You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Matteo Mario Carlà.
iOCT serves as a guidance tool for key surgical processes in endothelial keratoplasty, from scoring the Descemet membrane to guaranteeing graft apposition at the conclusion of the procedure. For this reason, both Descemet stripping anterior endothelial keratoplasty (DSAEK) and Descemet membrane endothelial keratoplasty (DMEK) are made easier by intraoperative OCT.
- anterior segment iOCT
- intraoperative optical coherence tomography
- Dsaek
- Dmek
- Endothelial keratoplasty
1. iOCT-Aided DSAEK
The first results of handheld intraoperative AS-OCT during DSAEK surgery were reported in 2010, when Knecht et al. highlighted the role of this technique to detect that there was still separation between the donor and host cornea at the conclusion of the procedure in two patients out of six, with an average width of interface fluid of 40 µm, despite following several published guidelines for good graft adhesion during surgery [8][1]. Other case studies have employed iOCT to demonstrate interface fluid outflow after corneal stab wounds [47[2][3],48], with Ide et al. focusing on the study of intraoperative fluid dynamics [7][4].
The PIONEER study was fundamental to highlight the utility of a microscope-mounted iOCT in different fields of ophthalmic surgery [9][5]. In DSAEK, iOCT allowed for clear visibility of fluid at the graft/host interface, in order to grant the best graft apposition, with the operation to be prolonged until optimum fluid evacuation was attained. On the first postoperative day, 3% (5/143) of the grafts dislocated. In all cases, a surgeon feedback form was completed and resulted in a 64% rate of uncertainty of graft apposition from the surgeon. Thanks to iOCT, persistent fluid was found in 48% of those cases, necessitating repeated procedures. Overall, in 65/135 (48%) eyes, iOCT determined surgical strategy changes, due to the evidence of persisting interface fluid necessitating further interventions, which was reported also in 29% of cases in which the surgeon believed the graft was completely apposed [9][5]. Hallahan et al. exploited the same PIONEER study cohort to analyze intraoperative interface fluid dynamics and correlate fluid parameters with graft non-adherence during the first week. iOCT measurements showed that total fluid volume was significantly higher when comparing completely or partially non-adherent grafts (0.22 and 0.17 µm2) with grafts that had no post-operative interface fluid (0.05 µm2). Similarly, the maximum area of fluid in the final iOCT also differed with the same trends (0.07 and 0.06 µm2 in eyes with subsequent displaced grafts and partial nonadherence grafts vs. 0.03 µm2 in eyes with totally adhered grafts) [49][6]. Despite the fact that there were no significant variations in results between the full and partial dislocation groups, the complete dislocation group tended to have higher interface fluid levels. Within the first week after surgery, many iOCT parameters were linked to whole and partial graft nonadherence, such as increased final interface fluid volume and area, maximum isolated interface fluid pocket volume, and mean and maximum interface fluid thickness, with volumetric and area assessments of final interface fluid more strongly associated with adherence graft rates rather than linear parameters. At last, there were no interface fluid characteristics linked to graft rebubbling and regrafting [49][6].
Successive studies were reported with the use of Mi-OCT, which offers better image stabilization compared to microscope-mounted or handheld iOCT in previous studies. Steverink et al. reported a case series of 8 eyes undergoing Mi-OCT-assisted DSAEK, which allowed the surgeon to identify persisting fluid interface in six cases, successfully managed with corneal swiping with a subsequent decrease in maximal interface width [50][7].
The most important research regarding microscope-integrated iOCT led to the publication of the DISCOVER study. In this research, Mi-OCT was reported to provide valuable feedback in 88.5% of cases, with interface fluid extent evaluation the most-cited area of iOCT feedback. The surgeon, by means of standardized questionnaires, declared the graft to be clinically apposed in 68.3% of patients in the DSAEK subgroup. In 46 cases (54.8%), however, residual interface fluid was seen on iOCT, thus permitting rescue procedures [11][8]. Further analyses were conducted with the same cohort of the DISCOVER study, with Ehlers et al. reporting that Mi-OCT aided decision-making during DSAEK surgery in 41% of cases, in which further maneuvers were necessary based on iOCT. When the surgeon thought the graft was entirely apposed clinically, iOCT revealed residual fluid in 19% of instances. Conversely, iOCT indicated full apposition in 47% of instances where the surgeon did not believe the graft was completely apposed, saving time and needless adjustments during surgery [10][9] (Figure 21).
