Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Sabine Berteina-Raboin and Version 4 by Vicky Zhou.
The structures composed of a pyridopyrimidine moiety which have shown a therapeutic interest or have already been approved for use as therapeutics, including pyrido[2,3-d]pyrimidines, pyrido[3,4-d]pyrimidines, pyrido[4,3-d]pyrimidines and pyrido[3,2-d]pyrimidines.
- pyridopyrimidines
- synthesis
- biological activity
- N-heterocycles
1. Introduction: Pyridopyrimidines and Their Scaffold
Depending on where the nitrogen atom is located in pyridine, itwe can be foufind four possible skeletons for the heterocyclic combination of pyrimidine and pyridine rings (Figure 1). Pyridopyrimidines and other N-heterocycles are of great interest due to their biological potential. The pyridopyrimidine moiety is present in relevant drugs and, in recent years, it has been studied in the development of new therapies, as evidenced by numerous publications, studies and clinical trials [1][2][3][1,2,3].
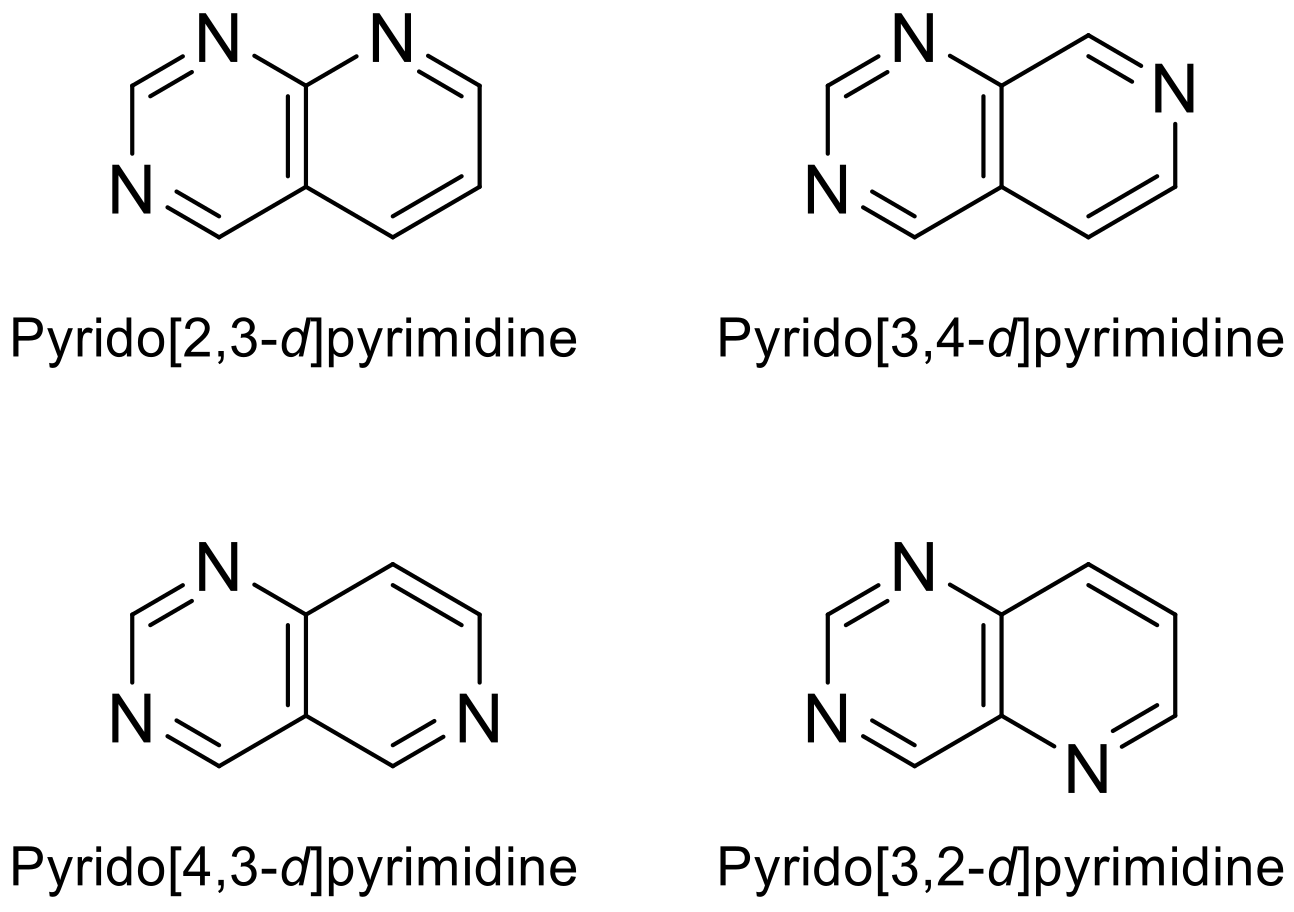
Figure 1. Various pyridopyrimidine structures types.
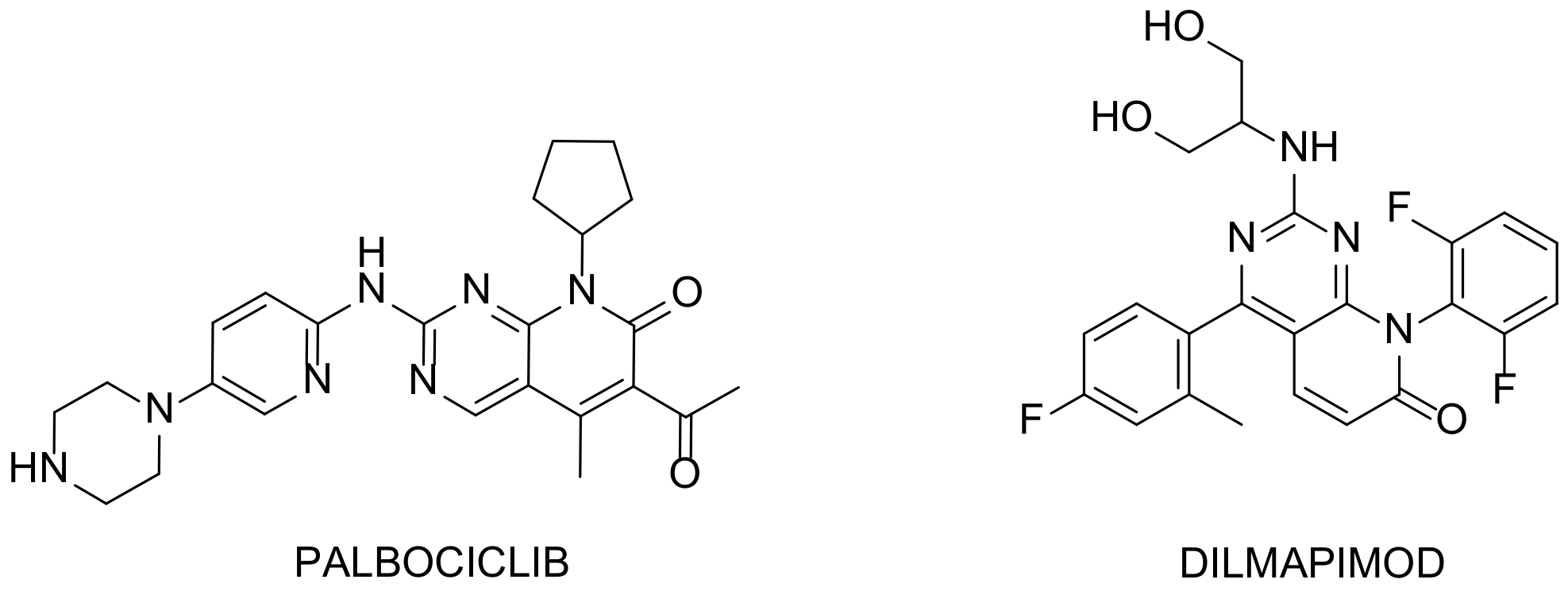
Figure 2. Examples of interesting molecules. Palbociclib: breast cancer drug developed by Pfizer and Dilmapimod: potential activity against rheumatoid arthritis.
