeutics for brain-related diseases intranasally.
|
Target Disease
|
Nanoformulation
|
Model Organism
|
Therapeutic Outcome
|
Ref. No.
|
|
Parkinsons
|
Selegiline nanoemulsion
|
Rat
|
Intranasally administered selegiline nanoemulsion improved the behavioral activities in comparison to oral administration.
|
[54]
|
|
Parkinsons
|
Resveratrol and curcumin nanoemulsion
|
Sheep
|
Intranasal delivery of hyaluronic acid-based lipidic nanoemulsion proven as a successful carrier to enhance the solubility, stability, and brain targetability of polyphenols.
|
[55]
|
|
Alzheimer’s disease
|
Rivastigmine-loaded nanoemulsion
|
Rat
|
Achieved higher drug delivery to the brain with enhanced safety, non-toxic and non-irritating to the nasal mucosa.
|
[56]
|
|
Alzheimer’s disease
|
Donepezil nanoemulsion
|
Pig
|
Effective strategy using polymers improved the adhesion and penetration of the drug through the nasal mucosa.
|
[57]
|
|
Alzheimer’s disease
|
Cholera Toxin B subunit-based nanoparticles
|
Mice
|
Delivered nanosystem exhibited a notable performance in accumulating in the hippocampus that further showed an excellent magnetic resonance imaging (MRI) potential in vivo.
|
[3]
|
|
Epilepsy
|
Letrozole loaded nanoemulsion
|
Mice
|
Intranasal administration of nanoemulsion improved the prolonged drug release profile in brain as compared to suspension.
|
[58]
|
|
Migraine
|
Zolmitriptan mucoadhesive nanoemulsion
|
Rat
|
In vivo delivery showed higher permeability through the nasal mucosa.
|
[59]
|
|
Neuroprotective
|
Kaempferol loaded chitosan nanoemulsion
|
Rat
|
In vivo delivery and biodistribution studies exhibited a higher drug concentration in the brain upon intranasal administration.
|
[60]
|
|
Glioblastoma
|
Bevacizumab-PLGA NPs
|
Mice
|
Bevacizumab-loaded PLGA NPs showed effective tumor reductions as accompanied by higher anti-angiogenic potentials than free drug.
|
[61]
|
|
Glioma
|
Ecto-50-nucleotidase (CD73 siRNA) nanoemulsion
|
Rat
|
Intranasal nasal administration of cationic nanoemulsion with CD73 siRNA delivery system improved glioblastoma therapy.
|
[62]
|
|
Glioma
|
Temozolomide-Anti-EPHA3 PLGA NPs
|
Rat
|
Study results indicated that anti-EPHA3-decorated PLGA NPs targeted the Glioma via a nose-to-brain drug delivery approach.
|
[63]
|
|
Glioblastoma
|
Farnesylthiosalicylicacid (FTA) loaded hybrid NPs
|
Rat
|
Intranasal delivery of FTA-NPs improved the glioblastoma therapy in vivo.
|
[64]
|
|
Glioblastoma
|
miR-100 and antimiR-21 loaded PolyGIONS
|
Mice
|
Intranasal delivery of NPs strategy potentiated the nano-theranostic effects in vivo.
|
[51]
|
|
Glioblastoma
|
siRNA + TMZ loaded chitosan NPs
|
Mice
|
Intranasal delivery of nanoparticle adjuvants increase the efficiency of immune-checkpoint blockade and chemotherapy in vivo.
|
[65]
|
|
Glioblastoma
|
Self-assembled BMP4 plasmid DNA with poly(beta-amino ester) NPs
|
Rat
|
Intransally administered NPs could target brain tumors to enhance targeted therapies.
|
[66]
|
|
Gliobastoma
|
Self-assembly of MPEG-PCL-Tat with siRaf-1/ Camptothecin
|
Rat
|
Nose-to-brain delivery proved the excellent therapeutic functions for treating glioblastoma.
