Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Joseph Oddy and Version 3 by Lindsay Dong.
Asparagine is an important plant metabolite, and since the discovery that it can be converted to acrylamide during the cooking and processing of food, there has been debate over how much its concentration could be reduced before effects were seen on other important traits. Breeding low-asparagine wheat could potentially be achieved in three main ways: directly, by using either existing or induced variation, or indirectly, through selection for related traits.
- wheat
- asparagine
- breeding
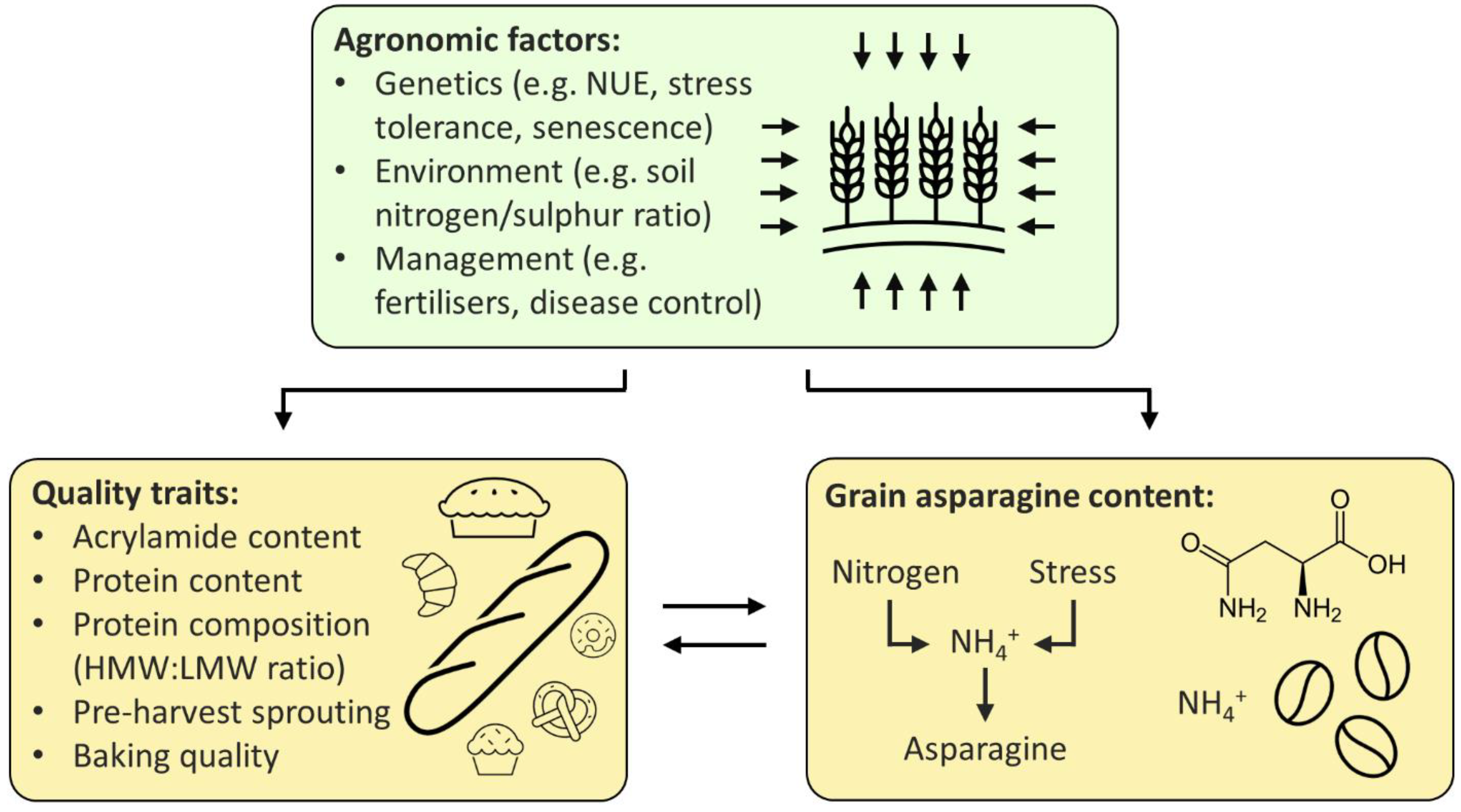
1. Relationships between Free Asparagine, Quality and Agronomic Traits
1.1. Free Asparagine Concentration and Quality Traits
Quality traits in wheat are those that impact the functionality of the end product (i.e., the baking and nutritional quality of the grain), so encompass traits such as pre-harvest sprouting (PHS), protein content and hardness. Grain-free asparagine content has sometimes been found to correlate with some of these quality traits, but this differs greatly between studies (
1.1. Free Asparagine Concentration and Quality Traits
Quality traits in wheat are those that impact the functionality of the end product (i.e., the baking and nutritional quality of the grain), so encompass traits such as pre-harvest sprouting (PHS), protein content and hardness. Grain-free asparagine content has sometimes been found to correlate with some of these quality traits, but this differs greatly between studies (
Table 1
and
Table 2
).
Table 1.
Association between free asparagine and selected quality traits.
| Asn Measurement | Trait | r | p | Reference |
|---|
Asn (asparagine), HFN (Hagberg falling number), Z-SDS (Zeleny sedimentation index).
One potentially interesting relationship is that between free asparagine and PHS, because of the potential for protein hydrolysis during PHS to release free asparagine. PHS negatively impacts wheat quality in a range of ways, reducing flour yield, the quality of baked products, and nutrient content [8]. Simsek et al. [9] reported a moderately strong (r = 0.6–0.7) positive correlation between free asparagine, sprouting score, and endoprotease activity in samples of sprouted wheat grain, suggesting that there was a relationship between asparagine and PHS at high levels of sprouting. Additionally, in a study designed to render the asparagine synthetase 2 