2. Pyridopyrimidines: Therapeutic Potential and Synthesis
TIn this section , we describes that, for each compound mentioned, the biological activity and the synthetic route reported. The 24 compounds described herein are presented according to the type of pyridopyrimidines (pyrido[2,3-d]pyrimidine, pyrido[3,4-d]pyrimidine, pyrido[4,3-d]pyrimidine and pyrido[3,2-d]pyrimidine). For each compound described, the target is indicated and some additional information has been added if different from that mentioned in the introduction.2.1. Pyrido[2,3-d]pyrimidine
HTherein study starts with some interesting pyrido[2,3-d]pyrimidines. The first one is 5-methyl-6-([methyl(3,4,5-trimethoxyphenyl)amino]methyl)pyrido[2,3-d]pyrimidine-2,4-diamine (Table 1, entry 1) which has been described to have DHFR dihydrofolate as the target [20][6].
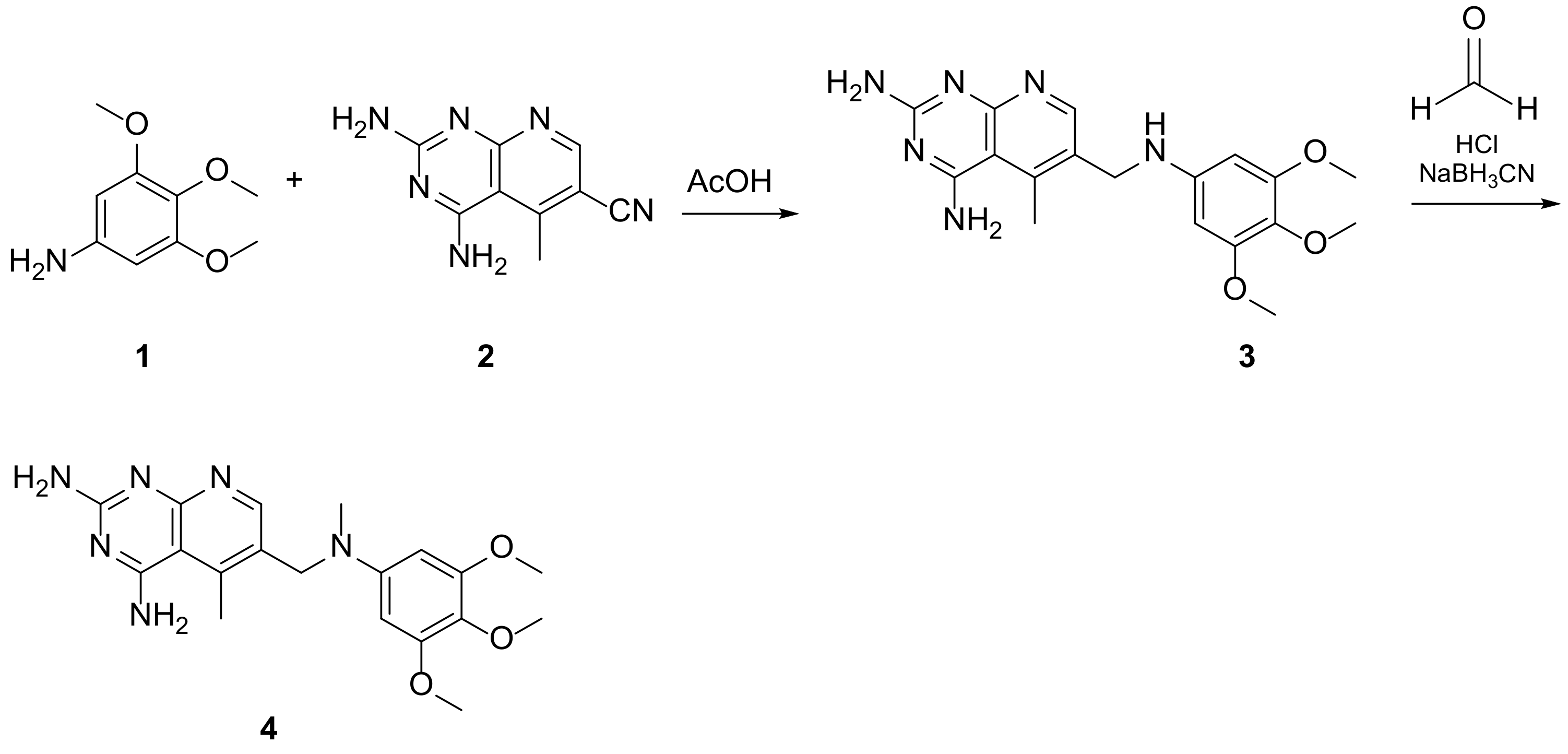
Kisliuk et al. described, in 1993, the synthesis of pyrido[2,3-d]pyrimidine-2,4-diamine (4). The reductive condensation of 6-cyano-5-methyl-pyrido[2,3-d]pyrimidine-2,4-diamine (2) with 3,4,5-trimethoxyaniline (1) in the presence of Raney Ni 70% in acetic acid gave the precursor 3 which underwent methylation at the N10 position by reductive alkylation with formaldehyde and sodium cyanoborohydride (Scheme 1) [20][6].