|
[67]
|
|
Glioblastoma
|
Extracellular vesicles (EVs) loaded with CXCR4 receptor, antimiRNA-21 and miRNA-100 biomaterials
|
Mice
|
Intranasally delivered EVs with miRNA sensitized the tumor cells to treat temozolomide, thereby improving mice’s survival rate.
|
[68]
|
|
Epilepsy
|
Carbamazepine loaded carboxymethyl chitosan nanoparticles
|
Mice
|
Enhanced drug bioavailability and brain targeting was achieved via nasal administration.
|
[69]
|
|
Central nervous systems disorders
|
Rabies Virus Glycoprotein (RVG29)-Modified PLGA Nanoparticles
|
Mice
|
Engineered nanoparticulate systems proved the viral delivery vectors to target and treat CNS via intranasal delivery.
|
[70]
|
|
Huntington’s disease
|
Chitosan nanoparticles loaded with anti-HTT siRNA
|
Mice
|
Intranasal delivery proved the promising therapeutic alternative for safe and effective which further decreases the mutant HTT expression.
|
[71]
|
|
Ischemic stroke
|
17β-estradiol (E2) loaded gelatin nanoparticles
|
Mice
|
The intranasally administered nanoparticles achieved higher delivery efficacy in vivo.
|
[72]
|
|
Newcastle disease and infectious bronchitis
|
Chitosan nanoparticles loaded with the combined attenuated live vaccine
|
Chicken
|
Intranasal adjuvant and delivery carrier made a mucosal vaccine and delivery of drugs for enhanced immune functions.
|
[73]
|
|
SARS-CoV-2
|
Receptor-binding domain (RBD) of SARS-CoV-2 spike glycoprotein loaded chitosan nanoparticles
|
Mice
|
An alternative route of intranasal vaccination mimics the natural route of SARS-CoV-2 infection and stimulates both mucosal and systemic compartments of the immune responses.
|
[74]
|
|
SARS-CoV2 vaccine mucosal immunization
|
Au-nanostar-chitosan loaded with SARS CoV-2 DNA vaccine
|
Mice
|
Intranasal administered SARS-CoV2 DNA vaccines encoded the spike protein antigen loaded nanomaterial achieved the humoral antibody responses and providing long-lasting immunity.
|
[75]
|
|
Respiratory infection
|
Chitosan Nanoparticles–Adjuvanted Chlamydia Vaccine
|
Mice
|
Intranasal adjuvants induced the humoral, mucosal, cell-mediated immunity against bacterial infections in vivo by acting as nano vaccines.