genes (TaASN2) non-functional through gene editing, Raffan et al. [10] observed a poor germination phenotype that could be rescued through exogenous application of asparagine to the soil, implying that low-grain asparagine content may inhibit germination and could perhaps also affect PHS. Further research is required to confirm the germination phenotype, but asparagine synthetases are known to play important roles in germination in other species [11][12]. No correlation has been observed to date between asparagine and Hagberg falling number (HFN) (
Asn (asparagine), HFN (Hagberg falling number), Z-SDS (Zeleny sedimentation index).
One potentially interesting relationship is that between free asparagine and PHS, because of the potential for protein hydrolysis during PHS to release free asparagine. PHS negatively impacts wheat quality in a range of ways, reducing flour yield, the quality of baked products, and nutrient content [30]. Simsek et al. [20] reported a moderately strong (r = 0.6–0.7) positive correlation between free asparagine, sprouting score, and endoprotease activity in samples of sprouted wheat grain, suggesting that there was a relationship between asparagine and PHS at high levels of sprouting. Additionally, in a study designed to render the asparagine synthetase 2 genes (TaASN2) non-functional through gene editing, Raffan et al. [25] observed a poor germination phenotype that could be rescued through exogenous application of asparagine to the soil, implying that low-grain asparagine content may inhibit germination and could perhaps also affect PHS. Further research is required to confirm the germination phenotype, but asparagine synthetases are known to play important roles in germination in other species [31,32]. No correlation has been observed to date between asparagine and Hagberg falling number (HFN) (
Table 1
), which is indicative of α-amylase activity and, therefore, PHS. However, it is possible that a relationship between grain asparagine content, germination and PHS could exist when asparagine concentration is very low (e.g., in TaASN2 edited lines) or very high (e.g., in artificially sprouted wheat samples).
In contrast to other quality traits, the relationship between grain asparagine content and protein has been tested numerous times and the results suggest that there is a positive correlation between the two traits, varying from weak to strong, under different conditions (
Table 2
).
