Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Amina Yu and Version 2 by Yvaine Wei.
Using the biological approach of halotolerant plant growth-promoting bacteria (HT-PGPB) as bio-inoculants provides a promising crop enhancement strategy. HT-PGPB has been proven capable of forming a symbiotic relationship with the host plant by instilling induced salinity tolerance (IST) and multiple plant growth-promoting traits (PGP).
- salinity issues
- halophytes
- HT-PGPB
- crop improvements
- rice
1. Introduction
Generally, soil salinization occurs by the localised increment of soluble salt in the surface layer of the soil, which leads to increased soil electrical conductivity (exceeding 4 dS/m) and salinity level. It also implies soils where natural leaching is no longer sufficient to remove the excess salts from the soil profile [1]. The process of land salinization can be classified into primary and secondary salinization. Primary salinization is due to natural causes and mainly originates from two sources, the weathering mineral rocks of the lithosphere and salts from seawater [2][3]. On the other hand, anthropogenic activities such as agricultural use and rapid urbanisation are responsible for secondary salinization [4].
The varying degree of soil salinity issues significantly impacts the agricultural industries by reducing the economic returns of cultivated land, leading the land to be barren and ultimately to mass land abandonment problems [5]. Hence, saline soil-based cultivation may be an alternate solution to mitigate such issues and cope with the ever-expanding salinity problems, and salt-tolerant crops are the key to this strategy. Previous development trials on salinity and water-deficit stress-tolerant crops were solely through selective breeding and high-tech genetic engineering [6]. Nonetheless, the progress of successful outcomes is mediocre, as proven by the limited number of effective salinity-tolerant genotypes made available so far [7].
Therefore, the biotechnological approach of utilizing beneficial bacteria to improve soil health is vital to ensure global food sustainability. In the natural environment, the interactions of intracellular and extracellular microorganisms are crucial in sustaining and promoting plant growth. Accordingly, multiple types of research and experiments have been undertaken over the years on such microbes, which have proven that these beneficial microbes do indeed qualify to act as bio-fertilizers, bio-stimulants, as well as bio-pesticides.
2. Salinization Effects on Plants
A salinized soil is defined as soil with electrical conductivity (EC) value of or exceeding 4 dS/m (40 mM sodium chloride, NaCl) in the root zone at 25 °C, with 15% exchangeable sodium, and a pH value less than 8.5 [8][9]. Various soluble salt ions contribute to soil salinization, and the varying concentration of these soluble salts depends on soil traits and the salinization process [4][10][11]. Despite that, sodium ion (Na+) and chloride (Cl−) are the most prominent and showed the most phytotoxicities [8][12]. Another variant of soil salinity is soil alkalinity. It occurs when the soil is primarily saturated in sodium carbonate, causing the soil pH to rise, and its effects are more