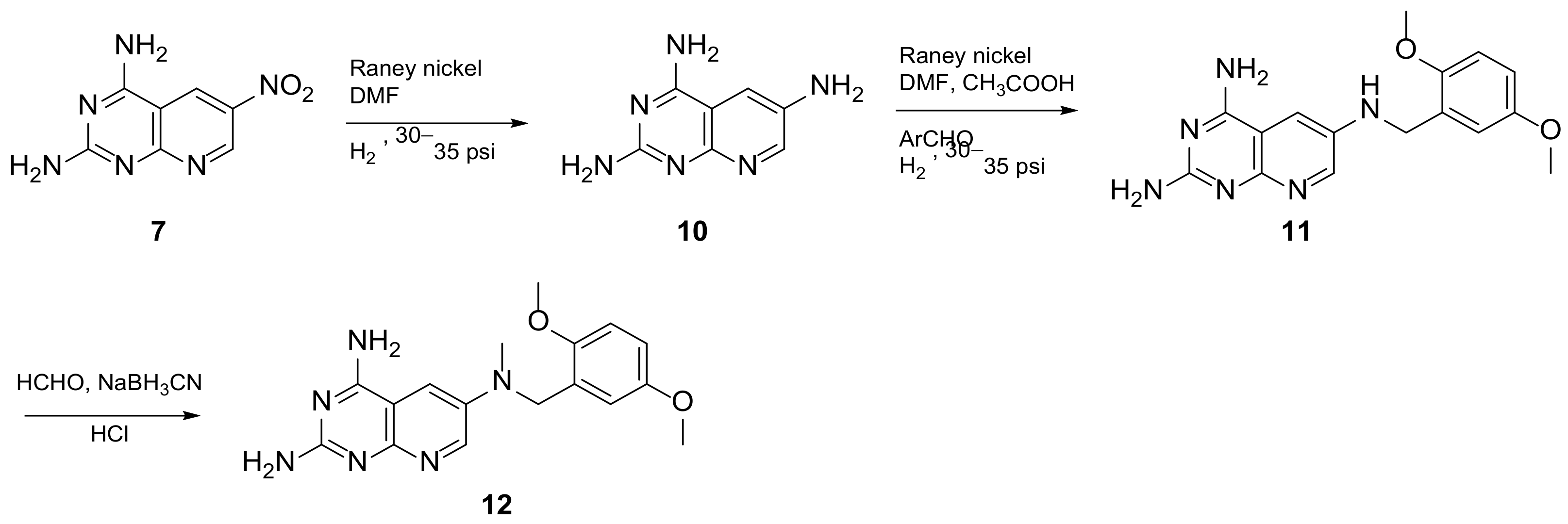
 In 2008, Queener et al. synthesized 12 starting from 2,4-diamino-6-nitroquinazoline 7 which underwent reduction with hydrogen and Raney nickel at 30-35 psi, providing the desired 2,4,6-triaminoquinazoline (10) (Scheme 3). Then, as described above, the 2,5-dimethoxybenzaldehyde ArCHO was added to generate the N9-H precursor 11. The following step was a reductive N9-alkylation using sodium cyanoborohydride which afforded the final compound [14][18]. In this study, Queener et al. conducted a biological evaluation of this compound 12 (Scheme 3, Table 1, entry 4) as a lipophilic inhibitor of dihydrofolate reductase.
In 2008, Queener et al. synthesized 12 starting from 2,4-diamino-6-nitroquinazoline 7 which underwent reduction with hydrogen and Raney nickel at 30-35 psi, providing the desired 2,4,6-triaminoquinazoline (10) (Scheme 3). Then, as described above, the 2,5-dimethoxybenzaldehyde ArCHO was added to generate the N9-H precursor 11. The following step was a reductive N9-alkylation using sodium cyanoborohydride which afforded the final compound [14][18]. In this study, Queener et al. conducted a biological evaluation of this compound 12 (Scheme 3, Table 1, entry 4) as a lipophilic inhibitor of dihydrofolate reductase.
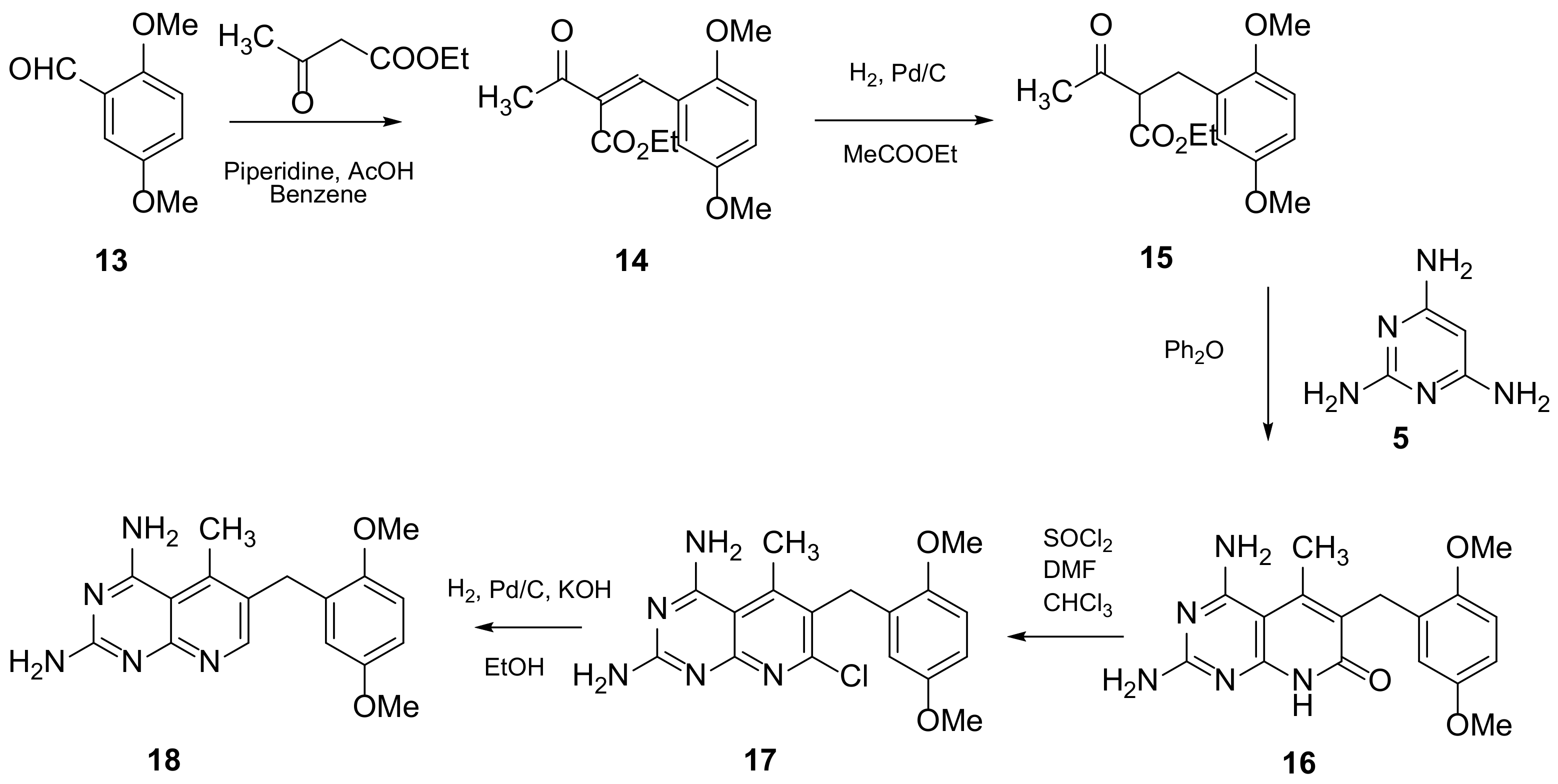
 Piritrexim (PTX) (Scheme 4 and Scheme 5, Table 1, entry 5) is a synthetic antifolate first synthesized by Grivsky, Sigel et al. [21][24] with anti-parasitic, anti-psoriatic and anti-tumor properties. Piritrexim inhibited dihydrofolate reductase (DHFR) and also showed good antitumor effects on the carcinosarcoma in rats. An advantage of this compound compared to some analogues is that it does not have effects as an inhibitor of histamine metabolism, reducing the potential risk of side reactions on metabolism. Its degree of lipophilicity, i.e., the affinity of this drug for a lipid environment, allows it to diffuse easily into the cells. The various therapeutical activities listed for piritrexim are on melanoma and urothelial cancer, and promising results in head and neck cancer were already obtained in combination with other molecules [16][20].