Table 2.
Associations between free asparagine and protein content.
| Asparagine Measure | Protein Measure | R | 2 | /r | p | Reference |
|---|
| Untransformed |
| Crude protein |
| 0.86 * |
| <0.001 |
| [ | ||||||||||
| 13 | ] | [ | 14 | ] | ||||||
| Nitrogen: sulphur grain content | 0.73 | <0.01 | ||||||||
| Untransformed | Protein content (2006 UN) | 0.93 | <0.01 | [14] | [16] | Nitrogen grain content | 0.62 | <0.05 | ||
| Protein content (2006 T) | Loge transformation |
) but, perhaps surprisingly, these correlations have not been consistent across studies. Xie et al. [44], for example, found that free asparagine (measured in milligrams per gram of protein) was negatively correlated with grain yield in one year when the yield was low (between two and four tonnes per hectare), but positively associated in another year, when the yield was higher (between four and eight tonnes per hectare), suggesting a non-linear relationship. A reduction in plant stress could explain the negative correlation observed over lower yield values, whilst the positive correlation could be due to greater nitrogen availability in the soil. However, the authors note that the relationship between absolute free asparagine content (measured without normalisation to protein) and yield was not as strong as the relationship when the normalisation of free asparagine to protein was performed. The lack of comprehensive yield/free asparagine studies does not provide strong support for hypotheses linking the two traits, but it could be worthwhile investigating the nature of the relationship between free asparagine and yield in more detail in future studies.
Table 3.
Associations between free asparagine and agronomic measurements.
| Asparagine Measure | Agronomic Measure | r | p | Reference |
|---|
| Log |
| e | ||||||||||||||||||
| back-transformed | Flowering time | −0.67 | <0.001 | [9] | [20] | |||||||||||||
| Untransformed | Plant height | 0.41 | <0.001 | [3] | [29] | |||||||||||||
| Sprouting score | ||||||||||||||||||
| 0.68 | ||||||||||||||||||
| TKW | 0.03 | 0.75 | <0.001 | [2] | [19] | |||||||||||||
| Endoprotease activity (sprouted) | 0.69 | <0.001 | ||||||||||||||||
| 0.63 | <0.05 | |||||||||||||||||
| Protein content (2007 UN) | 0.75 | >0.05 | ||||||||||||||||
| Mean kernel diameter | 0.13 | 0.11 | Protein content (2007 T) | 0.27 | ||||||||||||||
| Mean kernel weight | 0.06 | >0.05 | 0.45 | Endoprotease activity (ΔD) | 0.60 | <0.01 | ||||||||||||
| Untransformed | ||||||||||||||||||
| Yield | −0.14 | 0.09 | HFN | 0.07 | ||||||||||||||
| 0.39 | [ | 3 | ] | [29] | ||||||||||||||
| Z-SDS | 0.37 | <0.001 | ||||||||||||||||
| Gluten content | 0.44 | <0.001 | ||||||||||||||||
| Protein content (2006 N) | 0.73 | <0.01 | ||||||||||||||||
| Protein content (2007 N) | 0.89 | <0.01 | ||||||||||||||||
| Precipitation (HH) | −0.85 | <0.05 | Loge transformation | Protein content (non-sprouted) | NA | >0.05 | ||||||||||||
| [ | 2 | Temperature (HH) | ] | [19] | ||||||||||||||
| 0.74 | 0.10 | |||||||||||||||||
| Loge transformation | Protein content (sprouted) | NA | >0.05 | HLW | −0.40 | <0.001 | [4] | [34] | Starch content | −0.32 | ||||||||
| Protein content (ΔD) | <0.001 | |||||||||||||||||
| NA | >0.05 | 0.37 | <0.05 | [5] | [35] | Water absorption | 0.35 | <0.001 | ||||||||||
| Untransformed | Total protein content | 0.45 | <0.001 | [3] | [29] | Hardness index | ||||||||||||
| Mean kernel weight | 0.37 | <0.05 | 0.03 | 0.68 | ||||||||||||||
| Wholemeal protein content | ||||||||||||||||||
