N-Glycosylation (NG) and disulfide bonds (DBs) are two prevalent co/post-translational modifications (PTMs) that are often conserved and coexist in membrane and secreted proteins involved in a large number of diseases. Both in the past and in recent times, the enzymes and chaperones regulating these PTMs have been constantly discovered to directly interact with each other or colocalize in the ER. However, beyond a few model proteins, how such cooperation affects N-glycan modification and disulfide bonding at selective sites in individual proteins is largely unknown. More investigations should be encouraged to unveil the hidden relationships of NG and DBs in the majority of membranes and secreted proteins for pathophysiological understanding and biotherapeutic development.
1. Introduction
Both
N-glycosylation (NG) and disulfide bonds (DBs) can form co- and post-translational modifications (PTMs)
[1] on proteins in the endoplasmic reticulum (ER) while they pass through the secretory pathway. These two modifications are not only common but often evolutionarily conserved in membrane and secreted proteins from prokaryotes to eukaryotes. As two critical modifications, they facilitate protein folding and regulate protein structure, function, stability, and cellular localization. Defects in either one can fail protein ER quality control, trigger unfolded protein response, and cause pathological conditions ranging from heritable congenital disorders of glycosylation as an example to acquired disorders such as cancers, dementia, diabetes, autoimmune, infectious, and cardiovascular diseases
[2][3][2,3].
2. Promoting Relationship
Feng et al.
[4][37] demonstrated through kinetic studies of intracellular folding of the human chorionic gonadotropin (hCG)-β subunit that NG facilitated the rapid formation of DBs and the folding of the hCG-β subunit, which harbors six DBs
[5][39]. The relative positions of NG and DBs are shown in
Figure 1. Lacking the two NG sites slowed down the folding of the β subunit more than fourfold from 7 min to 33 min in CHO cells, and the slow formation of DBs retained the misfolded proteins up to 5 h in the ER before degradation
[4][37]. The co-expression of the α subunit could assist the appropriate folding and secretion of the β subunit of the hormone lacking the NG. Among the six DBs in the hCG-β subunit shown in
Figure 1, the formation of Cys
34–Cys
88 occurred earlier than that of Cys
9–Cys
57/Cys
38–Cys
90, while the remaining three pairs occurred later
[5][39]. The first three pairs are important in protein folding and secretion and
N-glycan processing. Eliminating these early formed DBs rendered part of the
N-glycans to be high mannose instead of complex glycans, which were sensitive to ER quality control and degradation.
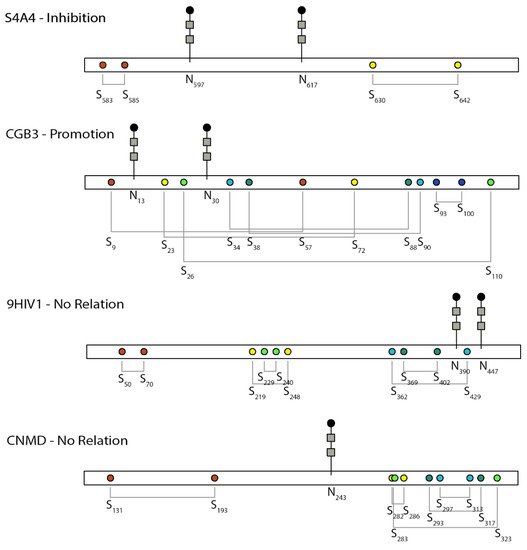
Figure 1. Schematic of the relative position between N-glycosylation and disulfide bonds and their relationship in the selected proteins discussed here. S4A4, UniProt ID of human SLC4 Na+-coupled transporter, NBCe1; CGB3, UniProt ID of human chorionic gonadotropin beta-subunit; 9HIV1, UniProt ID of human immunodeficiency virus envelope glycoprotein; CNMD, UniProt ID of human chondromodulin-1. Colors indicate different disulfide bond pairs. The N-glycan sign does not represent the actual sugar structure.