|
[76]
|
2. Nanomaterials Improving Theranostic Imaging Modalities
Theranostic nanoparticles in molecular imaging significantly impact non-invasive strategies to understand biological and biochemical events in intact cells within living subjects. It plays a prominent role in disease diagnosis and therapeutic monitoring outcomes in vivo
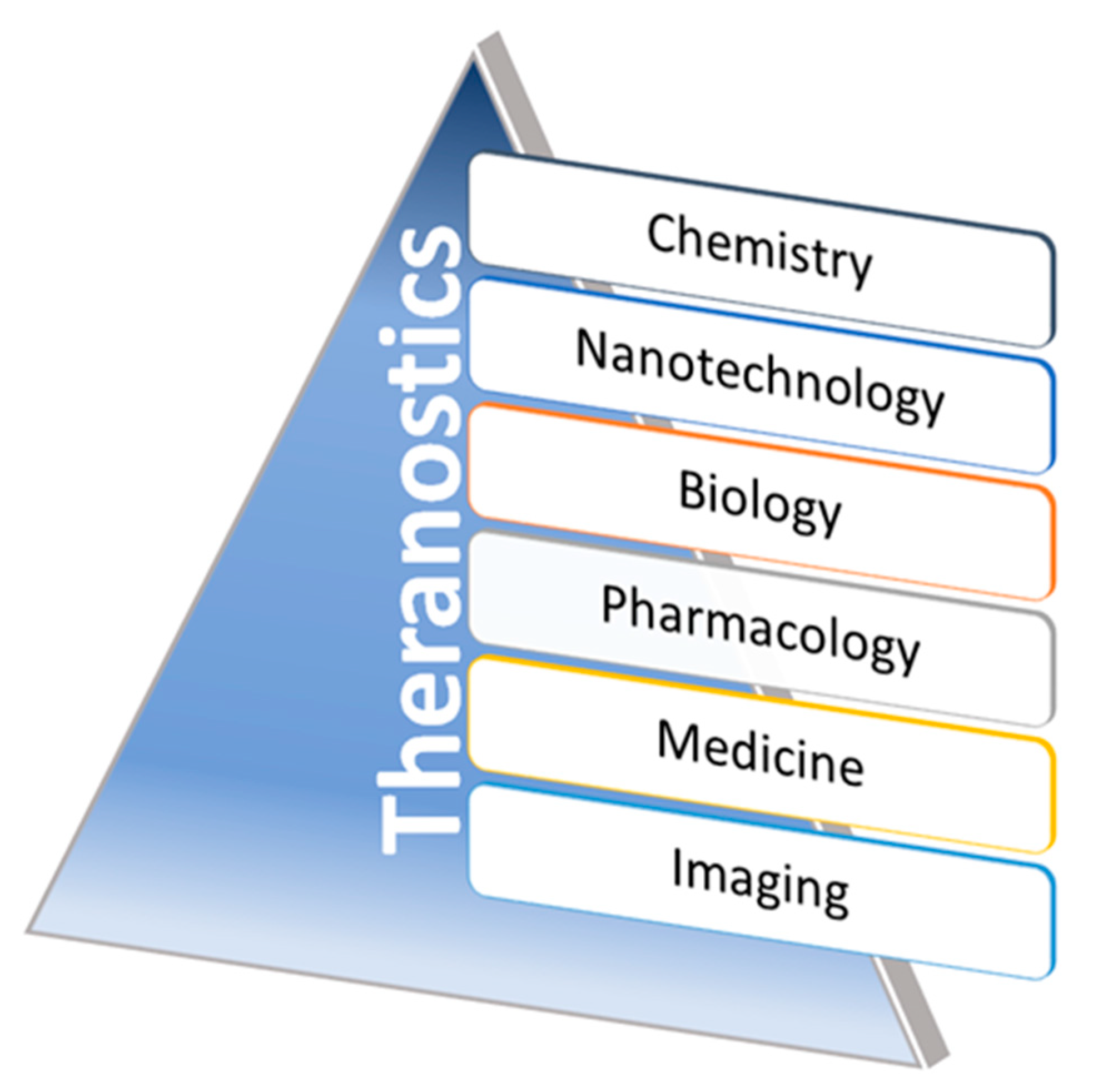
[27]. The theranostic application of nanomaterials can be classified into morphological and functional imaging based on their roles in image contrast abilities during applicable imaging methods. A wide range of multifunctional nanoparticles have been extensively proven for their properties as an agent for both therapeutic and diagnostic applications (theranostics). Promoting newer research directions are shown to explore those novel materials function in relevant animal disease models via improving their qualities towards clinical translations—recent approaches in non-invasive disease monitoring, biomarkers, and therapeutic drug deliveries are under investigation in advanced theranostics. In addition, some of the biomaterials, including magnetic NPs, QDs (Quantum dots), UCNPs (Upconverting nanoparticles), SLNs (silica nanomaterials), carbon nanoparticles, and organic dye coupled materials, have shown a significant role in theranostics with wide ranges of clinical translations. Variations in size and surface changes could modulate biocompatibility and interactions of these nanomaterials with target tissues. Hence, developing the contracted interest for improved disease monitoring/detections with improved chemotherapies along with clinically translatable innovative nanomaterials can be a significant driving force for theranostic agent research in the near future. The advancements made in, and the tie between, interdisciplinary scientific disciplines, such as nanotechnology, biology, pharmacology, chemistry, medicine, and imaging fields involving developing theranostics, have been significantly designed and evaluated over the past few years in clinical conditions, for growing nano-theranostics (
Figure 4).