| Yield | 0.51 | <0.001 | −0.32 | >0.05 | Loge transformation | Absorption | −0.03 | >0.05 | [ | |||||||||
| 4 | ] | Flour protein content | [34] | |||||||||||||||
| 0.38 | <0.001 | |||||||||||||||||
| Days to harvest | 0.61 | <0.001 | Untransformed | Hardness index | 0.15 | >0.05 | ||||||||||||
| Log10 back transformed | [ | 5 | ] | [35] | ||||||||||||||
| Loge transformation | Protein content | 0.43 | <0.001 | [4 | TKW | ] | [34] | −0.24 | <0.01 | [6] | [23] | Log10 back-transformed | Sulphur grain content | 0.14 | ||||
| Loge transformation | Protein content (rp | >0.05 | ) | −0.03 | [6 | >0.05 | ] | [23] | ||||||||||
| [ | 15 | ] | [ | 36] | −0.37 | |||||||||||||
| HLW | −0.21 | <0.01 | HFN | 0.03 | >0.05 | |||||||||||||
| Protein content ( | rg) | >0.05 | ||||||||||||||||
| Untransformed | Nitrogen application | 0.63 | NA | [16] | [40] | Z-SDS | −0.29 | Untransformed<0.001 | ||||||||||
| Total protein content | 0.52 | <0.01 | [ | 5] | [35] | |||||||||||||
| Log | ||||||||||||||||||
| Untransformed | Mean kernel diameter | |||||||||||||||||
| Untransformed | TKW | −0.27 | 0.15 | [7] | [28] | Untransformed | 10 | HFN | −0.17 | back transformed | 0.36 | [7 | Total protein content | ] | 0.23 | [ | <0.01 | 28 |
| ] | ||||||||||||||||||
| [ | 6 | ] | [ | 23] | ||||||||||||||
| HLW | −0.07 | 0.71 | Gluten index | −0.36 | <0.05 | |||||||||||||
| Untransformed | Crude protein | 0.36 * | NA | [16] | [40] | |||||||||||||
| Loge transformed responses | YDT | −0.73 | <0.05 | [22] | [45] | Flour starch damage | −0.18 | 0.33 | ||||||||||
| Untransformed | Crude protein | 0.04 * | NA | |||||||||||||||
| Per unit protein | Yield (2018) | [ | 0.74 | 17] | NA | [41] | ||||||||||||
| [ | 21 | ] | [ | 44] | Untransformed | Wholemeal protein content | −0.08 | 0.66 | [7] | [28] | ||||||||
| Flour protein content | −0.14 | 0.46 |
* These values refer to R2 values, not r values. rp (phenotypic correlation), rg (genotypic correlation).
Although the relationship between free asparagine content and the protein composition of grain is complex, there are two factors that are well known to affect both: nitrogen and sulphur fertilisers. Nitrogen application increases both the free asparagine and protein content of grain, whereas sulphur application decreases free asparagine content and improves protein composition (see [18]). This is reflected in the correlation between free asparagine, nitrogen, and the nitrogen to sulphur ratio in wheat grain (Although the relationship between free asparagine content and the protein composition of grain is complex, there are two factors that are well known to affect both: nitrogen and sulphur fertilisers. Nitrogen application increases both the free asparagine and protein content of grain, whereas sulphur application decreases free asparagine content and improves protein composition (see [42] for review). This is reflected in the correlation between free asparagine, nitrogen, and the nitrogen to sulphur ratio in wheat grain (
Table 1), and implies that wheat uses free asparagine as a nitrogen store in the grain when sulphur is limiting [19]. Application of more sulphur is, therefore, desirable for both traits, except for its environmental pollution effects [20], whereas a balance between higher protein/higher free asparagine and lower protein/lower free asparagine must be struck when it comes to nitrogen application. Similar trade-offs arise because of the association of nitrogen with desirable agronomic traits, but there may be solutions in breeding.