Another study was conducted in the β subunit of Na, K-ATPase. Na, K-ATPase is a plasma membrane transporter that is responsible for the maintenance of potassium and sodium homeostasis in animal cells
[6][59]. The functional β subunit is a type II glycoprotein composed of a large C-terminal ectodomain with three NG sites (Asn
158, Asn
193, Asn
265) and three conserved DBs (Cys
126–Cys
149, Cys
159–Cys
175, and Cys
213–Cys
276)
[6][59]. The mutation of Cys
126–Cys
149 increased the non-glycosylated proportion of the protein compared to the wildtype from the Western blot
[7][60], suggesting a promoting relationship. Mutating each of the three glycosylation sites indicated their involvement in initial folding
[6][59]. The acquisition of at least one sugar moiety was necessary for the β subunit to ensure its association with the α subunit through pulse chase. Interestingly, when all three
N-glycans were removed, the protein did not form aggregates through DBs but permanently associated with BIP from degradation
[6][59].
In addition to hCG and Na, K-ATPase, multiple other examples also indicate a “strengthening” relationship between NG and DB. Mirazimi and Svensson
[8][105] showed that the chief role of NG on rotavirus VP7 is to facilitate correct intermolecular DB formation in dimerization. Removal of NG induced VP7 misfolding through random intermolecular DBs. Similar effects were also observed for MUC2
[9][56], vWF
[10][36], meprin A
[11][52], and hemagglutinin
[12][47].
3. Inhibitory Relationship
The hemagglutinin-neuraminidase (HN) glycoprotein of Newcastle disease virus (NDV) is responsible for virus attachment to host cell receptors, thereby initiating infection
[13][4]. The HN protein is a type II membrane protein containing six potential NG sites: Asn
119, Asn
341, Asn
433, Asn
481, Asn
508, and Asn
538 [14][45]. Among them, only four (Asn
119, Asn
341, Asn
433, Asn
481) are utilized for NG
[15][106]. The protein also has 13 cysteine residues in the ectodomain
[15][106]. The cysteine residue closest to the membrane anchor (Cys
123) is involved in an intermolecular DB
[16][17][107,108], while the other 12 cysteine residues form intramolecular DBs
[16][107].
McGinnes and Morrison found that intramolecular DBs might play a critical role in the usage of glycosylation sites
[16][107]. They explored whether DB formation could be a determinant of the two unused glycosylation sites, Asn
508 (site 5) and Asn
538 (site 6), in HN protein
[14][45]. Removing Cys
531–Cys
542 flanking the unused glycosylation site Asn
538 by mutation or DTT promoted the NG of Asn
538 for an efficiency of 39–59% and 26–27%, respectively
[14][45]. The successful NG was supported by the deglycosylation analysis with endo H. Under similar conditions, the usage of the non-glycosylated site 5, Asn
508, which is far from any DB, was not improved. Together, these results suggest that the glycosylation of Asn
538 is under steric hindrance by the DB in the vicinity
[14][45], whereas the non-glycosylated Asn
508 could be caused by other factors not related to DBs
[14][45].
Another study investigating the lack of sequon utilization in tissue-type plasminogen activator (t-PA) reported similar findings that folding and DB formation of t-PA negatively impact the extent of core
N-glycosylation
[18][33]. As a result, they suggested that variable usage of glycosylation sites could be caused by the transient accessibility and appropriate orientation of the sequon relative to the transferase or dolichol-linked donor in a folding event
[18][33].
Human sodium bicarbonate cotransporter 1, NBCe1 (
SLC4A4 gene), is an electrogenic sodium/bicarbonate cotransporter localized in the plasma membrane
[13][4]. The malfunction of this gene is related to a series of diseases in the kidney, eye, ear, brain, and tooth. All SLC4 Na
+-coupled transporters are multipass transmembrane proteins containing a large extracellular loop (EL-3) with multiple NG consensus sites and four highly conserved cysteines
[19][109]. NBCe1-A, one of the three variants, is a homodimer, and its two EL-3 loops form unique conformations that are potentially critical to the function of the protein
[20][28]. In the EL-3 loop of NBCe1-A, two sequons are glycosylated (Asn
597 and Asn
617) but not Asn
592, and four conserved cysteines form two intramolecular DBs (Cys
583–Cys
585 and Cys
617–Cys
642)
, [20]as shown in Figure 3 [28].
