Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Arthur BAGEL.
Shiga toxin-producing Escherichia coli (STEC) are zoonotic Gram-negative bacteria. While raw milk cheese consumption is healthful, contamination with pathogens such as STEC can occur due to poor hygiene practices at the farm level. STEC infections cause mild to serious symptoms in humans. The raw milk cheese-making process concentrates certain milk macromolecules such as proteins and milk fat globules (MFGs), allowing the intrinsic beneficial and pathogenic microflora to continue to thrive. MFGs are surrounded by a biological membrane, the milk fat globule membrane (MFGM), which has a globally positive health effect, including inhibition of pathogen adhesion.
- STEC
- MFGM
- Milk Fat Globules
- Raw milk Cheese
- bacterial adhesion
1. Introduction
In the Code of Hygienic Practice for Milk and Milk Products (Codex Alimentarius, 2004), raw milk is defined as milk that has not been heated beyond 40 °C or undergone any treatment that has an equivalent effect. Raw milk is an oil-in-water type emulsion and contains almost 900 g of water and 130 g of dry matter, in varying proportions [1]. Milk is a major source of calcium, and also an important supply of proteins, for those who consume it (newborn mammals and humans). Milk contains all essential amino acids, lipids, fatty acids, vitamins, and lactose [2]. One liter of whole milk contains approximately 38 g of fat, which is present mainly in the form of milk fat globules (MFGs) [3].
Raw milk cheeses are made from raw cow, sheep, or goat milk. Different cheese-making processes can be applied to create the end-products. The various combinations of ingredients (such as milk and cultures) and cheese-making processes result in a large diversity of cheeses. No less than 1200 different cheeses are made in France [4]. These include ripened or unripened soft, semi-hard, hard, or extra-hard products, which may be coated, uncooked, or cooked pressed cheeses (with short or long ripening), blue-type cheeses, lactic cheeses, and white mold cheeses.
Raw milk is unstable due to the presence of microflora and, therefore, is often treated to optimize its conservation and to prevent microbiological hazards. The microbiota of raw milk is complex and is derived from many sources, including direct contact with the animal (teats, hides, and feces), the surface of milking equipment (particularly if hygiene practices are poor), housing, bedding, feed, air, and water [5,6,7][5][6][7]. On the one hand, this dynamic bacterial community plays several beneficial roles in subsequent dairy products such as facilitating fermentation and promoting the health of consumers. On the other hand, microbiota can contain spoilage or pathogenic bacteria [5]. The milk microbiota is almost eliminated during heat treatment, such as ultra-high temperature (UHT) sterilization, and microfiltration, while in raw milk the microbiota is preserved. Different studies have shown that the raw milk microbiota is useful to the human digestive system; therefore, it may be beneficial to preserve it [5,8][5][8]. In addition, the raw milk microbiota gives raw milk cheeses more varied and intense flavors than heat-treated milk cheeses. The raw milk microbiota can also influence the human intestinal microbiota, which contributes significantly to human health, for example, by modulating the immune system. The consumption of raw milk and raw milk cheeses can also reduce blood pressure in people with mild to moderate hypertension [9] and decrease allergies in infants [10,11][10][11]. However, the risk–benefit ratio of consuming raw milk products is difficult to estimate. Nevertheless, in France, it is strongly recommended to avoid giving raw milk products to children under 5 years old, pregnant women, and immunocompromised patients [12]. If poor hygiene procedures have been applied, particularly during milking, raw milk may be contaminated by pathogenic bacteria such as Shiga toxin-producing Escherichia coli (STEC).
STEC are foodborne zoonotic bacteria associated with large-scale epidemics that represent a major public health problem. Human STEC infection is most often linked to the ingestion of contaminated food and water, such as undercooked ground meat, raw milk cheeses, or raw vegetables. Raw milk and raw milk cheeses have been linked to foodborne infections associated with STEC in humans from different countries [13,14,15,16,17,18,19,20][13][14][15][16][17][18][19][20]. Ruminants are the primary reservoir of STEC. Milk is most often contaminated by feces (directly or indirectly) during the milking process. STEC are very frequently associated with severe forms of infection such as hemorrhagic colitis and, in very severe cases, systemic complications including hemolytic uremic syndrome (HUS). HUS is the leading cause of renal failure in children under 3 years of age. The estimated infectious dose is very low: between 5 and 50 viable cells [21,22][21][22].