1.2. Free Asparagine and Agronomic Traits
As a result of the positive association between free asparagine and nitrogen application, it might be expected that there would be a similar association between free asparagine and traits related to growth because of the positive relationship between plant growth and nitrogen. Positive correlations between free asparagine and yield have indeed been found (), and implies that wheat uses free asparagine as a nitrogen store in the grain when sulphur is limiting (reviewed in [8]). Application of more sulphur is, therefore, desirable for both traits, except for its environmental pollution effects [43], whereas a balance between higher protein/higher free asparagine and lower protein/lower free asparagine must be struck when it comes to nitrogen application. Similar trade-offs arise because of the association of nitrogen with desirable agronomic traits, but there may be solutions in breeding.
2.2. Free Asparagine and Agronomic Traits
As a result of the positive association between free asparagine and nitrogen application, it might be expected that there would be a similar association between free asparagine and traits related to growth because of the positive relationship between plant growth and nitrogen. Positive correlations between free asparagine and yield have indeed been found (
TKW (thousand kernel weight), HH (heading to harvest date), HLW (hectolitre weight), YDT (yield gap-based drought tolerance).
2. Breeding Wheat with Low Free Asparagine
Breeding low-asparagine wheat could potentially be achieved in three main ways: directly, by using either existing or induced variation, or indirectly, through selection for related traits (
Figure 1). New wheat varieties are commonly developed using existing variation; however, the only multi-environment quantitative trait locus (QTL) for low-asparagine known at present is the one in which the TaASN-B2 gene is either present or deleted, which has been shown to affect the free asparagine content of grain in two different field trials [24]. Selection for the TaASN-B2 deletion represents an easy gain for breeders, but further trials testing the effect of the deletion should be performed to confirm the stability of the effect across more environments. Other QTL controlling grain asparagine content have also been identified, but these have not yet been verified across more than one environment [9][6]. Identification of multi-environment QTL, in combination with genomic and marker assisted selection [6], could enable low-asparagine wheat to be developed, without the time-consuming or expensive need to screen large numbers of plants for asparagine concentration.

3). New wheat varieties are commonly developed using existing variation; however, the only multi-environment quantitative trait locus (QTL) for low-asparagine known at present is the one in which the TaASN-B2 gene is either present or deleted, which has been shown to affect the free asparagine content of grain in two different field trials [26]. Selection for the TaASN-B2 deletion represents an easy gain for breeders, but further trials testing the effect of the deletion should be performed to confirm the stability of the effect across more environments. Other QTL controlling grain asparagine content have also been identified, but these have not yet been verified across more than one environment [20,23]. Identification of multi-environment QTL, in combination with genomic and marker assisted selection [23], could enable low-asparagine wheat to be developed, without the time-consuming or expensive need to screen large numbers of plants for asparagine concentration.
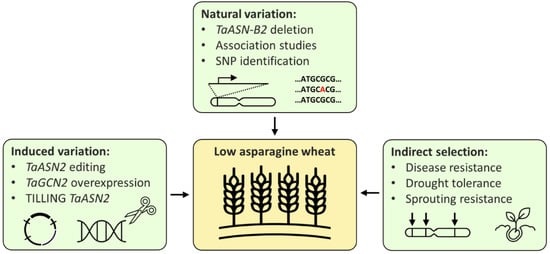

Figure 13. Strategies for the breeding of low-asparagine wheat.