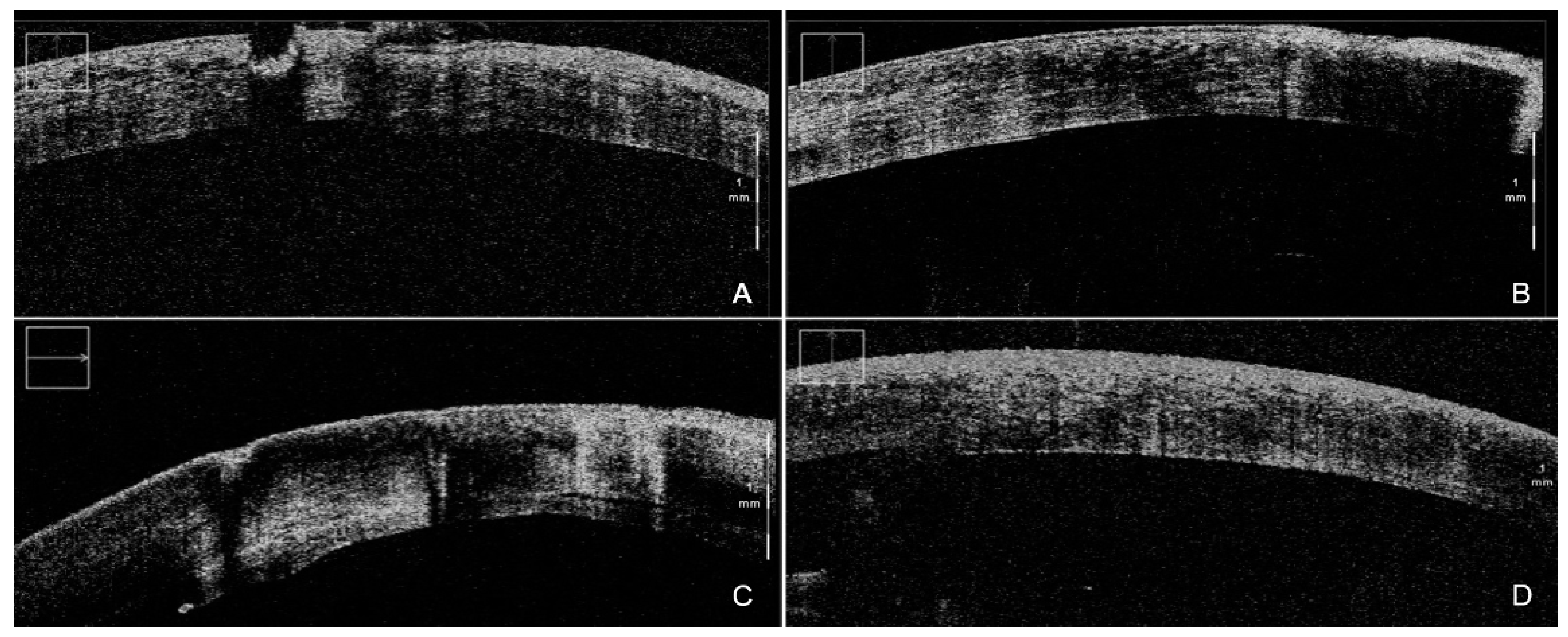
Figure 21. iOCT-assisted DSAEK performed by an expert surgeon. During DM stripping, iOCT aided debris visualization (A) in order to obtain the smoothest possible dissection plane (B). After the implantation of the graft, iOCT was able to identify even the smallest persisting interface fluid (C), thus allowing for complete apposition of the graft, clearly visible at the end of the surgery (D). iOCT = intra-operative optical coherence tomography; DSAEK = Descemet stripping automated endothelial keratoplasty; DM = Descemet membrane.