devastating than normal salinized soils [13]. Salinized soils are notoriously known to restrain salt intolerant plant growth worldwide by inducing salinity stress. This abiotic factor significantly affects almost every agricultural development aspect, including lowered productivity and yield potential, disrupting local ecological balance, impairing economic returns, and the precursor of soil degradations and erosions [14]. Typically, the adverse effects of salinity stress are depicted as two consecutive stages. Firstly, the excessive presence of salt reservoirs in the root zones will disrupt the regular water uptakes due to the altered osmotic pressure. This is known as the water-deficit effects of salinity stress, and the symptoms shown include stunted growth and developments due to reduced cell division and differentiation. Moreover, cell deaths will occur due to osmotic stress if there are excessive accumulations of salt ions in the cell walls [15].3. Salinity Alleviation Attempts
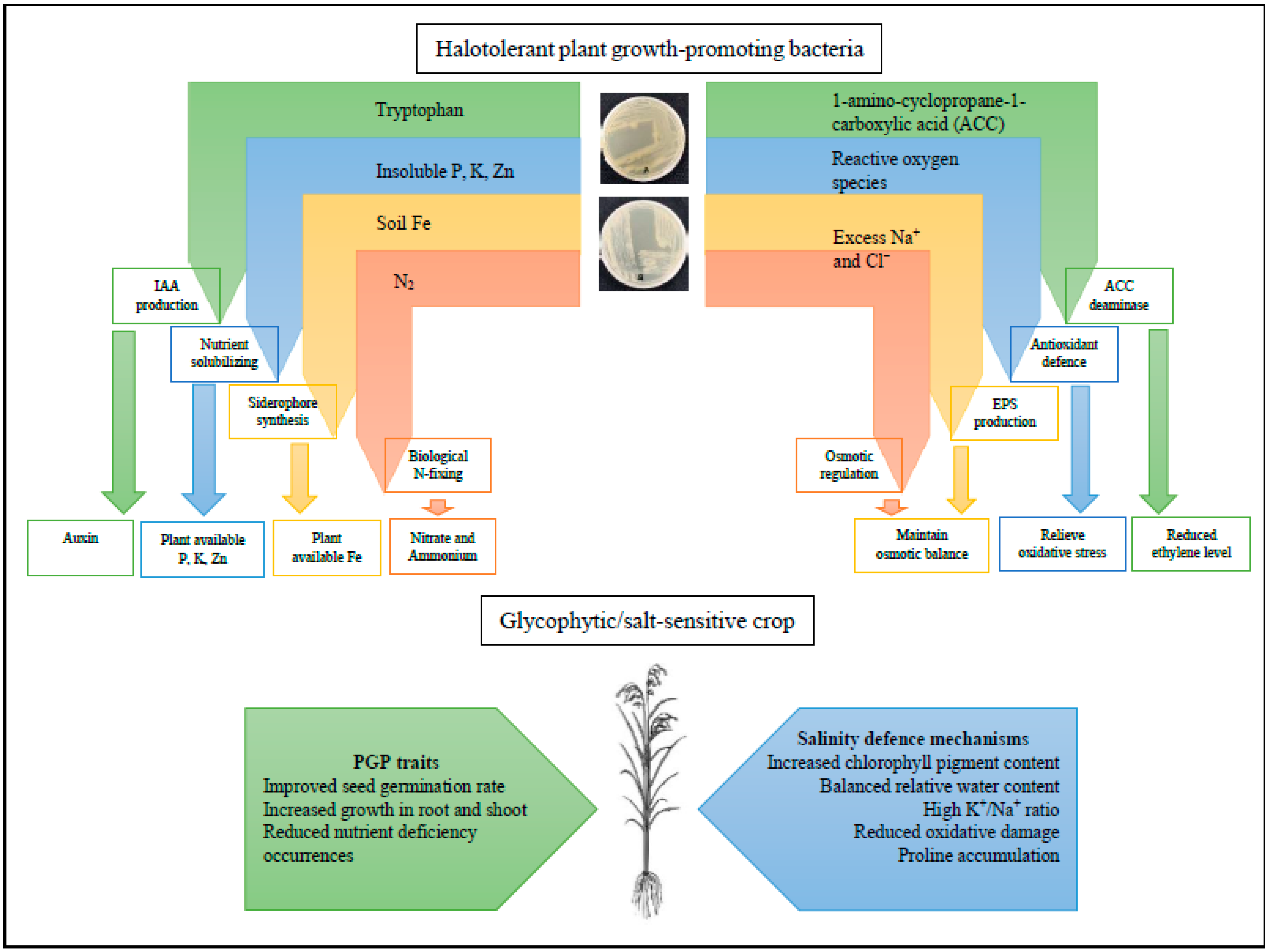
The standard methods on soil reclamations comprise of physical means (such as breaking down and disturbing the soil surface layer, scraping away the soil surface salt build-ups, and flooding and leaching), chemical amendments (including gypsum, calcium chloride, and lime), and some agricultural managements such as proper soil amendments, irrigation methods, doing crop rotations, intercropping, or precision farming [15][16][17][18]. Apart from soil reclamations, crop modifications via traditional selective breeding, primming agent applications, genetic engineering, and induced mutant breeding were also carried out by researchers aiming to augment crops with new potential qualities, such as heightened salt tolerance [19][20][21]. However, it has proven to be a difficult task as salinity stress is complex. It affects multiple facets of plant physiology, not to mention the time, effort, and costs needed [22]. Ironically, to counteract the low productivity and yield of salt-sensitive crops such as rice on salinized soils, farmers usually opted to apply chemical fertilizer to mitigate the problems because of convenience. Furthermore, due to the vulnerability of salinity-stressed plants to pests and disease, excessive usage of pesticides is usually involved. These temporary solutions will eventually lead to even more build-ups of soluble salts from the chemical amendments, hastening or worsening the salinity problems [23][24] Moreover, some pesticides such as parathion can attain further stability and become more structurally non-degradable under saline conditions, causing long-term soil contamination [25]. Hence, the biotechnological approach of bio-inoculants, namely HT-PGPB, is demonstrated to be an effective alternate measure of mitigating salinity stress effects and overall crop improvements [26]. Naturally, these beneficial microbes possess considerable growth-promoting and salinity-defensive traits that improve the inoculated host’s tolerance towards salinity and even biotic stresses [27]. The HT-PGPB alleviates salinity stress through various mechanisms evoking multipronged physiological, biochemical, and molecular plant responses to promote plant growth, as shown in Figure 1.