The proportion of milk and dairy products involved in Rapid Alert System for Food and Feed (RASFF) notifications issued due to food contamination with STEC is very low compared to those involving meat products [23]. The RASFF is a European communication tool used when public health microbiological hazards are detected in the food chain and food products. In 2013, two RASFF notifications were related to STEC-contaminated dairy products versus 68 for meat products. In 2014, four RASFF notifications of dairy products contaminated with STEC were listed, compared with 53 for meat products. Likewise, there were 7 versus 16, 8 versus 26, and 4 versus 49 dairy-related and meat-related notifications, respectively, in 2015, 2016, and 2017 [23]. Furthermore, epidemiological studies have shown that this class of product is only a minor source of human enteric infection [24,25,26][24][25][26]. Interestingly, prevalence data on these enteropathogens in dairy matrices and ingestion-related outbreaks do not fit overall foodborne-related outbreak figures [26]. A study led by Douëllou et al. [27] showed that there were no differences in the key virulence properties of dairy STEC isolates compared with human isolates. The same authors hypothesized that this phenomenon might be related to an association between STEC and MFGs, thus inhibiting STEC adhesion to enterocytes.
Milk fat globules (MFGs) have a positive impact on the immune system, and their antimicrobial properties have been largely described [28,29,30,31][28][29][30][31]. The positive action of MFGs on human health seems to be carried out by the membrane (and membrane components) surrounding the globules. MFGs are small lipid droplets formed by a core of triacylglycerols (TAGs) and enveloped by a biological phospholipid triple membrane, the milk fat globule membrane (MFGM), which is derived from mammary epithelial cells [32]. The outer bilayer of the MFGM contains diverse (glyco)-proteins and (glyco)-lipids on its surface [33]. These glycoconjugates make up the glycocalyx and act as a source of specific bacterial and viral ligands [29,34][29][34].
2. Raw Milk Sector and STEC
2.1. Importance of Raw Milk Cheeses
Cheeses are products with high added value and significant economic importance in France and Europe. In 2019, the annual cheese consumption per inhabitant was 26.8 kg in France and 19.1 kg in all of Europe [35]. In total, 10,630,000 t of cheese were produced in Europe in 2019 [35]. In France, cheeses represent one of the main food industries, worth approximately EUR 38.7 billion in 2017 [36]. In 2019, with all milk processing combined, French cheese production included 1,664,632 t of cow’s milk cheese, 99,265 t of goat’s milk cheese, and 59,638 t of sheep’s milk cheese [35]. The production of raw milk cheese accounted for 172,128 t of the cow’s milk cheese, 20,872 t of the sheep’s milk cheese, and 9691 t of the goat’s milk cheese produced in France in 2019 [35]. Raw milk cheeses represent the vast majority of farmhouse dairy products and approximately 75% of the volume of cheeses marketed under quality and origin identification signs (SIQO), including protected designations of origin (PDO) and protected geographical indications (PGI). Finally, a study has shown that 75% of French people consume raw milk cheeses at least once a month and that 33% of French people consume raw milk cheeses every week [37]. In contrast, no data on the worldwide consumption or production of raw milk cheeses are currently available.
Raw milk cheeses are part of French and European food heritage and are an essential tool to enhance product value and to create dynamism in our territories. They are part of a dynamic of rural development and land use planning; their production avoids the desertification of certain areas by providing a significant source of income for farmers. They are often produced under a PDO quality label. Raw milk cheeses encourage variety and diversification of production and contribute to the sustainability of rural economies. They protect traditional production areas, enhance the recognized know-how of operators, and facilitate, especially for small producers, the marketing of differentiated products with specific and clearly identifiable characteristics. In France, the competent authorities as well as scientists recognize the importance of raw milk cheese both in terms of gastronomic heritage and regional socio-economic development. At the same time, authorities and scientists seek to support the industry by improving scientific knowledge about STEC and developing methods for the control and surveillance of this bacteria from farm to fork.
2.2. STEC
STEC are foodborne zoonotic bacteria associated with large-scale epidemics that represent a major public health problem. Ruminants (cattle, buffalo, goats, and sheep) are the main reservoir of STEC [38,39][38][39]. Infected ruminants can be asymptomatic, harboring the bacteria in their gastrointestinal tract and shedding bacteria in their feces [40,41,42][40][41][42]. Detailed investigations have shown that without proper cleaning methods and udder hygiene practices, feces can contaminate the teats and udders of animals and cause milk contamination during milking [43]. When STEC-contaminated milk is used to produce raw milk cheeses, STEC can survive and be isolated in some cheeses.