A recent retrospective study by Asif et al. focused on Mi-OCT-assisted DSAEK in congenital hereditary endothelial dystrophy (CHED) [51][10]. Corneal transplantation in infants may be difficult owing to a variety of ocular characteristics, including a tiny eyeball, a very low anterior chamber, enhanced positive posterior pressure, poor scleral stiffness, phakic status, and limited intraocular space. These variables may increase the likelihood of problems [52][11]. Moreover, CHED patients often have poor visibility due to corneal edema and stronger adherence of the DM to the underlying stroma, in contrast to decompensated corneas of Fuchs’ endothelial dystrophy [53][12]. This might cause DM residues to remain in the graft, obstructing graft apposition. iOCT proved useful in these conditions, thereby improving the outcomes of the procedure. Among the 39 eyes undergoing DSAEK, the graft was attached intraoperatively in all cases, while graft detachment was seen in the early postoperative phase in 4 eyes, requiring a rebubbling procedure [51][10].
2. iOCT-Aided DMEK
For the treatment of corneal endothelial disorders, Descemet membrane endothelial keratoplasty (DMEK) outperforms Descemet stripping automated endothelial keratoplasty (DSAEK). DMEK, in particular, has been demonstrated to speed up visual rehabilitation, improve visual acuity, and reduce corneal rejection [54,55,56,57][13][14][15][16]. Despite its benefits, DMEK necessitates the acquisition of new skills by the surgeon, which may result in a high learning curve [55,58][14][17]. DMEK scroll orientation may be seen and identified in real time using iOCT, avoiding the use of potentially hazardous exterior markers (such as the “S” stamp or peripheral notches), and may be useful in cases of corneal opacity or advanced corneal edema when vision is impaired [21,27,59][18][19][20].
Saad et al., in 2015, were the first to describe iOCT-assisted DMEK in a prospective case series of 14 eyes. Using the iOCT integrated into a surgical microscope (RESCAN 700; Carl Zeiss Meditec), which offered an 840-nm central wavelength that performed 27,000 A scans per second, graft orientation was correctly evaluated in all cases. The success rate was 100%, with only one patient needing rebubbling at 1 week. The mean unfolding time calculated from the moment the graft was inserted into the anterior chamber to the time the air bubble was injected into the anterior chamber, including the time spent looking at and evaluating the OCT pictures, was reported to be 6.1 min, faster when compared to the 8.9 min needed by the same surgeon without this technology [27][19]. Even in difficult situations with severe corneal edema, when direct visibility of the graft was hindered, OCT pictures aided graft orientation assessment, suggesting that live OCT aid may also assist in broadening DMEK indications in severe corneal edemas. Moreover, in the instance where the graft was in the incorrect orientation, OCT pictures enabled the surgeon to confirm graft rotation [27][19].
The aforementioned DISCOVER study evaluated the role of iOCT even in DMEK procedures. In the report on the first eight eyes, iOCT-assisted DMEK allowed completion of intraoperative graft attachments in seven eyes, while one graft exhibited a linear region of non-adherence matching a large posterior stromal irregularity, clearly visible on iOCT during descemetorhexis. The median “unscrolling time” was 6 min and 15 s, and the graft orientation was rapidly identifiable in 100% of cases [59][20].
These results were confirmed at 3-year follow-up in the DISCOVER study, in which surgeons reported iOCT-enabled graft orientation feedback in 63% of instances in DMEK, based on the scrolling arrangement of the tissue. Moreover, iOCT was commonly used to establish orientation even before viewing of the orienting “S” stamp, thus allowing surgeons to abandon the use of this stamp [11][8]. In addition, following first implantation, the surgeons reported clinical graft apposition in 54 patients (90%). Intraoperative imaging revealed persisting interface fluid in 7% of cases, allowing for further corneal sweeping before the surgery was completed. In this research, the trend of preferred iOCT visualization was analyzed: surgeons used the external screen in 30% of cases in the first year, the heads-up display in the oculars in 25% of cases, and both kinds of vision in 42% of instances. In years 2–3, a substantial change toward using the screen occurred in 82% of instances, compared to oculars in 10% and both systems in 8% [11][8].