Figure 1. The roles of potential halotolerant plant growth-promoting bacteria (HT-PGPB) with plant growth (PGP) and salinity defense mechanisms to alleviate salinity stress in glycophytic crops. The depicted plates are potential HT-PGPB (rhizobacteria and endophytes, respectively) isolated from native halophytes.
4. Halophytes and Halotolerant Plant Growth-Promoting Bacteria (HT-PGPB)
The salinity tolerance revolves around a complex of physiological, morphological, and molecular processes, alongside the determined factors of intensity and duration of exposures. To illustrate, the capability of a plant to limit toxic salt intake and accumulations, regulate cells’ ionic and osmotic balance, and control leaf development and the onset of senescence are examples of salinity-tolerant mechanisms [28]. For instance, halophytes are plants that thrive and complete their life cycle in an environment with elevated salinity (up to 1 M sodium chloride, NaCl) without suffering from any sign of salinity distress [24][29]. Additionally, some coastal region halophytes can even reach optimum growth at 5 to 25% salinity level of standard seawater. Nevertheless, their growths can still be affected by either absence or over accumulations of salt [30].
Consequently, halophytes are equipped with diverse natural strategies to combat the adverse effects of excessive salt accumulations. The halophyte’s primary mechanisms are involved in their morphological (thickened leaves, foliar salt glands), physiological (salt elimination mechanisms), biochemical (accumulation or synthesizing compatible solutes), and salt-responsive genet characteristics such as senescence-associated genes (SAG), homeostasis genes (SOS), molecular chaperones (HSP), and dehydration-related transcription factors (DREB) [31].
5. Plant Growth-Promoting Mechanisms by HT-PGPB
5.1. HT-PGPB Mediated Soil Nutrient Bio-Availabilities
Deficiencies of plant-available soil nutrients are critical, especially for plants under salinity stress. The physicochemical properties of salinized soil reduces various nutrient bioavailability, and thus symptoms of deficiencies are common among salinity-stressed plants [32][33][34]. To prevent such problems, temporary solutions of continuous and unregulated applications of chemical fertilizer are practiced, which will lead to environmental hazards, soil health deterioration and, ironically, further increase the soil salinity level [35]. On that account, the applications of beneficial microorganisms to increase nutrient bio-availability rather than chemical amendments are stated to be more sustainable and greener solutions for crop production systems.
Nitrogen (N) is one of the macronutrients that plays multiple essential roles in plant growth and productivity, mainly involved in the cellular synthesis of proteins, enzymes, DNA, and RNA. Although there is an abundance of atmospheric N (78% of the atmosphere), plants cannot utilize it in such form. Therefore, bacteria with nitrogen-fixing ability are crucial in metabolizing and conversion of atmospheric N into plant-available ammonium (NH4+) and nitrate (NO3−) forms [36]. Moreover, the high Cl− uptake from the saline soil significantly reduced the absorption of nitrogen and sulfur [32].
Application of HT-PGPB with phosphate-solubilizing capability, also known as phosphate-solubilizing bacteria (PSB), can significantly aid in increasing bio-availabilities of soil P [37]. It is proven that the application of PSB in a field can reduce the required P-type fertilizers usage by approximately 50 % without affecting the final yield [38].