Relying on natural variation is limited by the availability of existing variation, whereas techniques that induce or increase variation in the wheat genome could generate new variants with free asparagine content below the normal range. This has been demonstrated by the use of CRISPR/Cas9 technology to ‘knock out’ the TaASN2 genes, reducing grain asparagine content by up to 90% in glasshouse experiments [10]. The edited lines still need to undergo trials to confirm the stability of this phenotype in the field, but the stability of the ‘natural’ TaASN-B2 deletion phenotype under field conditions [24] is encouraging, suggesting that the TaASN2-edited phenotypes may be similarly stable. However, the interaction between the TaASN-B2 deletion and sulphur deficiency implies that TaASN2 variants may not be sufficient to control grain asparagine content during sulphur deficiency or other stresses, again highlighting the effects of E and G × E. On the other hand, the varieties carrying the TaASN-B2 deletion have intact TaASN-A2 and TaASN-D2 genes, whereas the edited lines lack any functional TaASN2 genes, so the edited lines will be valuable for investigating whether this prevents free asparagine accumulation under conditions of sulphur deficiency or other stresses.
The benefits of inducing variation in candidate genes was also recently demonstrated in a preprint by Alarcón-Reverte et al. [25], in which wheat plants possessing EMS-induced null TaASN-A2 alleles were grown in the field and tested for grain-free asparagine content. Reductions of between 9% and 34% were achieved, without any negative side effects on quality traits, demonstrating again the utility of induced variation and the lack of strong associations between free asparagine and quality traits.
As a result of the potential loss or partial loss of the low-asparagine phenotype of TaASN2 knockouts under stress, a third, complementary option for controlling grain asparagine content can also be adopted: breeding for stress tolerance. As discussed above, stress and grain asparagine content are closely linked, and it is often during stress that the highest grain asparagine contents are observed [26][27]. Breeding for stress tolerance could, therefore, ensure that a low-asparagine phenotype would be retained under stress. Selection for other related traits, such as those discussed above (e.g., PHS resistance, delayed senescence), could also provide indirect selection for lower-grain asparagine, but these traits are not as clearly linked with asparagine as asparagine is with stress.
Strategies for the breeding of low-asparagine wheat.
Relying on natural variation is limited by the availability of existing variation, whereas techniques that induce or increase variation in the wheat genome could generate new variants with free asparagine content below the normal range. This has been demonstrated by the use of CRISPR/Cas9 technology to ‘knock out’ the TaASN2 genes, reducing grain asparagine content by up to 90% in glasshouse experiments [25]. The edited lines still need to undergo trials to confirm the stability of this phenotype in the field, but the stability of the ‘natural’ TaASN-B2 deletion phenotype under field conditions [26] is encouraging, suggesting that the TaASN2-edited phenotypes may be similarly stable. However, the interaction between the TaASN-B2 deletion and sulphur deficiency implies that TaASN2 variants may not be sufficient to control grain asparagine content during sulphur deficiency or other stresses, again highlighting the effects of E and G × E. On the other hand, the varieties carrying the TaASN-B2 deletion have intact TaASN-A2 and TaASN-D2 genes, whereas the edited lines lack any functional TaASN2 genes, so the edited lines will be valuable for investigating whether this prevents free asparagine accumulation under conditions of sulphur deficiency or other stresses.
The benefits of inducing variation in candidate genes was also recently demonstrated in a preprint by Alarcón-Reverte et al. [62], in which wheat plants possessing EMS-induced null TaASN-A2 alleles were grown in the field and tested for grain-free asparagine content. Reductions of between 9% and 34% were achieved, without any negative side effects on quality traits, demonstrating again the utility of induced variation and the lack of strong associations between free asparagine and quality traits.
As a result of the potential loss or partial loss of the low-asparagine phenotype of TaASN2 knockouts under stress, a third, complementary option for controlling grain asparagine content can also be adopted: breeding for stress tolerance. As discussed above, stress and grain asparagine content are closely linked, and it is often during stress that the highest grain asparagine contents are observed [39,63]. Breeding for stress tolerance could, therefore, ensure that a low-asparagine phenotype would be retained under stress. Selection for other related traits, such as those discussed above (e.g., PHS resistance, delayed senescence), could also provide indirect selection for lower-grain asparagine, but these traits are not as clearly linked with asparagine as asparagine is with stress.