In the last update of the DISCOVER study, those findings were confirmed. Patel et al. noted that iOCT offered helpful real-time feedback in all instances (100%) and did not interfere with the surgical operation in any manner [43][21]. In all instances, the iOCT picture on the linked external video display was preferred, because of the smaller, lower-definition picture on the inside display. iOCT also confirmed its effectiveness by allowing for quick and safe detection of tissue orientation in 99% of cases, thus avoiding other reported marking techniques [60,61][22][23]. Moreover, average unscrolling time of 4.4 ± 4.1 min was competitive when compared to reported times for S-stamped grafts (5.7 ± 3.9 min) and for non-stamped grafts (6.4 ± 4.4 min) [59,62][20][24]. The rebubbling rate reported in this study was 6.4%, suggesting that iOCT-assisted DMEK may yield equivalent or better early outcomes than previous reported outcomes, with a mean rebubbling rate of 28.8% reported in a recent thorough literature analysis across 17 DMEK case series without iOCT support [63][25].
iOCT favorability was also claimed by Muijzer et al., who reported recent experience with 22 iOCT-aided DMEKs with a reduced over-pressurization time (<2 min, compared to the >12 min of the standard procedure) [64][26]. Thanks to the capacity of iOCT in reducing unscrolling times, as reported by previous studies, they decided to avoid over-pressurizing the globe for an extended duration during surgery, since a high intra-ocular pressure (IOP) may harm corneal endothelial cells and the retinal nerve fiber layer [65,66][27][28]. Refraining from over-pressurization enabled reducing the entire surgical time by 24% without endangering its safety or compromising clinical outcomes. Moreover, iOCT provided the surgeon with useful information, including an unclear graft orientation in 12 cases and interface fluid and minor graft detachments in 8 instances. Ultimately, iOCT altered the surgical decision-making process in 42% of cases, and its role in the management of graft misorientation or in the prevention of unnecessary manipulation was crucial [64][26].
A recent prospective study was conducted by Sharma et al., specifically targeting 25 cases of corneal decompensation with poor visualization due to pseudophakic bullous keratopathy, Fuchs endothelial corneal dystrophy, failed graft or iridocorneal endothelial (ICE) syndrome that underwent Mi-OCT-guided DMEK [42][29]. Prior to descemetorhexis, regions of lacking DM in the host cornea were identified thanks to Mi-OCT, preventing needless DM scraping, which might have resulted in postoperative stromal haze. After completing the descemetorhexis, retained DM tags, otherwise undetectable, were detected on Mi-OCT in 92% of instances (23 eyes) and immediately removed using micro-vitreoretinal forceps and a vitrectomy cutter in all of these patients. In the case of ICE syndrome, Mi-OCT guided evaluation and release of peripheral and mid-peripheral anterior synechiae. In this research, Mi-OCT was able to identify the position and form of the DM roll in the cartridge in every case, with the majority of the instances having a double scroll (76%), and minor percentages of tight scroll and trifold. Following graft implantation, the correct orientation was always detectable, even in cases of severe stromal haze, thus overcoming the shortfalls of the numerous techniques previously proposed in cases of poor visualization, such as chandelier or trans-corneal illumination [67,68,69][30][31][32]. iOCT proved useful to assess whether or not to try to unfold the peripheral DM folds based on their precise position and extent, avoiding inadvertent damage to the graft. This study reported graft attachment success rates of 72% at day 3, with 16% of eyes requiring rebubbling, leading to a 100% rate of attachment at the 6-month follow-up, thus underlining how Mi-OCT assistance allowed for the safe and effective conduct of the procedure, otherwise rendered unsuitable for DMEK due to the presence of severe corneal edema and poor visibility of the anterior segment details [42][29].