The mechanisms of PSB involve mineralizing organic P (tricalcium phosphate, aluminium phosphate, rock phosphate, etc.) in the soil by solubilizing their complex structure, releasing P as inorganic form. The multiple solubilizing mechanisms that PSB utilizes include chelation, acidification, ion-exchange reactions, and organic acid productions [39]. Primarily, the PSB secretes low molecular organic acids, which are the by-product of metabolized sugars from the root exudates [40]. Examples of these low molecular weight organic acids are gluconic acid, citric acid, succinic acid, propionic acid, and lactic acid [41]. These acids act as chelators of divalent calcium cations (Ca2+), thus releasing the insoluble bonded P ions in inorganic forms. The secreted acids also help lower the surrounding soil pH to maintain the mobilisation of available P [42]. Besides, some PSB directly lowers soil pH by directly releasing hydrogen ions. Furthermore, PSB also synthesis phosphatases or phytase enzymes that hydrolyses organic P in the soil [43]. Additionally, HT-PGPB possesses the ability to solubilize organic potassium (K) and zinc (Zn) in the soil similarly via organic acid secretions and altering the surrounding soil pH [11][44][45].
5.2. HT-PGPB Mediated Indole-3-Acetic Acid (IAA) Production
Generation of phytohormones is not exclusive to plants as plant-accompanied HT-PGPB possess the ability to alter plant phytohormone levels by producing exogenously [46]. Under normal circumstances, IAA is involved in cellular division and enlargement, translating into seed germination, shoot growth, and root initiation. However, plants will generally suffer from a drastic drop in IAA concentrations under salinity stress. For example, IAA cumulation of tomato is affected at 100 mM NaCl and further diminishes at 300 mM and above [47]. Consequently, such a decline will reduce germination rate, retarded root formations, and stunt plant growth and development [48]. Rice plants are no exceptions, as the stated symptoms are also shown on studied rice seedlings under simulated saline conditions. Therefore, the application of HT-PGPB with IAA synthesizing ability can mitigate the low cumulation by producing exogenously to be taken up by the host plant [49]. The production of exogenous bacterial IAA involved utilizing L-tryptophan (L-Trp amino) secreted among root exudates or decaying cells as precursors [50]. There are currently five L-tryptophan-dependent pathways documented. The biosynthesis process is subject to root exudate contents and environmental factors such as soil salinity level, pH, and osmotic or matrix stress [51].6. Salinity Mitigating Mechanisms by HT-PGPB
6.1. HT-PGPB Modulations of Stress Ethylene
Ethylene is one of the stress-signalling phytohormones that, at a low amount, initiates various response mechanisms to counteract biotic or abiotic stress effects, including salinity stress [52]. However, prolonged and severe stress will lead to the second peak synthesis of ethylene, also known as excessive or stress ethylene. The deleterious effect of stress ethylene includes stunted root development, which subsequently impairs root functioning, reduces vegetative growths, and eventually affects productivity and yields [53][54].
Ethylene synthesis in plants begins with its precursor, 1-amino-cyclopropane-1-carboxylic acid (ACC) that is converted by the enzyme ACC oxidase to the final product of ethylene. Due to that, some HT-PGPB with the possession of acdS genes can metabolise ACC, which is secreted among root exudates into ammonia and α-ketobutyrate via the production of enzyme ACC deaminase (ACC-D). These bacteria then utilize the end products as their unique C and N source [55].