Piritrexim (PTX) (Scheme 4 and Scheme 5, Table 1, entry 5) is a synthetic antifolate first synthesized by Grivsky, Sigel et al. [21][24] with anti-parasitic, anti-psoriatic and anti-tumor properties. Piritrexim inhibited dihydrofolate reductase (DHFR) and also showed good antitumor effects on the carcinosarcoma in rats. An advantage of this compound compared to some analogues is that it does not have effects as an inhibitor of histamine metabolism, reducing the potential risk of side reactions on metabolism. Its degree of lipophilicity, i.e., the affinity of this drug for a lipid environment, allows it to diffuse easily into the cells. The various therapeutical activities listed for piritrexim are on melanoma and urothelial cancer, and promising results in head and neck cancer were already obtained in combination with other molecules [16][20].
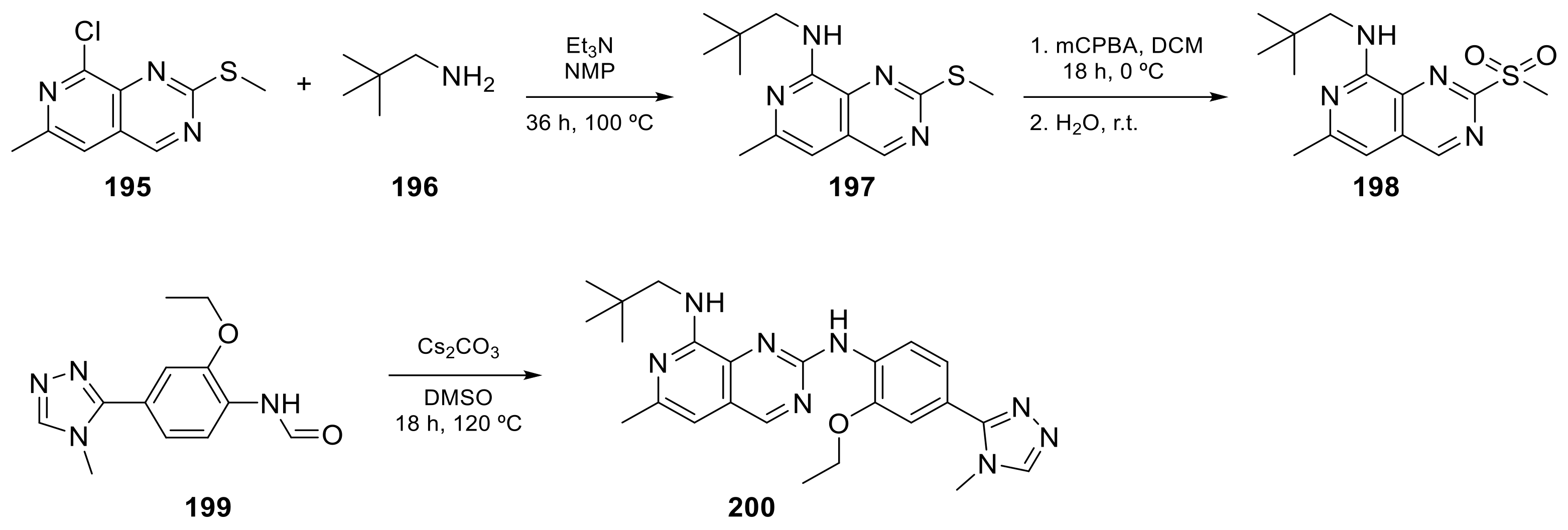
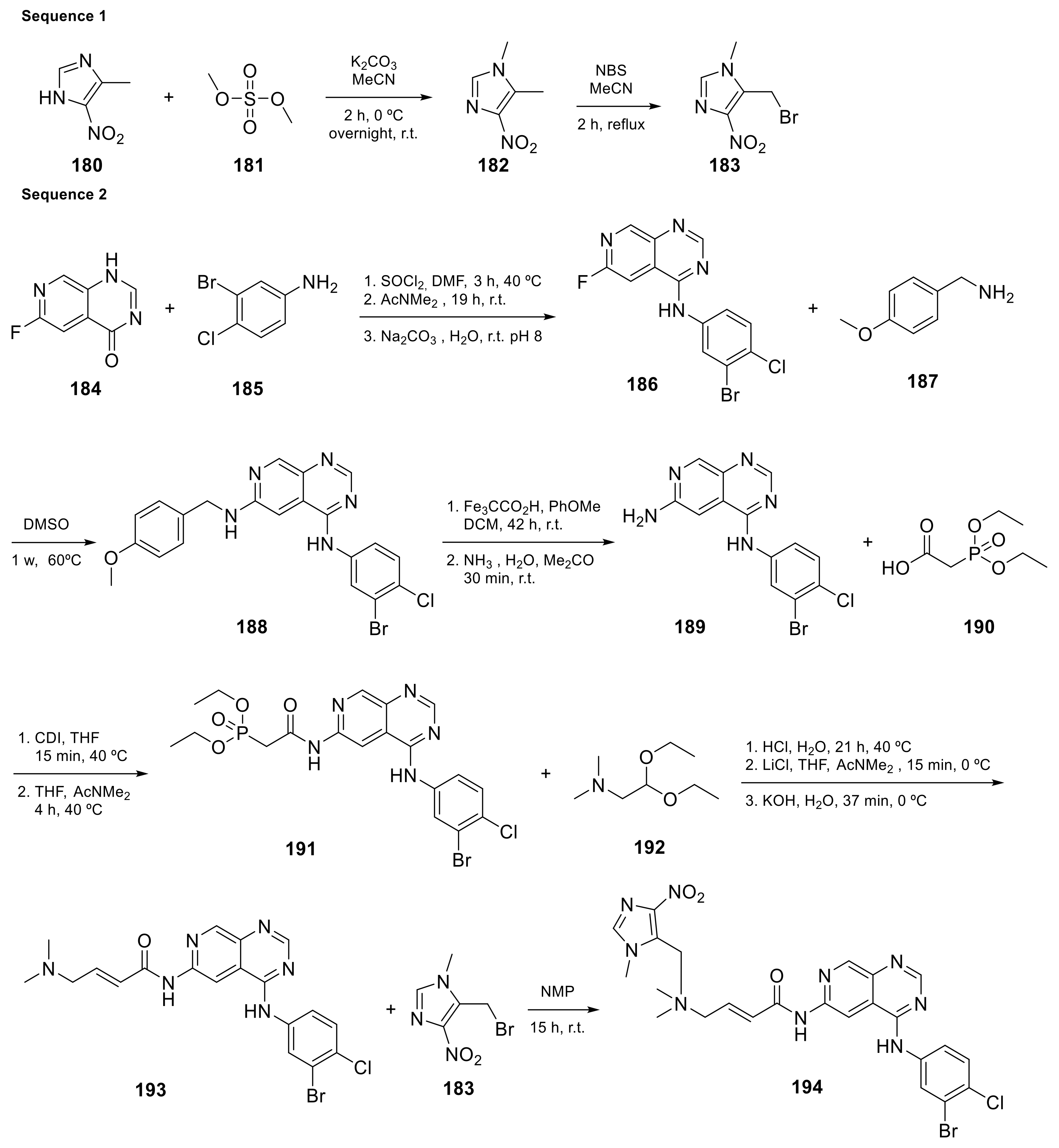 Carlin et al. [23][61] patented in 2015 the preparation of 4-anilinopyrido[3,4-d]pyrimidine prodrugs (Scheme 619, Table 1, entry 19) as kinase inhibitors useful for cancer treatment. The procedure is described in Scheme 619 with classical synthetic methodologies affording the expected compound 194 in twelve steps.