The pathogenesis of STEC-related disease generally involves three phases: (i) ingestion of contaminated food; (ii) colonization of the intestinal epithelium by STEC; and (iii) production of Shiga toxins (Stx) that disrupt normal cellular functions and damage cells. Stx are the main virulence factors of STEC. The Stx family includes all toxins with a similar structure and biological activity. Based on their different in vitro and in vivo toxicity, amino acid sequences, or nucleotide sequences of the stx genes, two major types of Shiga toxins, Stx1 and Stx2, and numerous variants (Stx1a to Stx1d and Stx2a to Stx2k) have been identified [44,45,46,47][44][45][46][47]. To effectively colonize a host and cause disease, STEC have evolved mechanisms and strategies for attaching or adhering to host cells and tissues [48]. Adhesion is required so that STEC cells are not swept away by the host’s natural self-cleaning mechanisms. An arsenal of STEC surface adhesion factors have been described (Figure 1).
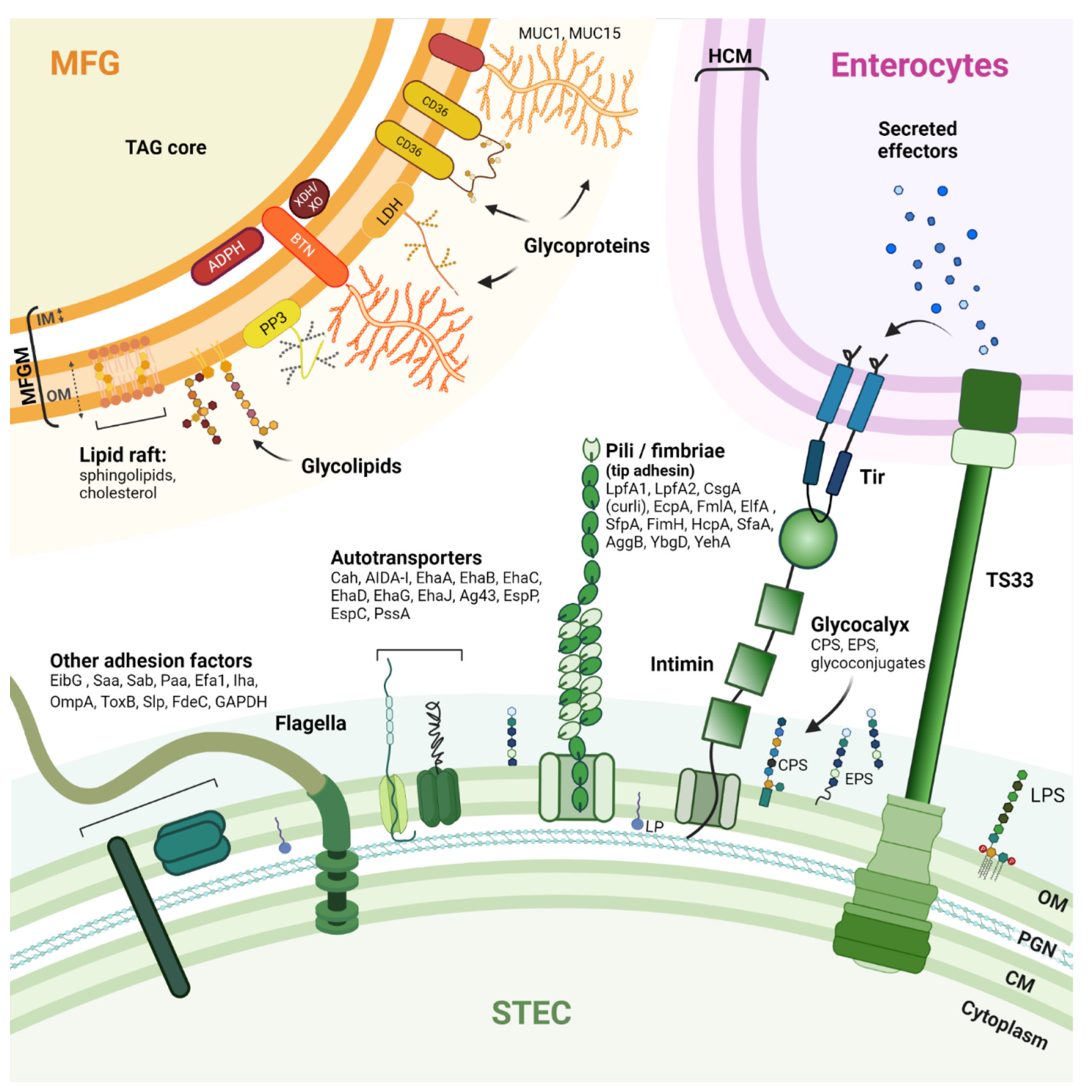

Figure 1. Schematic drawing of STEC adhesion factors and the bovine MFGM. STEC have an arsenal of protein structures involved in host cell adhesion. However, the adhesion mechanisms that contribute to the pathogenicity of STEC are not fully understood, and the receptors recognized by these adhesion factors are not all known. Nevertheless, some pili, autotransporters, and flagella can bind to host extracellular matrix (ECM) components such as fibronectin and laminin (glycoproteins). The MFGM is a complex trilayered structure, comprising a monolayer of polar lipids derived from endoplasmic reticulum (IM: inner membrane) and a bilayer of polar lipids originating from the apical plasma membrane of mammary secretory cells (OM: outer membrane). The structures drawn do not necessarily reflect the actual structures of the macromolecules and are not to scale. CM: Cytoplasmic membrane; PGN: Peptidoglycan; OM: Outer membrane; LPS: Lipopolysaccharide; LP: Lipoprotein; Tir: Translocated intimin receptor; T3SS: Type 3 secretion system; HCM: Host cytoplasmic membrane; CPS: Capsular polysaccharide; EPS: Extracellular polysaccharide; LpfA: Long polar fimbria subunit A; CsgA: Major curlin subunit; EcpA: E. coli common pilus subunit A; FmlA: Type-1 fimbria subunit A; ElfA: laminin-binding fimbria subunit A; SfpA: sorbitol-fermenting fimbria subunit A; FimH: Type 1 fimbrin D-mannose specific adhesin; HcpA: Hemorrhagic coli pilus subunit A; SfaA: S-fimbria subunit A; AggB: Aggregative adherence fimbria I subunit B; YbgD: Putative fimbria Ybg subunit A; YehA: Putative fimbria Yeh subunit A; Cah: Calcium-binding antigen 43 homologue; AIDA-I: Adhesin involved in diffuse adherence; Eha: Enterohaemorrhagic E. coli autotransporter; Ag43: Antigen 43; EspP: Extracellular serine protease plasmid encoded; EspC: EPEC-secreted protein C; PssA: Protease secreted by STEC; EibG: E. coli immunoglobulin-binding protein G; Saa: STEC autoagglutinating adhesion autotransporter; Sab: