Premenstrual symptoms are very common, affecting about half of women in reproductive age worldwide
[22][9]. However, prevalence rates vary widely in different studies and countries depending on samples, methods of investigation and diagnostic criteria. Disparities may also derive from genetic and socio-cultural factors, including diet and life-style, stressors, personal attitudes, coping behaviors, workload and family responsibilities
[22][9]. Available surveys in community populations indicate that PMS affects 20–30% of women, whereas PMDD ranges between 1.2 and 6.4%
[23][10], with black women being significantly less likely to experience PMDD and PMS than white women (odds ratio (OR) 0.44, 95% confidence interval (CI) 0.25–0.79 and OR 0.64, 95% CI 0.47–0.88, respectively), similarly to what is observed in other mental health disorders
[24][11]. Both conditions significantly reduce quality of life and raise societal costs associated with decreased work productivity, work absenteeism and increased use of health care services
[25][12]. Prevalence and impact of PMS/PMDD are strong priorities to implement preventive strategies in young women
[26][13]. Health care providers (HCPs) should be aware that premenstrual symptoms might fluctuate over time with no clear impact of age or reproductive stage, apart from menopausal transition
[27,28][14][15]. Another relevant factor is that combined oral contraceptives (COCs), the most studied type of combined hormonal contraception (CHC), may improve overall premenstrual symptomatology in women with PMS/PMDD, but not premenstrual depressive symptoms
[29][16]. Behavioral risk factors, especially smoking and adiposity, are overrepresented in women with PMS/PMDD, confirming their link to emotional vulnerability. Indeed, smoking was associated with an increased risk of premenstrual disorders (OR = 1.56 (95% CI: 1.25–1.93)). Stratified by diagnosis, the effect size estimate was higher for PMDD (OR = 3.15 (95% CI: 2.20–4.52)) than for PMS (OR = 1.27 (95% CI: 1.16–1.39))
[30][17]. A strong linear relationship between body mass index (BMI) at baseline and risk of incident PMS, with each 1 kg/m
2 increase in BMI associated with a significant 3% increase in PMS risk (95% confidence interval (CI) 1.01–1.05), was evident
[31][18]. In particular, women with BMI ≥ 27.5 kg/m
2 at baseline had significantly higher risks of PMS than women with BMI < 20 kg/m
2, following adjustment for age, smoking, physical activity, and other factors
[31][18]. Intake of alcohol was associated with a moderate increase in the risk of PMS (OR = 1.45, 95% CI: 1.17 to 1.79), especially heavy drinking (OR = 1.79, 95% CI: 1.39 to 2.32) as compared to no or light drinking
[32][19]. Studies on the effect of exercise have many methodological biases with some suggesting improvement of premenstrual symptoms
[33][20]. Other proven risk factors include traumatic events, which greatly increased the odds of developing PMDD at follow-up (OR = 4.2, 95% CI = 1.2 to 12.0). Likewise, a history of anxiety disorder (OR = 2.5, 95% CI = 1.1 to 5.5) and elevated daily conflict scores (OR = 1.6, 95% CI = 1.1 to 2.3) predicted PMDD
[34][21]. Depression may be strongly comorbid
[17[22][23],
18], in particular postnatally
[35][24], and women with PMDD should be considered a high-risk group for suicidality, including increased vulnerabilities for suicidal thoughts, ideation, plans and attempts
[36][25]. Other comorbidities include eating disorders, mainly bulimia and binge eating
[37][26], and migraine
[38][27]. The co-occurrence with pathological manifestations displaying premenstrual exacerbations supports a common neuroendocrine etiology
[2,3][2][3]. Medical conditions such as anemia and endocrine disorders (namely thyroid and adrenal dysfunctions and hyperprolactinemia)
[13][28], as well as chronic pelvic pain, fibromyalgia and any other inflammatory disorders
[39,40][29][30], may mimic PMS/PMDD symptoms. HCPs should make a differential diagnosis to establish an individualized treatment plan
[3,8,13][3][8][28].
3. Neuroendocrine Aspects of PMS/PMDD
The most characteristic aspect of PMS/PMDD is the temporal relation between the appearance of symptoms and the menstrual phase, indicating a role for gonadal steroid hormones and their metabolites in influencing the plethora of biological systems that contribute to the adjustments required to fulfil reproductive goals. However, women with PMS/PMDD do not show abnormalities in the reproductive hormone release pattern; rather, they seem to display a more sensitive neuroendocrine threshold to cyclical variations of estrogens and progesterone
[41[31][32],
42], which may give origin to catamential symptoms and exacerbation of mood disorders during reproductive transitions
[43,44][33][34]. Data on other circulating hormones (prolactin, testosterone, cortisol, dehydroepiandrosterone sulphate, and thyroxine) are discordant and fail to separate women with PMS/PMDD from controls. However, they may be relevant to some individual somatic symptoms, for instance cyclic mastalgia or water retention
[13][28].