The second example is the BOS172722 derivative (200 in Scheme 720, Table 1, entry 20). This compound, in combination with paclitaxel, was tested in vivo for the treatment of triple hormone receptor-negative breast cancer demonstrating a promising synergy. This selective monopolar spindle 1 (Mps1) kinase inhibitor has been identified as a potential anti-cancer agent because it is involved in the division of cancer cells. This is, therefore, an attractive target for cancer therapy [24][25][62,63]. It has the dual specificity protein kinase TTK as the target.
Carlin et al. [23][61] patented in 2015 the preparation of 4-anilinopyrido[3,4-d]pyrimidine prodrugs (Scheme 619, Table 1, entry 19) as kinase inhibitors useful for cancer treatment. The procedure is described in Scheme 619 with classical synthetic methodologies affording the expected compound 194 in twelve steps.
The second example is the BOS172722 derivative (200 in Scheme 720, Table 1, entry 20). This compound, in combination with paclitaxel, was tested in vivo for the treatment of triple hormone receptor-negative breast cancer demonstrating a promising synergy. This selective monopolar spindle 1 (Mps1) kinase inhibitor has been identified as a potential anti-cancer agent because it is involved in the division of cancer cells. This is, therefore, an attractive target for cancer therapy [24][25][62,63]. It has the dual specificity protein kinase TTK as the target.

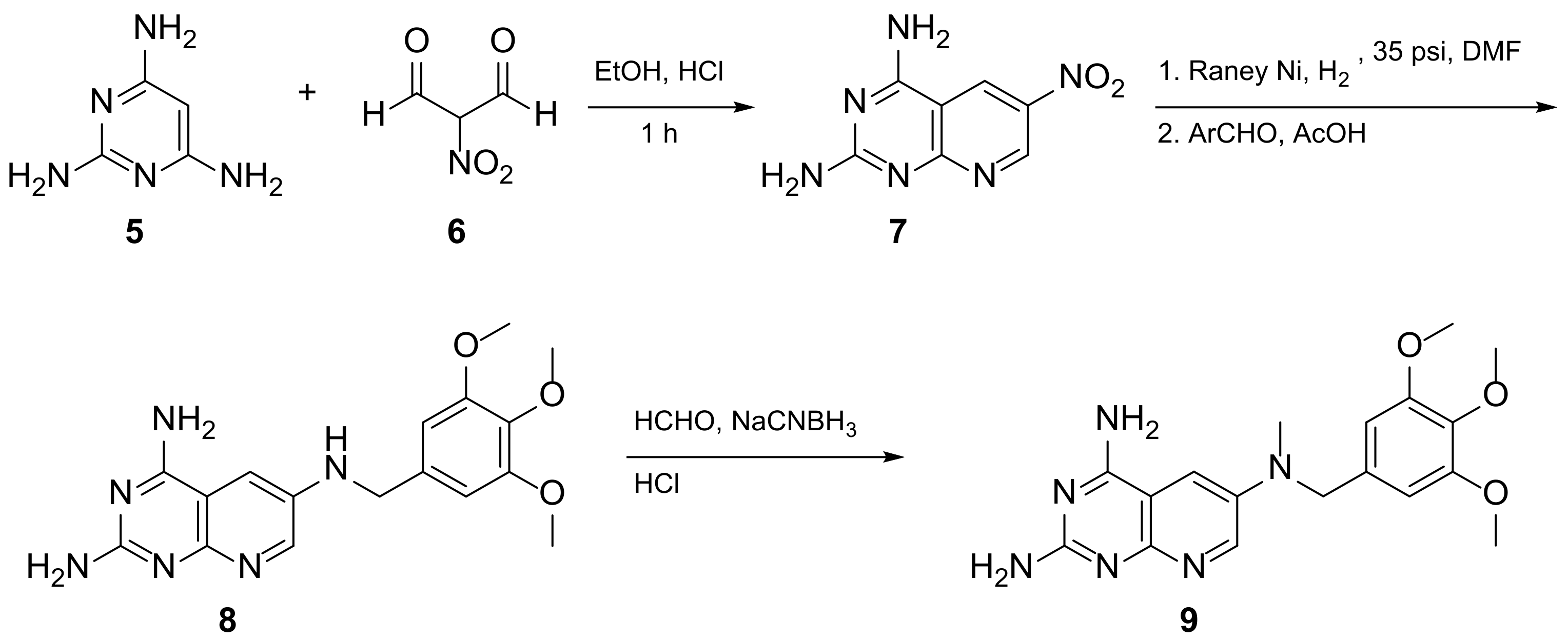
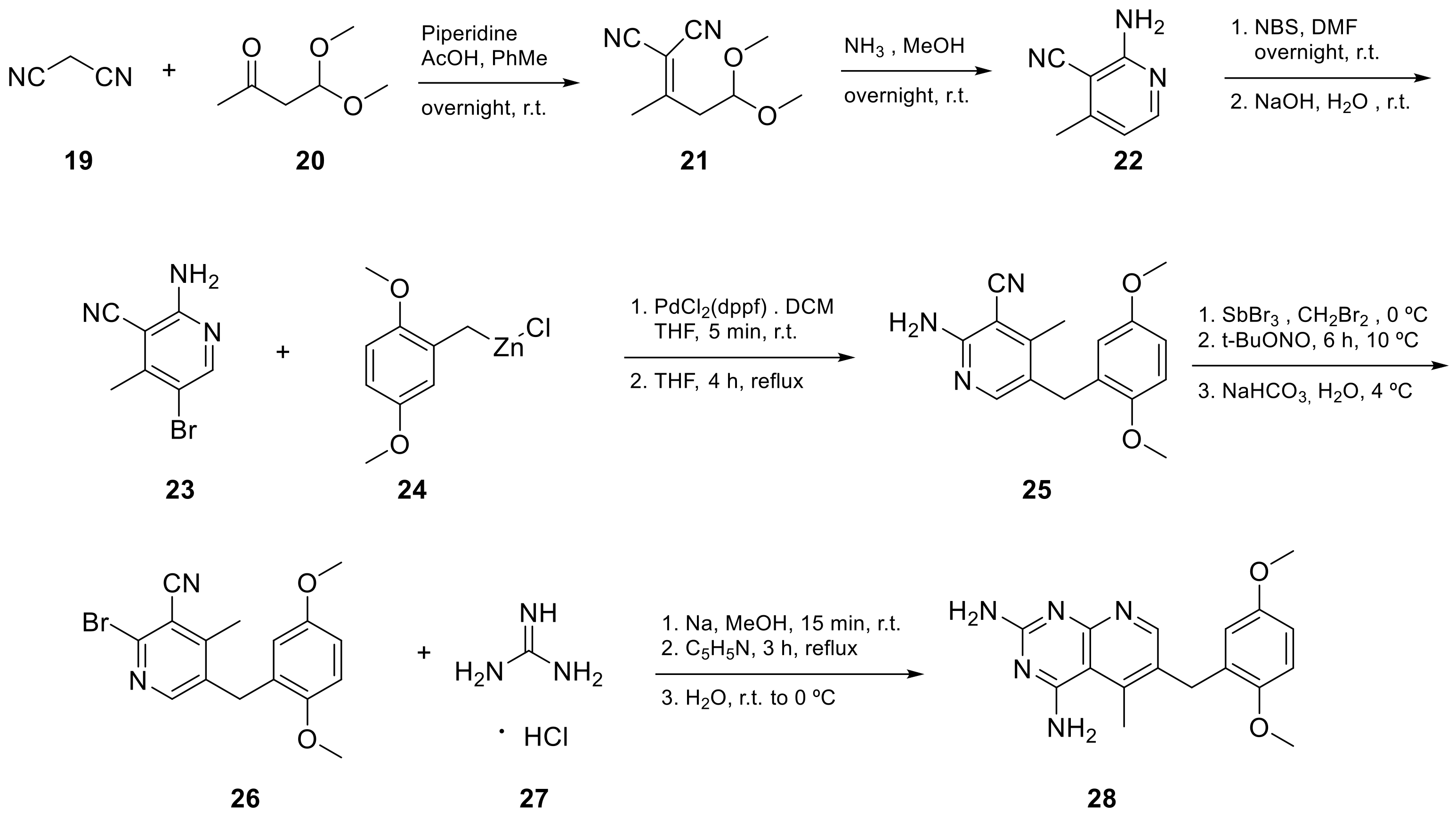
Kisliuk et al. also developed another strategy to synthesize pyrido[2,3-d] pyrimidine-2,4-diamines as compound 9 (Scheme 2, Table 1, entry 2). Starting from 2,4,6-triaminopyrimidine (5) with the sodium salt of nitromalonaldehyde, they obtained in a single step the 2,4-diamino-6-nitropyrido [2,3-d]pyrimidine (7) which was then reduced to its corresponding 6-amino analogue using Raney Ni in DMF. The reductive amination with various aldehydes (ArCHO, in this case 3,4,5-trimethoxybenzaldehyde) provided the desired product 8. In the last step, 8 was N-methylated by treatment with formaldehyde in the presence of sodium cyanoborohydride [15][19] (Scheme 2). An analog compound (Table 1, entry 3) was obtained following the same synthetic pathway (Scheme 2) using 3,5-dimethoxybenzaldehyde.