STEC autotransporter contributing to biofilm formation; Paa: porcine A/E-associated protein; Efa1: EHEC factor for adherence; Iha: IrgA homologue adhesin; OmpA: Outer membrane protein A; ToxB: Toxin B; Slp: Carbon starvation-inducible lipoprotein; FdeC: Factor adherence E. coli; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase. MUC1/MUC15: Mucin 1/15; LDH: Lactadherin; ADPH: Adipophilin; BTN: Butyrophilin; XDH/XO: Xanthine dehydrogenase/oxidase; CD36: Cluster of differentiation 36; PP3: Proteose peptone 3; TAG: triacylglycerols.
The ability to adhere to the intestinal epithelium and colonize the intestine undeniably contributes to the pathogenic processes of STEC cells. Thus, the vast majority of clinical isolates known to cause bloody diarrhea or HUS have one or more virulence factors that allow their adhesion to intestinal epithelial cells [44]. The major adhesion factor of clinical STEC isolates is intimin [49], a protein encoded by the eae gene that resides in the locus of an enterocyte effacement pathogenicity island (LEE). The pathophysiology of clinical isolates possessing the eae gene is characterized by the development of enterocyte attachment-effacement (A/E) lesions. These lesions are responsible for the diarrhea observed in patients [49]. Intimin attachment to the host cell requires an upstream connection of the STEC cell to the host cell cytoplasm [48]. Intimin binds to the translocated intimin receptor (Tir) protein, which is encoded by STEC and translocated into the host cell cytoplasm using a type III secretion system (T3SS) and then inserted into the host cell membrane. Although STEC isolates carrying the eae gene represent the vast majority of human infections, some STEC lacking the eae gene have been isolated from patients [50,51][50][51]. An early adhesion phase involving other adhesion factors may occur before, or in parallel with, the formation of the highly specific intimin/Tir bond. Intimin can also bind, with less specificity and strength, to certain host cell surface components such as integrin and nucleolin, and this may contribute to STEC–host cell adhesion [52,53,54][52][53][54]. Some studies suggest involvement of the long polar fimbriae (LPF), which recognizes moieties of eukaryotic extracellular matrix (ECM) components [55,56][55][56]. Molecular characterization studies of STEC isolates have also identified paa, efa1, ompA, saa, sab, toxB, and aggR as genes encoding virulence factors involved in adhesion [57,58,59][57][58][59]. Flagella are also involved in STEC adhesion by binding to mucus and mucin proteins [60]. Other proteins can interact with immunoglobulins, for example, E. coli immunoglobulin-binding protein (Eib) [61,62][61][62]. The complete list of virulence factors, the timing of their expression, and the mechanisms involved in STEC pathogenicity are not yet fully known. Current knowledge of STEC surface proteins is summarized in Figure 1. STEC adhesion mechanisms are further detailed in these articles: [48,59,63][48][59][63].