Many genetic and epigenetic factors influence the neuroendocrine threshold of premenstrual symptoms according to the biopsychosocial model. Severity of mood symptoms and associated distress should guide clinical judgement
[8,13][8][28]. However, both HCPs and women have their personal view of the set point of such threshold, explaining variable epidemiology of PMS/PMDD symptoms
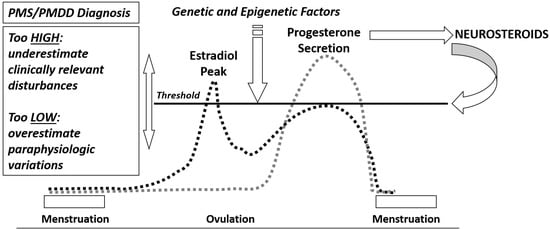
[23][10]. If the diagnostic threshold is too high, clinically relevant premenstrual symptoms may be underestimated and PMS/PMDD remain untreated. If it is too low, paraphysiologic variations in menstrual cycle-related well-being can be over-treated (
Figure 1). The central nervous system (CNS) is one of the main target tissues for reproductive hormones but it is also a source of neurosteroids, which are involved throughout genomic and non-genomic mechanisms in a vast array of CNS functions
[45,46,47][35][36][37] far beyond the scope of the present overview. Here,
wthe researche
rs report the key concepts relevant to the current understanding of pathophysiology and potential treatment targets of PMS/PMDD.
Figure 1.
Neuroendocrine threshold and PMS/PMDD diagnosis.
Estrogens and Progesterone
Hormonal transitions are associated with reproductive mood disorders, whereas premenarchal girls and postmenopausal women do not experience PMS/PMDD in the absence of gonadal steroid fluctuations. The same is true when gonadal steroids are high and rather stable, as occurs in pregnancy
[48][38]. In addition, premenstrual symptoms do not occur during anovulatory cycles and disappear in chemically and/or surgically castrated women
[9][39]. Several mechanisms involving estrogen receptors polymorphisms may explain the vulnerability to reproductive mood disorders
[49][40]. Fluctuations of gonadal steroids, in particular progesterone produced by the corpus luteum, are key factors for PMS/PMDD
[42][32], given the synchrony with post-ovulatory phase and the reinstatement of symptoms during GnRH agonist treatment when add-back progesterone is administered
[50][41]. However, many women experience premenstrual symptoms immediately after ovulation and during the early luteal phase, while others report an exacerbation only a few days before menstruation, irrespective of progesterone fluctuations
[9][39]. Therefore, the importance of the progesterone to estrogens ratio has been also investigated, because estrogens may exert an antidepressant effect
[51][42], and women with PMS/PMDD and healthy women have similar progesterone serum concentrations
[42,52][32][43]. A recent study prospectively evaluated estrogen and progesterone levels in both early and late luteal phases in women with PMDD and the association of these levels with PMDD symptom severity
[53][44]. In women with PMDD, estrogen levels were lower than the controls during the early luteal phase and displayed a significant interaction with early luteal progesterone, suggesting that low estrogen level could moderate the severity of PMDD symptoms following exposure to progesterone
[53][44]. On the other hand, estradiol administration may provoke PMS-like complaints, similarly to progesterone administration alone or together with estrogens
[50,54][41][45]. Moreover, PMS-like symptoms often persist after anovulation has been induced with COCs, suggesting that both the dose of estrogens and the type of progestins may be relevant to mood symptoms in vulnerable women
[55][46]. Finally, postmenopausal women receiving combined hormonal replacement therapy (HRT) may experience PMS-like complaints despite stable levels of estradiol and progesterone
[56][47]. Of note, administration of mifepristone, a progesterone receptor antagonist, did not reduce physical, emotional and/or behavioral manifestation of PMS or change the timing of these symptoms
[57,58][48][49]. More recently, ulipristal acetate (UPA), a second-generation SPRM already employed for emergency contraception and for the treatment of uterine fibroids
[59][50], was tested as a suitable option to ameliorate symptoms in women with PMDD. The first proof-of-concept randomized controlled trial on UPA at low chronic dosing (5 mg/day) showed improvement in emotional and behavioral symptoms of PMDD
[60][51]. Interestingly, brain-imaging studies demonstrated a specific sensitivity to gonadal steroids, confirmed at the cellular level in women with PMDD
[61[52][53],
62], that appeared regulated by UPA in response to behavioral stimuli
[63][54]. Whether UPA displays a positive effect on PMDD by blocking the progesterone receptor-mediated signaling or by preventing ovulation with more stable levels of gonadal steroids remains to be determined.
At present, the most commonly prescribed hormonal treatment for the management of both physical and affective symptoms of PMS is CHC, with the rationale of suppressing ovulation
[13][28]. Temporary chemical castration with gonadotropin releasing hormone (GnRH) agonists also appeared to be an effective treatment in the management of PMS/PMDD, more on physical than psychological symptoms
[64][55]. However, add-back therapies using a combination of estrogen and progestogen to minimize negative effects of prolonged low estrogenic state in fertile women may restore symptoms in PMDD women who are intolerant especially to progestogens
[65][56].