Pregnancy is a delicate state, during which timely investigation of possible physiological anomalies is essential to reduce the risk of maternal and fetal complications. Medical imaging encompasses different technologies to image the human body for the diagnosis, course of treatment management, and follow-up of diseases. Ultrasound (US) is currently the imaging system of choice for pregnant patients. However, sonographic evaluations can be non-effective or give ambiguous results. Therefore, magnetic resonance imaging (MRI), due to its excellent tissue penetration, the possibility of acquisition of three-dimensional anatomical information, and its high spatial resolution, is considered a valid diagnostical alternative.
- MRI
- magnetic resonance imaging
- pregnancy
1. Indications for MRI during Pregnancy
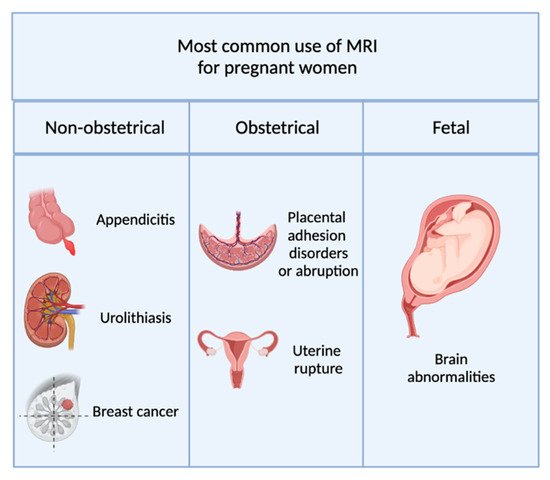
1.1. Non-Obstetric Indications
1.2. Obstetric and Gynecological Indications
-
Placenta accreta, in which placental villi adhere to the myometrium.
-
Placenta increta, where placental villi invade the myometrium.
1.3. Fetal Indications
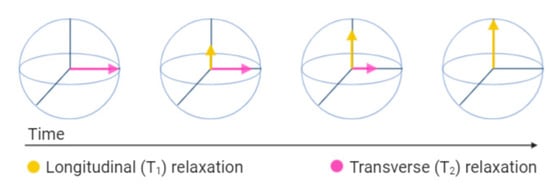
2. Safety Issues of Non-Contrast-Enhanced MRI during Pregnancy
-
It is not prudent to delay the examination until the completion of pregnancy;
-
The information needed cannot be obtained through other non-ionizing techniques;
-
The data gained may influence the management of the patient or the fetus.