6.2. HT-PGPB Modulations of Exopolysaccharides
Under undesirable conditions, soil bacteria secrete polysaccharides to promote adherence to available environmental surfaces and form an organo-mineral sheath, also known as a biofilm. These polysaccharides consist of complex mixtures of polymers with high molecular weight (MW ≥ 10,000) that provide both physical and functional protection against desiccating conditions and constraints of high salinity [56]. These extracellular polysaccharides are vital components of biofilm formations and effectively alleviate salinity stress [57]. Furthermore, a rhizosheath is a form of EPS that layers around the root surface. The rhizosheath serves as a barrier against toxic ions, site of nutrient cycling, cation uptakes, symbiotic reactions, and maintaining osmotic equilibrium [58][59]. EPS productions are typical for bacteria under heavy metal and high salinity stresses [60]. However, high-quality EPS can only be produced by halo- or drought-tolerant rhizobacteria to tolerate and survive under harsh conditions [61].6.3. HT-PGPB Modulation of Antioxidant Defences
Oxidative stress is the follow-up secondary effect of salinity stress. The reduced photosynthetic activity caused by salinity stress effects led to the over-reduction of photosynthetic electrons, thus generating reactive oxygen species (ROS) [62][63]. The generated ROS such as hydrogen peroxide (H2O2), superoxide ions (O2−), singlet oxygen (1O2), and hydroxyl radical (OH−) are toxic molecules that are highly reactive [64]. They tend to cause oxidative damage to plant biomolecules such as membranous lipids and proteins and nucleic acids, which results in disrupted metabolic enzyme activities and cell homeostasis [57][65][66]. Kim et al. reported that membrane deterioration due to ROS, which leads to cellular toxicity, has been discovered in salinity-stressed rice, citrus, and tomato plants [67].6.4. HT-PGPB Modulations of Osmotic Balance Regulation
Homeostasis of ion concentration is crucial in plant cells under salinity stress. The excessive uptake of toxic salt ions like Na+ and Cl− can upset the balances of other vital ion intakes, namely vital potassium ion (K+) accumulations. Choudhary stated that the chemical physiological properties of K+ are highly similar to Na+, meaning that Na+ can compete with K+ binding sites of various crucial enzymatic reactions, protein synthesis, and ribosome functions. By improving the plant’s selective uptake of K+, the accumulation of toxic Na+ ions diminish significantly, thereby maintaining an optimal high K+/Na+ ratio [53]. The high K+/Na+ ratio is able to suppress the osmotic stress of the plant by preserving higher stomatal conductance as well as photosynthetic processes [47].7. Future Developments and Challenges
Overall, the plant growth-promoting traits and salinity defence conferred by inoculated HT-PGPB have been proven capable of instilling IST among targeted glycophytic crops. In some cases, the isolated HT-PGPB has been proven to be non-specific to the original host or source. Notwithstanding this, there are observed cases when a control HT-PGPB has no significant effect on other crops, showing that certain factors such as rhizospheric environment, root exudate content, or root morphology need to be fulfilled before successful colonization, and thus inoculation is achievable [68]. Kamilova and others discovered that the disparity in the efficacy of an HT-PGPB could also be due to the multiplicity of climatic and environmental factors that vary from one farm to another or even within fields [69]. Hence, the discoveries of more rice plant-compatible HT-PGPB from native halophyte plants as bio-inoculants can potentially provide breakthroughs in future sustainable agriculture prospects and mitigate the ongoing salinization issues. Lastly, the guideline states that an ideal PGPB should be an aggressive colonizer, possess multiple PGP traits with non-host specificity, not be antagonistic to local microbes, be isolated from indigenous salt-affected soils, and be compatible with inoculant carriers [70]. Moreover, the PGPB should not benefit nearby wild or invasive plants and be stabilized enough to not further genetically evolve with undesirable traits [71]. Thus, the success of HT-PGPB helps to determine an alternative strategy of saline soil-based rice cultivation, aiming to preserve future food security.8. Conclusions
Soil salinity has become a menace to soil that poses a significant threat to worldwide agricultural industries, and the go-to unsustainable chemical treatments exacerbate the situation. Moreover, Malaysia’s rice-producing sectors are at a stagnant level, with land salinization as part of the problem while waiting for any scientific breakthroughs. Thus, the exploration of HT-PGPB as bio-inoculants would make significant advancements toward sustainable agriculture. The benefits of HT-PGPB have gained great interest in past decades, with multiple studies having demonstrated the capabilities, mechanisms involved, and potentials of HT-PGPB as an optimal and eco-friendly alternative to remediate salinity stress and growth enhancements among salt-sensitive crops. Even so, further in-depth research is required to elucidate and illustrate the varying plant–microbe interactions under complex stresses elicited by soil salinity. A better understanding could set the plausible prospects of advanced saline soil-based cultivation, developing a novel market-available HT-PGPB-based biofertilizer, or even genetically engineering HT-PGPB to obtain the ideal strains.References
- Schofield, R.V.; Kirkby, M. Application of salinization indicators and initial development of potential global soil salinization scenario under climatic change. Glob. Biogeochem. Cycles 2013, 17, 1–13.
- Rengasamy, P. Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 2010, 37, 613–620.