2.3. Milk Fat Globules
MFGs can be distinguished from other forms of fat by the milk fat globule membrane (MFGM) that surrounds a core of triacylglycerols (TAGs) (Figure 1). Complex fat supramolecular organizations are also found in egg yolk or oilseeds in the form of oleosomes [64]. The MFGM is made of phospho- and sphingolipids, cholesterol, and proteins [65,66][65][66]. As a consequence of the mechanism of milk fat secretion from mammary epithelial cells, the MFGM is a complex trilayered structure, comprising a monolayer of polar lipids derived from the endoplasmic reticulum and a bilayer of polar lipids originating from the apical plasma membrane of the mammary secretory cells [67,68,69][67][68][69]. The MFG size is dependent on the origin of the milk. Bovine MFGs have a mean diameter of 4 µm, while MFGs from goat (3.19 µm), camel (2.99 µm), and sheep raw milk (3.78 µm) are all smaller, and MFGs from buffalo (8.7 µm) are much larger [70]. The main MFGM (glyco)-proteins include glycoproteins mucin 1 and 15 (MUC1; MUC15), the redox enzyme xanthine dehydrogenase/oxidase (XDH/XO), butyrophilin (BTN), cluster of differentiation 36 (CD36), lactadherin (LDH); and two proteins: adipophilin (ADPH) and fatty-acid binding protein (FABP) [29,71][29][71]. For a more complete description of MFGs and MFGM, we refer the reader to these articles: [69,72,73].
3. STEC in Raw Milk Cheeses
3.1. Prevalence and Behavior of STEC in Raw Milk Cheeses
The most comprehensive studies on the prevalence of STEC in cheeses have been conducted in Europe and show that the prevalence of STEC varies from 0% to 13.1%, depending on the study [74][72]. In France in 2009, 2014, and 2018, surveillance plans assessed the prevalence of specific STEC isolates (E. coli possessing the eae and stx genes and belonging to O157:H7, O26:H11, O103:H2, O111:H8, and O145:H28 serotypes) in raw milk cheeses. These studies showed a prevalence of 0.9%, 0.2%, and 0.8% in the raw milk cheese studied in 2009, 2014, and 2018, respectively. In 2016, researchers in a French study evaluated the genetic diversity and virulence gene profiles of STEC isolated from dairy products [27]. They showed that the 197 studied isolates displayed a high genetic diversity regardless of their serotype, with Simpson’s Diversity Index ranging from 1.0 to 0.9615. In addition, their results suggested that the virulence gene profiles of the dairy isolates are a potential hazard. Nevertheless, for the isolate most frequently found in dairy products, O26:H11 STEC, gene expression was similar between human and dairy isolates except for stx1 (44% vs. 87%) and stx2 (81% vs. 23%) expression. It is important to keep in mind that Stx2 has stronger cytotoxicity than Stx1 [75][73]. A French team showed that during the manufacture of different types of cheese experimentally contaminated with STEC strains, there was no statistically significant strain effect for the same serotype [76,77][74][75]. However, only a few different strains were tested for each serotype. Interestingly, a serotype effect was observed in certain types of cheese. Researchers observed less growth of the serotype O157:H7 strains than of the serotype O26:H11, O103:H2, and O145:H28 strains. They hypothesized that strains belonging to serotypes O26:H11, O103:H2, and O145:H28 could be more adapted to the conditions (physicochemical parameters and microbiota) encountered in cheeses.