Scheme 4. Synthesis of 6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3-d]pyrimidine-2,4-diamine (18) by Grivsky, Sigel et al. [21][24].

Scheme 5. Synthesis of 6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3-d]pyrimidine-2-amine (28) by Chan and Rosowsky [17][21].
2.2. Pyrido[3,4-d]pyrimidine
This class of pyridopyrimidine is mainly referenced with kinase activity. The first example mentioned herein is Tarloxotinib (194 in Scheme 619). It is being studied in the clinical trial NCT03743350 (NSCLC exon 20 or HER2 activating mutation) [22][60]. This molecule is a kinase inhibitor targeting all members of the HER family, with a novel mechanism of action. It is a hypoxia-activated prodrug that releases an active metabolite irreversibly targeting the kinase. The goal is to inhibit only HER kinases in tumor cells. Tarloxotinib is a Pan-HER kinase inhibitor.

Scheme 720.
N
8-(2,2-dimethylpropyl)-N
2-[2-ethoxy-4-(4-methyl-4H
-1,2,4-triazol-3-yl)phenyl]-6-methylpyrido[3,4-d
]pyrimidine-2,8-diamine (), BOS172722 [62].
2.3. Pyrido[4,3-d]pyrimidine
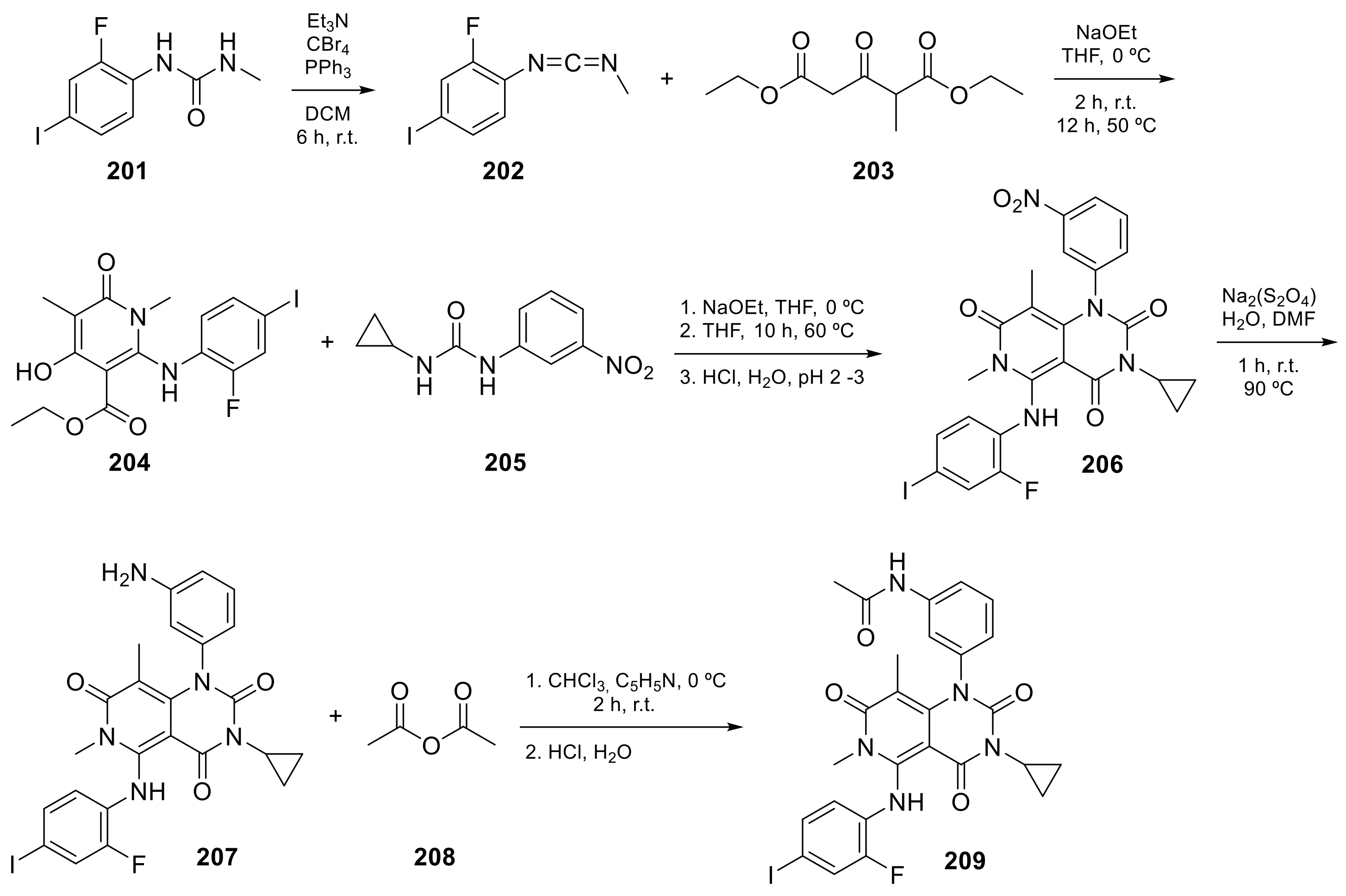
Trametinib (209 in Scheme 821, Table 1, entry 21) is a kinase inhibitor used for specific types of melanoma. This compound, associated with other molecules such as Dabrafenib (Tafilnar) and/or Mekinist (trametinib), has been approved by the FDA in particular for the treatment of degenerative thyroid cancer (ATC) [26][27].