- Paul, D.; Lade, H. Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: A review. Agron. Sustain. Dev. 2014, 34, 737–752.
- Rengasamy, P. World salinization with emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023.
- Yasin, N.A.; Akram, W.; Khan, W.U.; Ahmad, S.R.; Ahmad, A.; Ali, A. Halotolerant plant growth promoting rhizobacteria modulate gene expression and osmolyte production to improve salinity tolerance and growth in Capsicum annum L. Environ. Sci. Pollut. Res. 2018, 25, 236–250.
- Etesami, H.; Adl, S.M. Can interaction between silicon and non–rhizobial bacteria benefit in improving nodulation and nitrogen fixation in salinity-stressed legumes? A review. Rhizosphere 2020, 15, 100229.
- Golan, Y.; Shirron, N.; Avni, A.; Shmoish, M.; Gepstein, S. Cytokinin induce transcriptional reprograming and improve Arabidopsis plant performance under drought and salt stress conditions. Front. Environ. Sci. 2016, 4, 63.
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, M.R. Gene expression profiling of plants under salt stress. Crit. Rev. Plant Sci. 2011, 30, 435–458.
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; Agriculture Handbook No. 60; Government Printing Office: Washington, DC, USA, 1954; pp. 1–2.
- Sha Valli Khan, P.S.; Nagamallaiah, G.V.; Dhanunjay Rao, M.; Sergeant, K.; Hausman, J.F. Abiotic stress tolerance in plants: Insights from proteomics. Emerg. Technol. Manag. Crop Stress Toler. 2014, 2, 23–68.
- Vaishnav, A.; Varma, A.; Tuteja, N.; Choudhary, D.K. PGPR-mediated amelioration of crops under salt stress. In Plant-Microbe Interaction: An Approach to Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; Volume 10, pp. 205–226.
- Tavakkoli, E.; Fatehi, F.; Coventry, S.; Rengasamy, P.; McDonald, G.K. Additive effects of Na+ and Cl− ions on barley growth under salinity stress. J. Exp. Bot. 2011, 62, 2189–2203.
- Day, A.D.; Ludeke, K.L. Soil Alkalinity. In Adaptations of Desert Organisms; Springer: Berlin/Heidelberg, Germany, 1993; pp. 35–37.
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549.
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250.
- Raychev, T.; Popandova, S.; Jozefaciuk, G.; Hajnos, M.; Sokolowska, Z. Physicochemical reclamation of saline soils using coal powder. Int. Agrophys. 2001, 15, 51–54.
- Cucci, G.; Lacolla, G.; Pallara, M.; Laviano, R. Reclamation of saline and saline-sodic soils using gypsum and leaching water. Afr. J. Agric. Res. 2012, 7, 6508–6514.
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131.
- Aziz, A. Biotechnological tools and trends for production and rice quality improvement: Agrobacterium-mediated genetic transformation practices for improvement of rice quality and production. In Rice Science: Biotechnology and Molecular Advancements; CRC Press: Boca Raton, FL, USA, 2018.
- Aziz, A.; Verma, D.K.; Srivastav, P.P.; Nadaf, A.B. Role of genetic engineering and biotechnology as a molecular advance tool and trend in quality improvement of rice crop. In Rice Science: Biotechnology and Molecular Advancements; CRC Press: Boca Raton, FL, USA, 2018; p. 208.
- Arora, N.K. Impact of climate change on agriculture production and its sustainable solutions. Environ. Sustain. 2019, 2, 95–96.
- Fita, A.; Rodríguez-Burruezo, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Breeding and domesticating crops adapted to drought and salinity: A new paradigm for increasing food production. Front. Plant Sci. 2015, 6, 978.
- Egamberdieva, D.; Lugtenberg, B. Use of Plant Growth-Promoting Rhizobacteria to Alleviate Salinity Stress in Plants; Springer: Berlin/Heidelberg, Germany, 2014; Volume 1, pp. 73–96.
- Cheeseman, J.M. The evolution of halophytes, glycophytes and crops, and its implications for food security under saline conditions. New Phytol. 2015, 206, 557–570.
- Reddy, B.R.; Sethunathan, N. Salinity and the persistence of parathion in flooded soil. Soil Biol. Biochem. 1985, 17, 235–239.
