You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Bruce Ren and Version 1 by Serafeim Papadopoulos.
Cryopreservation is a technique that offers various advantages, especially in fish, among others, that makes the reproduction of species easier through a constant supply of sperm, synchronization of the gamete availability of both sexes, storage of semen for genetic improvement programs, reduction in the cost by eliminating the need to maintain male broodstock, and conserving the gametes of endangered species. However, freezing and warming procedures for cryopreservation lead to a reduction in the quality and viability of cryopreserved sperm because of oxidative stress.
- cryopreservation
- fish
- farm animals
- human
- semen
- melatonin
- antioxidant
- oxidative stress
1. Introduction
The use of the technology of sperm cryopreservation offers many benefits extensively described in previous reviews for fish [1,2[1][2][3],3], farm animals [4[4][5][6],5,6], and humans [7,8][7][8]. In addition, extensive reviews have been published that include detailed protocols for fish species [9,10,11,12][9][10][11][12].
The development of fish sperm cryopreservation protocols for marine species is not as extensive for freshwater species, and the plethora of research work concerns the latter [2]. The main goal of most work on new species is to maintain stocks to ensure production, to optimize genetic improvement programs, and to properly manage offspring [2,13][2][13]; therefore, the development of cryopreservation protocols would help to achieve the above objectives [2]. In addition, cryopreservation of gametes can be used to protect endangered species [14].
2. Cryodamage of Spermatozoa
The cryopreservation of sperm provokes a decrease in its quality and viability, mainly due to the increase in the production of reactive oxygen species (ROS) and the alteration of oxidative metabolism during the process of freezing and warming [15].
Although the sperm of fish, like all biological systems, are provided with protective antioxidants agents [16[16][17],17], in the cryopreservation technique, the antioxidant defense of the sperm is almost insufficient due to the reduced amount of these factors after the dilution of sperm [18,19][18][19]. As a consequence, during cryopreservation, an imbalance is observed between ROS production and the inherent antioxidant system [20[20][21],21], known as oxidative stress. Scientific studies in fish have shown that ROS production during cryopreservation contributes to the occurrence of lesions in sperm [22[22][23],23], resulting in lipid peroxidation (LPO) [24[24][25],25], DNA fragmentation [18[18][26],26], mitochondrial damage and dysfunction [23[23][27][28][29],27,28,29], protein oxidation [30], and loss or inactivation of enzymes associated with sperm motility [24,31,32][24][31][32]. Due to the aforementioned problems, it has become common practice to enrich the cryopreservation diluents of the sperm of many fish species with enzymatic and non-enzymatic antioxidants [18,33,34,35][18][33][34][35]; nevertheless, their use is often controversial.
3. Antioxidant Supplementation of Semen Extenders: The Case of Melatonin
Considering that the increased production of ROS during the cryopreservation process is partly responsible for the poor quality of sperm after thawing, various antioxidants have been proposed and tested for the cryopreservation of sperm of various terrestrial animals and fish [36]. Recent published studies focus on the protective role of various antioxidants, especially melatonin, in maintaining male fertility in both productive animals [37,38][37][38] and fish species [39], thus demonstrating the increased interest in this hormone.
Melatonin, the principal hormone secreted by the pineal gland, has been suggested as a free radical scavenger and antioxidant [40]. Mainly due to the fact of its amphiphilic nature that allows it to pass through all morphophysiological barriers of the cell; it is one of the most effective antioxidants protecting cells from oxidative stress caused by reactive species [41]. In addition, its lipophilic nature allows it to easily cross cell membranes and act directly in various organs including those of the reproductive system [42,43][42][43]. Of particular interest is the fact that melatonin’s metabolites, which are formed when the hormone functions as a scavenger, are likewise equally as good or better than the parent molecule in neutralizing toxic oxygen-based and nitrogen-based reactants [44].
The cytoprotective action of melatonin and its metabolites is due to the fact of its direct and indirect antioxidant properties [45]. The direct properties include the scavenging of both ROS and RNS (reactive nitrogen species) [46], while the indirect effects cover the stimulation of antioxidative enzymes and inhibition of pro-oxidative enzymes [47], probably through epigenetic mechanisms [48]. This molecule with its strong detoxifying effect at the mitochondrial level, could be an appropriate candidate for improving the quality of animal sperm during cryopreservation [49,50][49][50]. It has been observed that this substance could protect sperm from oxidative damage [51], maintain its viability [42,52][42][52], and reduce morphological abnormalities [53,54][53][54] and DNA fragmentation [55]. Improvement in sperm quality due to the high levels of endogenous melatonin has been found in humans [56], while in vitro treatment with this hormone can improve human sperm motility [56] and several quality parameters of ram [57] and pig [58] sperm. Finally, melatonin has been used as an additive antioxidant in the cryopreservation of sperm [59,60][59][60] helping to increase its quality after thawing [61].