Scheme 8. Synthesis of N-(3-(3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-6,8-dimethyl-2,4,7-trioxo-1H,2H,3H,4H,6H,7H-pyrido[4,3-d]pyrimidin-1-yl)phenyl)acetamid, Trametinib (209) [28].
2.4. Pyrido[3,2-d]pyrimidine
Seletalisib (229 in Scheme 9[66, Table 1, entry 22) is a novel small-molecule inhibitor of PI3Kδ that was evaluated in clinical assays to study the treatment and basic science of Primary Sjogren’s Syndrome [29]67]. This molecule is an ATP-competitive and highly selective PI3Kδ inhibitor. Phosphoinositide 3-kinases (PI3K) are enzymes regulating cellular survival, development, and function. They play a key role in immune cell development and function.
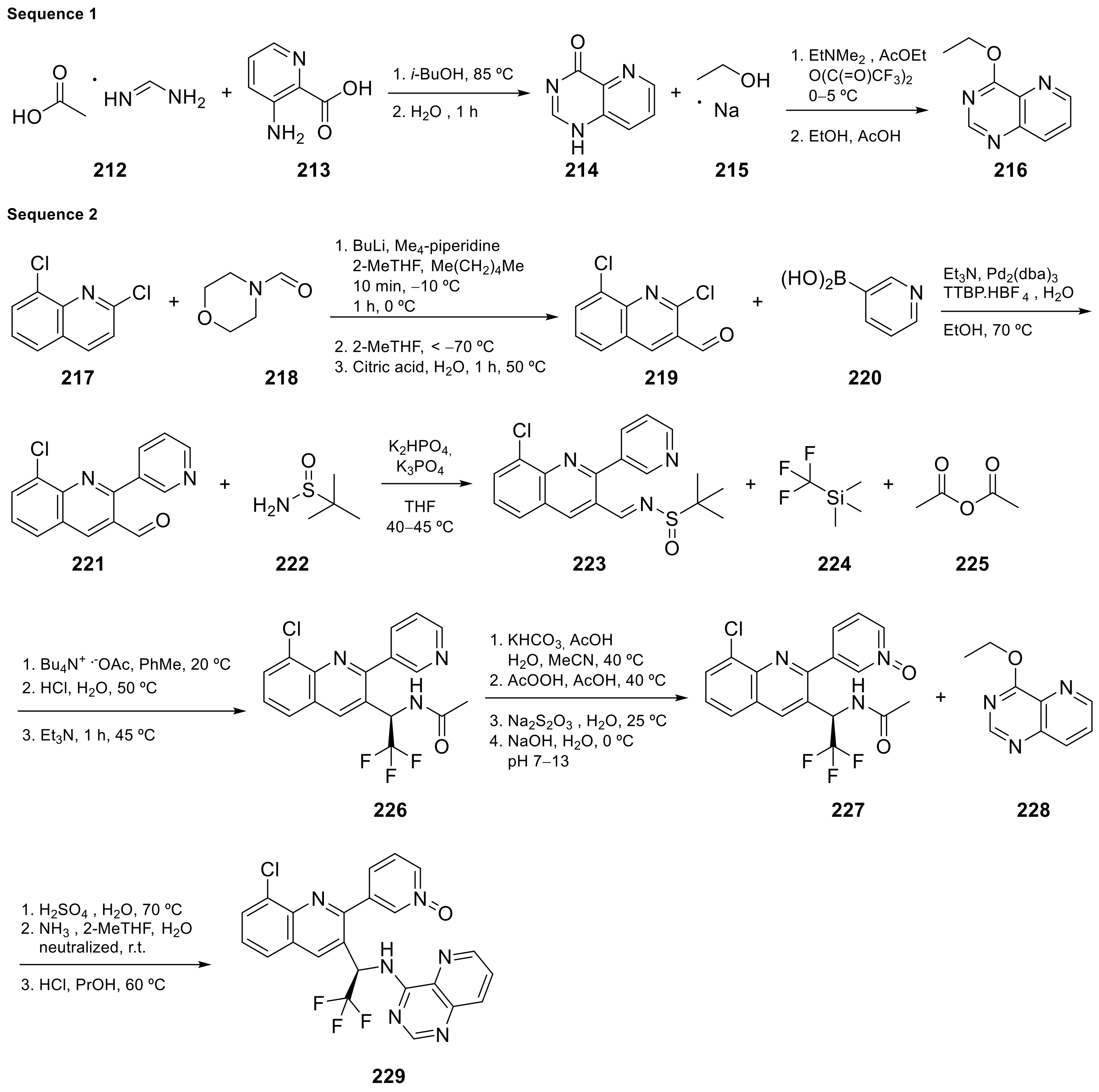

Scheme 922.
Synthesis of 3-[8-chloro-3-[(1
R
)-2,2,2-trifluoro-1-([pyrido[3,2-
d
]pyrimidin-4-yl]amino)ethyl]quinolin-2-yl]pyridin-1-ium-1-olate (
Le Meur et al. [30][71] patented the synthesis of Seletalisib 229 following the procedure summarized in Scheme 922, and described crystalline forms for the treatment of various pathologies.
All compounds described herein with their target were listed below (Table 1).
Table 1. The 24 pyridopyrimidines described here.
| Entry | Structure | Name | Target | Ref. |
|---|---|---|---|---|
| Pyrido[2,3-d]pyrimidine | ||||
| 1 | 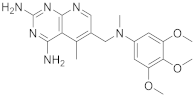 |
5-methyl-6-([methyl(3,4,5-trimethoxyphenyl)amino]methyl)pyrido[2,3-d]pyrimidine-2,4-diamine | DHFR Dihydrofolate reductase |
[31][32][20][4,5,6] |
| 2 | 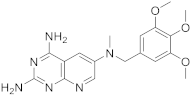 |
N6-methyl-N6-[(3,4,5-trimethoxyphenyl)methyl]pyrido[2,3-d]pyrimidine-2,4,6-triamine | DHFR Dihydrofolate reductase |
[15][19] |
| 3 | 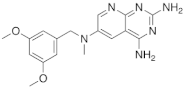 |
N6-[(3,5-dimethoxyphenyl)methyl]-N6-methylpyrido[2,3-d]pyrimidine-2,4,6-triamine, | DHFR Dihydrofolate reductase |
[15][19] |
| 4 | 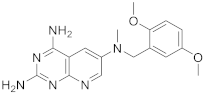 |
N6-[(2,5-dimethoxyphenyl)methyl]-N6-methylpyrido[2,3-d]pyrimidine-2,4,6-triamine | DHFR Dihydrofolate reductase |
[14][18] |
| 5 | 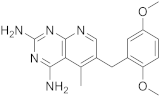 PIRITREXIM |
6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3-d]pyrimidine-2,4-diamine | DHFR Dihydrofolate reductase |
[16][17][21][20,21,24] |
| 6 | 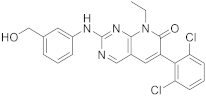 |
6-(2,6-dichlorophenyl)-2-([3-(hydroxymethyl)phenyl]amino)-8-ethyl-7H,8H-pyrido[2,3-d]pyrimidin-7-one | Tyrosine kinase activity | [33][34][25,26] |
| 7 | 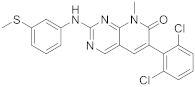 PD-173955 |
6-(2,6-dichlorophenyl)-8-methyl-2-([3-(methylsulfanyl)phenyl]amino)-7H,8H-pyrido[2,3-d]pyrimidin-7-one | Kinase activity: Tyrosine-protein kinase transforming protein Abl |
[13][17] |
| 8 | 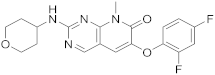 |
6-(2,4-difluorophenoxy)-8-methyl-2-[(oxan-4-yl)amino]-7H,8H-pyrido[2,3-d]pyrimidin-7-one | Kinase activity: Mitogen-activated protein kinase 14 |
[35][36][37][27,28,29] |
| 9 | 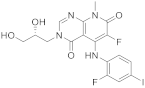 TAK-733 |
3-[(2R)-2,3-dihydroxypropyl]-6-fluoro-5-[(2-fluoro-4-iodophenyl)amino]-8-methyl-3H,4H,7H,8H-pyrido[2,3-d]pyrimidine-4,7-dione | Kinase activity: Against MEK and ERK |
[38][39][40][41][30,31,32,33] |
| 10 | 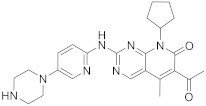 Palbociclib |
6-acetyl-8-cyclopentyl-5-methyl-2-([5-(piperazin-1-yl)pyridin-2-yl]amino)-7H,8H-pyrido[2,3-d]pyrimidin-7-one | Kinase activity: Cyclin-dependent kinase 4/Cyclin-dependent kinase 6 Breast cancer drug |
[42][43][44][45][46][34,35,36,37,38] |
| 11 | 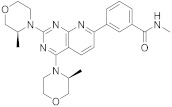 Vistusertib |
3-(2,4-bis[(3S)-3-methylmorpholin-4-yl]pyrido[2,3-d]pyrimidin-7-yl)-N-methylbenzamide | Kinase activity: Vistusertib (AZD2014) is a novel mTOR inhibitor |