In a detailed study evaluating the interplay between DBs and NG to define the EL-3 loop topology in NBCe1, it was found that the two EL-3 loops of the dimer formed a unique clove conformation
[20][28]. This conformation was “finely tuned” by glycosylation
[20][28]. In the absence of Cys
583–Cys
585 or the two NG sites, the third NG site, Asn
592, became glycosylated. With glycans, the two DBs were deeply buried from the external surface of the EL-3 loop, which can sustain DDT-induced denaturation and enzymatic digestion under basic conditions. Losing both DBs and NG made the loop adopt an extended structure that could not be recognized by the designated antibody and was susceptible to chymotrypsin digestion
[20][28]. Instead of considering the steric hindrance between DBs and NG at Asn
592,
rthe
searche authors hypothesized that Asn
592 was originally glycosylated at the nascent polypeptide chain of NBCe1-A and later removed when DBs and other glycosylation sites were formed
[20][28]. Removing NG did not affect the formation of two DBs; however, an additional removal of one cysteine in the DBs by mutagenesis promoted the formation of intermolecular DBs in the homodimer, which maintained transport function
[20][28]. A complex relationship must exist between NG and DBs in determining the final folding of EL-3 during NBCe1-A protein maturation; however, no kinetic experiments were performed to monitor protein folding, and ER maturation was not specifically probed. It is, therefore, difficult to elucidate how ER resident enzymes facilitate these processes.
The study, on the other hand, had systematic structural delineation by a combinatorial mutation of all four cysteines in two DBs for a total of 12 mutants.
4. Independent Relationship
Envelope glycoprotein 160 (gp160) on human immunodeficiency virus (HIV) is critical for viral binding to the CD4 receptor and fusion with CD4
+ cells. The precursor gp160 needs to be cleaved to gp120 and gp41 to activate the binding domain on gp120 with CD4. A study on the linkage region between gp120 and gp41, which is also the future binding site of gp120 to CD4, suggested that DBs and NG in this region function independently
[21][27]. The relative position between NG and DBs in this region is shown in Figure 3. Cys
402 and Cys
429 are both located in the linker region but form separate DBs, of which Cys
402 is critical for cleavage. Mutation of Cys
402 not only prevented the cleavage but also affected the transport of gp160 and the future binding of gp120 to CD4
+ cells
[22][23][110,111]. Around Cys
402, there are two occupied NG sites Asn
390 and Asn
447. Mutating these NG sites did not affect disulfide bonding through Cys
402 or the relevant functions, suggesting an independent relationship between DBs and NG.
In another study of the 25 kD extracellular matrix protein chondromodulin-I (ChM-I), NG was critical in its solubility but had no effect on DBs
[24][69]. ChM-I is a secreted protein and has two separate domains, in which the hydrophilic
N-terminal domain is heavily glycosylated by one
N-glycan and two
O-glycans, whereas the hydrophobic C-terminal domain harbors four DBs. As the two domains are separated, the removal of either the NG or the
N-terminal domain seems to have no effect on the formation of DBs in the C-terminal domain, as shown in
Figure 1.
5. Unknown Relation
For most
of our s
earched studies that concerned both DBs and NG, the exact relationship between the two PTMs was not experimentally examined. Half of these studies focused on experimental mapping of their sites without functional studies. One-third of the remaining studies only predicted the potential DBs and NG by sequence alignment or computational modeling without experimental data. For the articles that did examine the functions of both modifications, many of them did not study or discuss their interactions but rather examined them separately. For a very small number of papers, the potential interactions were hypothesized but not experimentally verified.
For example, a very nice study investigated the role of NG and DB in the rat G protein-coupled receptor class C, group 6, member A (GPRC6A)
[25][112], a widely expressed GPCR that functions importantly in many diseases ranging from metabolic syndrome to cancer
[26][27][113,114]. This protein is a class C GPCR with a large
N-terminal extracellular domain (ECD), which contains a Venus-flytrap (VFT) domain and a cysteine-rich domain (CRD)
[13][4]. The VFT domain is for ligand binding, and the CRD domain is for signal transfer
[25][112].