Figure 4. Schematic representations of the growing contributing fields of theranostics. Representative illustration showing the contributing interdisciplinary fields of nanomaterials associated with theranostics. Via adopting these multidisciplinary fields, the innovative nanomaterial formulations aim to involve disease monitoring, diagnosis, and therapy through the researcher’s intersections of multiple scientific fields.
The development of novel nanoparticles consisting of both diagnostic and therapeutic components has increased over the past decade. These theranostic nanoparticles have been tailored toward one or more types of imaging modalities. They have been developed as imaging probes in optical imaging, magnetic resonance imaging (MRI), photoacoustic imaging (PAI), computed tomography (CT), and nuclear imaging comprising both single-photon computed tomography (SPECT) and positron emission tomography (PET). Here, the researchers focused on the brain theranostic nanoparticles capable of both delivering therapy and self-reporting/tracking disease through imaging. Generally, imaging modalities, such as optical imaging, SPECT, and PET, are performed using a broad range of probes with high sensitivity
[81][77]. The other primary imaging modalities, CT and MRI, are also reduced by image contrast properties during the probe conjugation or the tailored agents in the surface modification to impact resolution and sensitivity further. The growing interest in applying CQD (carbon quantum dots) with functionalized nanomaterials attention to various brain-related drug delivery approaches is emerging towards the clinical need of the situation. Utilizing these types of nanomaterials in combination with external stimuli, such as light or photoacoustic waves, at the delivery site, could efficiently modulate the functional properties of chemotherapeutic agents with diagnostic abilities
[82,83][78][79]. The broader utilization of CQD in clinical applications is increasing because of their role in various aspects, including imaging and drug/gene delivery with therapeutics. The use of CQD in drug delivery across the BBB was achieved via nanoparticles after several functionalization processes in their structure that further internalized to the glioma cells, thereby envisaging their potentials in theranostic modalities in vitro and in vivo
[84,85,86][80][81][82]. Thus, the novel nanomaterials in various categories could overcome these limitations by enhancing the tissue penetration, biodistribution at the desired sites, and the target specificity while adopting them in molecular imaging application
[87][83]. Moreover, the nanomaterials are economically affordable and accurately deliverable for molecular-level quantitative imaging information towards translational approaches. Several interdisciplinary areas that range from novel targeting strategies, combination therapies, and unique imaging prospects via multiple multidisciplinary fields are emerging in recent decades to enhance theranostics (
Figure 4). Despite significant progresses in developing MRI-targeted nanotheranostic platforms and their undeniable potential in predictive, preventive, and personalized medicine, gaps in knowledge continue to hinder their translations from bench to bedside, and a few nanotheranostic systems have undergone clinical trials
[88][84]. This can be due to several factors, including the complexity of the developed hybrid nanosystems, difficulty in predicting their complex effects and interactions with biological systems, species-dependent immune responses and toxicity profiles, difficulty in controlling the pharmacokinetics and biodistribution properties, premature release of the therapeutic cargos in blood and healthy tissues, toxicity concerns, and the significant differences between animal models and humans. Recent research has focused on using imaging data to understand better the interactions between nanoparticles and biological systems to optimize tumor targeting and biodistribution
[89][85].
In recent years, the growing interest in multimodal theranostics has been getting broader to overcome most contrast-associated limitations; nanomaterials with image contrast functions can provide more optimal materials to study the physiological and anatomical data retrieval in disease diagnosis with treatments (theragnosis). Hence, molecular engineers and nanotechnologists are trying to improve the existing nanomaterials contrast in disease detections in several human diseases, including neurological disorders
[90][86]. Furthermore, various research groups work towards brain targeting and imaging with multifunctional nanomaterials strategies for suitable optical and MR-imaging modalities
[24,91,92][24][87][88]. In practice, the multimodal targeting of tumors in the brains or the microenvironments could reach via engineered nanomaterials to enhance their theranostic functions (
Table 1). In addition to integrating the disparity of dosage requirement between diagnostic and therapeutic entities within a single nanoparticle platform, the emerging tailored theranostic technologies could be applied to optimize the differences by estimating their circulation times that will further necessitate to realize the potentialities.