4. Melatonin Supplementation in Farm Animals and Human Freezing Medium
The majority of recent studies evaluating the effect of melatonin on the cryopreservation of sperm focus on cattle compared to those on sheep, pigs, or goats [38].
In summary, the effect of melatonin on sperm is shown in Table 1 and the pathway of action in Figure 1.
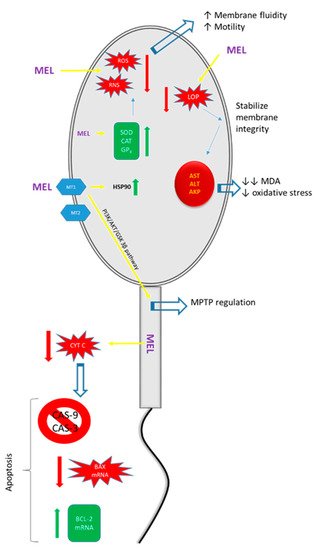

Figure 1. Illustration of the positive effects of melatonin (MEL) on spermatozoa. MT1: melatonin type 1 receptor; MT2: melatonin type 2 receptor; ROS: reactive oxygen species; RNS: reactive nitrogen species; LOP: lipid peroxidation; SOD: superoxide dismutase; CAT: catalase; GPX: glutathione peroxidase; HSP90: heat shock protein 90; AST: aspartate amino transferase; ALT: alanine aminotransferase; AKP: alkaline phosphatase; MDA: malondialdehyde; MPTP: mitochondrial permeability transition pore.
Table 1. Effects of melatonin on sperm.
| Pathway of Action | Εffects on Spermatozoa | References |
|---|---|---|
| Reduction in excessive production of free radicals. | Positive effects on the function and morphometry parameters of sperm in humans and various farm animals. | [62,63,64][62][63][64] |
| Upregulation of the expression of heat shock protein (HSP) 90. | Resistance to stress factors in frozen–thawed sperm. | [62] |
| Upregulation of antioxidant enzymes, e.g., superoxide dismutase, glutathione peroxidase, and catalase. | Elimination of ROS levels causing preservation of membrane fluidity and motility. | [62,65,66][62][65][66] |
| Regulation of mitochondrial permeability transition pores (MPTPs) as a result of binding to the MT1 receptor and the activation of the PI3K/AKT/GSK 3β pathway. | Improvement in the quality and fertilizing capacity of frozen–thawed ram sperm. | [67] |
| Reduction of LPO production leads to (a) stabilization of membrane integrity and (b) prevention of leakage of intracellular enzymes, e.g., aspartate transaminase (AST), alanine transaminase (ALT), and phosphatase. | Decreased malondialdehyde (MDA) concentrations and oxidative stress. | [68,69][68][69] |
| Enhancement of the functions of antioxidant enzymes. | Protection against oxidative modifications of DNA. DNA becomes more resistant to fragmentation, reducing the rate of sperm degradation and enhancing its viability and functions. | [42,59,70,71][42][59][70][71] |
| Action as an anti-apoptotic molecule. |
|
[72] |
|
[73,74][73][74] |
Several studies have shown that the cryoprotective effects of melatonin depend on its concentration [59,75][59][75]. In the international literature, many different concentrations have been defined as being optimal for the cryoprotection of sperm. Among others, some studies have shown that 1 and 2 mM are the optimal concentrations, while another study found that the best concentration was 0.25 mg/mL in various species [76]. In humans, a better range of sperm viability and motility were observed at a 3 mM melatonin concentration, and intracellular ROS levels were reduced [51]. The vitality of thawed human sperm was found to be improved after the supplementation of 0.1 mM melatonin, while it was adversely affected by other concentrations (i.e., 0.001 and 1 mM) [62]. Karimfar et al. [75] reported that the best protection for human sperm against cryopreservation damage was observed at a 0.01 mM melatonin concentration.
Studies have shown that parameters, such as membrane integrity, motility and velocity, capacitation, antioxidant protein quantity, and developmental competence of fresh and frozen sperm improved after administration of moderate melatonin concentrations [62,77,78,79][62][77][78][79]. The addition of melatonin to cryopreservation solutions of bovine sperm [71], sheep [80[80][81],81], goats [82[82][83],83], rams [70], buffalo [84], and pigs [85,86][85][86] increased the number of live sperm with normal quality after thawing, including the normal length and movement of the tail, and reduced morphological defects of the sperm. In addition, in farmed animals, the frozen sperm with membrane integrity showed greater motility [60,86][60][86].
The scientific work on the role of melatonin in the mitigation of oxidative damage, mostly concerns humans and fewer farm animals. Significant progress has been made in understanding the action of melatonin against oxidative damage caused by cryopreservation [38]. In studies of farm animals, the available data focus on the positive effects of melatonin on sperm quality indicators but without clearly identifying the mechanisms by which it acts. It would therefore be crucial to further investigate these mechanisms in both fresh and frozen sperm, especially in sheep, goats, and pigs [38].