[47][48][39,40] |
| 12 | 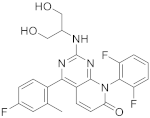 Dilmapimod (SB-681323) |
8-(2,6-difluorophenyl)-2-[(1,3-dihydroxypropan-2-yl)amino]-4-(4-fluoro-2-methylphenyl)-7H,8H-pyrido[2,3-d]pyrimidin-7-one | Kinase activity: P38 MAPK inhibitor, Tumor necrosis factor/Interleukin-1 beta/Interleukin-6. Potential activity against rheumatoid arthritis | [49][50][51][52][53][54][55][41,42,43,44,45,46,47] |
| 13 | 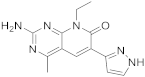 Voxtalisib |
2-amino-8-ethyl-4-methyl-6-(1H-pyrazol-5-yl)-7H,8H-pyrido[2,3-d]pyrimidin-7-one | Kinase activity: PI3K/mTOR Inhibitor |
[56][57][48,49] |
| 14 | 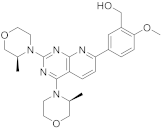 AZD8055 |
(5-(2,4-bis[(3S)-3-methylmorpholin-4-yl]pyrido[2,3-d]pyrimidin-7-yl)-2-methoxyphenyl)methanol | Kinase activity: Selective ATP-competitive mTOR kinase inhibitor. Induction of MEK/ERK |
[58][59][50,51] |
| 15 | 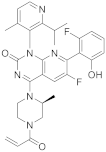 AMG-510 |
6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-[4-methyl-2-(propan-2-yl)pyridin-3-yl]-4-[(2S)-2-methyl-4-(prop-2-enoyl)piperazin-1-yl]-1H,2H-pyrido[2,3-d]pyrimidin-2-one | Kinase Activity: KRAS inhibitor implicated in the RAS/MAPK pathway GTPase KRas |
[60][61][62][63][64][52,53,54,55,56] |
| 16 | 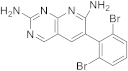 |
6-(2,6-dibromophenyl)pyrido[2,3-d]pyrimidine-2,7-diamine | Biotin carboxylase | [65][57] |
| 17 | 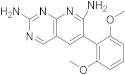 |
6-(2,6-dimethoxyphenyl)pyrido[2,3-d]pyrimidine-2,7-diamine | Biotin carboxylase | [33][66][25,58] |
| 18 | 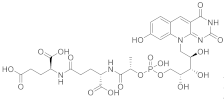 |
(2S)-2-[(4S)-4-carboxy-4-[(2S)-2-([hydroxy(([(2R,3S,4S)-2,3,4-trihydroxy-5-(8-hydroxy-2,4-dioxo-2H,3H,4H,10H-pyrimido[4,5-b]quinolin-10-yl)pentyl]oxy))phosphoryl]oxy)propanamido]butanamido]pentanedioic acid | Methanobacterium redox coenzyme Factor 420 (F420) | [67][59] |
| Pyrido[3,4-d]pyrimidine | ||||
| 19 | 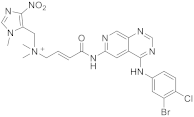 Tarloxotinib |
[(2E)-3-((4-[(3-bromo-4-chlorophenyl)amino]pyrido[3,4-d]pyrimidin-6-yl)carbamoyl)prop-2-en-1-yl]dimethyl[(1-methyl-4-nitro-1H-imidazol-5-yl)methyl]azanium | Kinase Activity: Pan-HER kinase inibitor |
[23][61] |
| 20 | 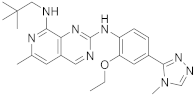 BOS172722 |
N8-(2,2-dimethylpropyl)-N2-[2-ethoxy-4-(4-methyl-4H-1,2,4-triazol-3-yl)phenyl]-6-methylpyrido[3,4-d]pyrimidine-2,8-diamine | Kinase Activity: Dual specificity protein kinase TTK |
[24][25][68][69][62,63,64,65] |
| Pyrido[4,3-d]pyrimidine | ||||
| 21 | 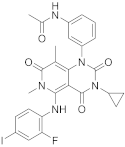 Trametinib |
N-(3-(3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-6,8-dimethyl-2,4,7-trioxo-1H,2H,3H,4H,6H,7H-pyrido[4,3-d]pyrimidin-1-yl)phenyl)acetamide | Dual specificity mitogen-activated protein kinase kinase 1/Dual specificity mitogen-activated protein kinase kinase 2 | [26][27][70][71][66,67,68,69] |
| Pyrido[3,2-d]pyrimidine | ||||
| 22 | 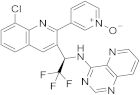 Seletalisib |
3-(8-chloro-3-[(1R)-2,2,2-trifluoro-1-((pyrido[3,2-d]pyrimidin-4-yl)amino)ethyl]quinolin-2-yl)pyridin-1-ium-1-olate | selective PI3Kδ inhibitor | [72][30][70,71] |
| 23 | 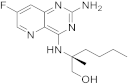 |
(2S)-2-((2-amino-7-fluoropyrido[3,2-d]pyrimidin-4-yl)amino)-2-methylhexan-1-ol | Chronic hepatitis B TLR8 receptor |
[73][74][72,73] |
| 24 | 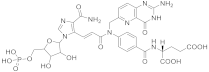 β-DADF |
(2S)-2-((4-[(2E)-N-((2-amino-4-oxo-1H,4H-pyrido[3,2-d]pyrimidin-6-yl)methyl)-3-(4-carbamoyl-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-[(phosphonooxy)methyl]oxolan-2-yl]-1H-imidazol-5-yl)prop-2-enamido]phenyl)formamido)pentanedioic acid | Bifunctional purine biosynthesis protein PURH | [75][76][74,75] |