3.2. Impact of Cheese-Making Parameters on STEC and MFGs
The different processing steps applied and the origin of the raw milk used (e.g., cow, buffalo, goat, or sheep) can influence the behavior and survival of STEC [76][74]. The behavior of STEC (survival, growth, or inactivation) can also be influenced by temperature and by the intrinsic physicochemical properties (pH, aw, and % lactic acid) of, and the other microflora present in, the raw milk microbiota and added starters used in different cheeses during their manufacture. At the initial stages of cheese-making, the temperature (around 30 °C) and aw value of milk provide favorable conditions for the growth of STEC and an increase in STEC level by 1–3 log CFU/g can occur [76,77][74][75]. Then, the rapid acidification (pH > 4.3) encountered during the manufacture of certain cheeses can reduce STEC cell counts by 1–4 log CFU/g, depending on the STEC serotype and the type of cheese [76,78][74][76]. Various studies have shown that when ripening is long and, therefore, the aw is low, STEC numbers decrease [76][74]. Nevertheless, while ripening can reduce the number of STEC cells, it cannot ensure the safety of the product if the raw milk is contaminated with STEC [79,80][77][78]. The environmental conditions during cheese processing generate stress for STEC, which can affect gene expression. Although little is known about the physiological state of STEC in cheese at different stages of production, it has been shown that the cheese-making process can trigger the production of Stx phages [81][79].
The various milk treatments for cheese processing also impact MFG and MFGM integrity and, consequently, the molecules involved in the MFGM–bacteria association [29,82][29][80]. Such treatments include high-temperature treatments [83[81][82],84], homogenization, and enzymatic reactions [85][83]. One must keep in mind that for raw milk cheeses, the curd is never heated above 54 °C. Heat treatments that kill or limit bacterial growth can also damage heat-sensitive compounds, such as glycoconjugates and associated oligosaccharides, located on the MFGM surface. Carbohydrate epitopes are well-known targets of bacterial adhesion [86][84]. Homogenization reduces the diameter of MFGs (ranging from 0.1 to 0.5 µm [82][80]) and increases the total MFGM surface area available to bacteria. Furthermore, homogenization can alter MFGM composition [84,85,87][82][83][85]. Treatments applied during cheese production may also alter the environment and the ability of STEC to adhere to the MFGM. In addition, physical forces applied in some cheese processing (e.g., pressed cheeses) can lead to detachment of the MFGM and their dispersion either in the product or in the whey, which will be eliminated. Nevertheless, glycoconjugates may remain intact through to end-consumption in non-pasteurized dairy products.
3.3. Location of STEC in Raw Milk Cheeses
Douëllou et al. used epifluorescence microscopy and a specific antibody coupled to FITC to assess bovine raw milk and concluded that both STEC strains assayed were localized near MFGs [88][86]. A natural raw milk creaming saturation assay with STEC revealed a strain-dependent tropism for the bovine raw milk cream layer and a distinct half-saturation point (8.5–9 log10 CFU.mL−1). Similar observations were presented by Brewster and Paul, suggesting that the cream layer exhibits a high capacity for E. coli O157:H7, Listeria monocytogenes, and Salmonella enterica [89][87]. Bacterial purification from milk by creaming seems to be not species-specific but rather general to bacteria.
The discrimination of specific bacteria in a complex product, such as raw milk cheese, is a true challenge. Furthermore, due to the modifications to milk and the MFG structure that occur during the cheese-making process, bacterial localization can vary. At the macroscopic scale, Miszczycha et al. showed that in experimentally inoculated cheeses, the levels of STEC strains belonging to serotypes O103:H2 and O145:H28 were statistically similar in the core and in the rind, regardless of the ripening conditions (traditional or industrial) [76][74]. The levels of serotype O26:H11 were also statistically similar in the rind. Bacterial localization in raw milk cheese has also been realized by morphologic observation on electronic micrographs [90,91,92,93][88][89][90][91] or by nonspecific DNA staining and assessment by fluorescent microscopy [90,94,95][88][92][93]. One study observed fluorescent mCherry-tagged Lactobacillus reuteri in raw milk [96][94]. However, to the authors’ knowledge, no study focusing on the localization of STEC or other pathogenic bacteria during the cheese production process has been published.
At the microscopic scale, the localization of bacteria in other dairy products, such as fermented milk, is poorly documented. Overall, few studies have focused on the localization of STEC in dairy products. More work is needed to understand STEC behavior in raw milk cheeses and their interaction with MFGs. Based on the available literature describing other bacteria [95,97,98][93][95][96], STEC could potentially localize near MFGs, in serum pockets, or the protein network.