It was observed that the ECD domain of GPRC6A consists of nine sequons
[25][112]. Only seven asparagine residues carry
N-glycans. Five of them are in the VFT domain, and Asn
555 and Asn
567 are located in the CRD
[25][112]. The VFT domain also has two conserved cysteines (Cys
122 and Cys
131), whereas the CRD domain has nine conserved cysteines, eight of which form intra-CRD DBs
[26][113]. Through analysis of different mutants by SDS-PAGE, it was found that Asn
555 is important for protein surface expression and that Asn
567 regulates receptor function. Furthermore, from the studies of two cysteines, Cys
122 and Cys
131, C
131 contributed to the formation of a homodimer through an intermolecular disulfide bridge
[25][112], and Cys
122 contributed to the interdomain DB between VFT and CRD
[28][115]. Mutation of C131A abolished the intermolecular DB and homodimer formation but did not impair receptor surface expression and its function, whereas mutation of C122A was responsible for the lowered signal response (40%) and higher (50%) surface expression
[25][112]. This result suggested that the C122A mutation causes certain conformational changes. Not only is Cys
122 next to Asn
121, but the DB between CRD and VFT domains can also be largely shaped by the seven
N-glycans carried by these two domains. It is likely that NG plays a role in the potential conformation changes or intermolecular DB formation; however, no experiments or discussion were presented on the relationships between NG and cysteine disulfide bridges or cysteines in the paper.
Another study explored the role of the conserved cysteines and NG sites among all alphaherpesviruses such as herpes simplex virus 1 (HSV-1) in virus production and membrane fusion by single- and double-site directed mutagenesis
[29][116]. Glycoprotein K (gK) is a conserved virion protein in all alphaherpesviruses
[30][117]. The
N-terminal extracellular domain of gK is important for HSV-1 to enter neurons via axonal termini. This domain contains two conserved NG sites at Asn
48 and Asn
58 and four conserved cysteines for two potential disulfide pairs of Cys
37–Cys
114 and Cys
82–Cys
243 according to single-cysteine mutation and computational modeling
[29][116].
It was found that viruses lacking Asn
58 or lacking both sites (Asn
48, Asn
58) had enhanced fusion
[29][116]. Interestingly, deletion of Cys
37 or Cys
114 led to a gK-null phenotype of few plaques, whereas mutation of Cys
82 or Cys
243 caused enhanced cell fusion.
RThe
searche authors provided an extensive discussion on the potential interactions between NG and DB on the basis of the known studies and hypothesized that the removal of NG at Asn
58 could displace the DB formation, as the deletion of the Cys
82–Cys
243 disulfide recapitulated a similar fusion phenotype of Asn58A. However,
rthe
searche authors did not further verify this hypothesis, such as examining the presence of DBs through gel shift assays, labeling assays for free thiols, or MS characterization. Therefore,
researchethe authors in the end did not entail the specific relationship more than stating the presence of “a potentially important relationship”
[29][116].
Related to the ongoing COVID-19 pandemic, the immunogen SARS-CoV-2 spike protein and its endogenous binding target ACE2 are both heavily glycosylated with numerous DBs. The SARS-CoV-2 S protein has a total of 22 sequons that to various extents are all glycosylated
[31][32][33][34][35][36][37][38][118,119,120,121,122,123,124,125], seven
O-glycosylations
[31][38][118,125], and 40 cysteines with 15 DBs
[31][118]. Similarly, ACE2 was mapped to have seven NG sites
[10][38][36,125], one
O-glycosylation
[38][125], and four DBs
[39][40][126,127]. According to mutations, molecular dynamics simulations of protein structures, and sequence alignment studies, eliminating certain DBs or NG on both ACE2 and SARS-CoV-2 S proteins can alter binding affinity to each other and change virus infectivity. For example, Cys
480–Cys
488 is considered the most important pair in the receptor-binding domain (RBD) of SARS-CoV-2 S proteins, and this pair participates in binding to the
N-terminal of the host receptor that forms a stable SARS-COV-2 and ACE2 complex
[39][40][41][126,127,128]. In addition, Cys
133–Cys
141 of ACE2 is responsible for making the loop at dimer interference
[42][41][76,128] and is predicted to be crucial for making interactions with the spike protein of SARS-CoV-2
[40][127]. Deletion of Asn
90 glycosylation of ACE2 increased the binding to S proteins, while removal of Asn
322 of ACE2 decreased virus binding and infection
[38][43][125,129]. From the perspective of SARS-CoV-2 S protein, the Ser
309 neutralizing antibody binds Asn
234 glycosylated RBD
[44][32], and double mutations of N165A/N234A
[45][35] and N331Q/N343Q
[46][53] in S protein both reduced the binding between the immunogen and the receptor. Despite the extensive and rapid studies of NG and DBs in S proteins and ACE2 in the past 2 years, no studies have examined the relationship between DBs and NG in these two proteins
. This phenomenon clearly indicates the severe understudy in this important field.