- Ahanger, M.A.; Tyagi, S.R.; Wani, M.R.; Ahmad, P. Drought tolerance: Role of organic osmolytes, growth regulators, and mineral nutrients. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Springer: Berlin/Heidelberg, Germany, 2014; pp. 25–55.
- Porcel, R.; Redondo-Gómez, S.; Mateos-Naranjo, E.; Aroca, R.; Garcia, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis ameliorates the optimum quantum yield of photosystem II and reduces non-photochemical quenching in rice plants subjected to salt stress. J. Plant Physiol. 2015, 185, 75–83.
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663.
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963.
- Ball, M.C.; Pidsley, S.M. Growth responses to salinity in relation to distribution of two mangrove species, Sonneratia alba and S. lanceolata, in northern Australia. Funct. Ecol. 1995, 9, 77–85.
- Etesami, H.; Beattie, G.A. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 2018, 9, 148.
- Cheng-Song, X.I.N.; He-Zhong, D.; Zhen, L.; Wei, T.; Zhang, D.M.; Wei-Jiang, L.I.; Kong, X.Q. Effects of N, P, and K fertilizer application on cotton growing in saline soil in yellow river delta. Acta Agron. Sin. 2010, 36, 1698–1706.
- Thomine, S.; Lanquar, V. Iron transport and signalling in plants. In Transporters and Pumps in Plant Signalling; Springer: Berlin/Heidelberg, Germany, 2011; Volume 7, pp. 99–131.
- Etesami, H.; Alikhani, H.A. Halotolerant plant growth-promoting fungi and bacteria as an alternative strategy for improving nutrient availability to salinity-stressed crop plants. In Saline Soil-Based Agriculture by Halotolerant Microorganisms; Springer: Berlin/Heidelberg, Germany, 2019; pp. 103–146.
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148.
- Raymond, J.; Siefert, J.L.; Staples, C.R.; Blankenship, R.E. The natural history of nitrogen fixation. Mol. Biol. Evol. 2004, 21, 541–554.
- Safdarian, M.; Askari, H.; Nematzadeh, G.; Sofo, A. Halophile plant growth-promoting rhizobacteria induce salt tolerance traits in wheat seedlings (Triticum aestivum L.). Pedosphere, 2020; 30, 684–693.
- Etesami, H. Enhanced phosphorus fertilizer use efficiency with microorganisms. In Nutrient Dynamics for Sustainable Crop Production; Springer: Berlin/Heidelberg, Germany, 2020; pp. 215–245.
- Parmar, P.; Sindhu, S.S. Potassium solubilization by rhizosphere bacteria: Influence of nutritional and environmental conditions. J. Microbiol. Res. 2013, 3, 25–31.
- Goswami, D.; Pithwa, S.; Dhandhukia, P.; Thakker, J.N. Delineating Kocuria turfanensis 2M4 as a credible PGPR: A novel IAA-producing bacteria isolated from saline desert. J. Plant Interact. 2014, 9, 566–576.
- Choudhary, D.K. Microbial rescue to plant under habitat-imposed abiotic and biotic stresses. Appl. Microbiol. Biotechnol. 2012, 96, 1137–1155.
- Patel, K.; Goswami, D.; Dhandhukia, P.; Thakker, J. Techniques to study microbial phytohormones. In Bacterial Metabolites in Sustainable Agroecosystem; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–27.
- Khan, M.S.; Ahmad, E.; Zaidi, A.; Oves, M. Functional aspect of phosphate-solubilizing bacteria: Importance in crop production. In Bacteria in Agrobiology: Crop Productivity; Springer: Berlin/Heidelberg, Germany, 2013; Volume 10, pp. 237–263.
- Tariq, M.; Hameed, S.; Malik, K.A.; Hafeez, F.Y. Plant root associated bacteria for zinc mobilization in rice. Pak. J. Bot. 2007, 39, 245–253.
- Richardson, A.E.; Hocking, P.J.; Simpson, R.J.; George, T.S. Plant mechanisms to optimise access to soil phosphorus. Crop Pasture Sci. 2009, 60, 124–143.
- Chaiharn, M.; Lumyong, S. Screening and optimization of indole-3-acetic acid production and phosphate solubilization from rhizobacteria aimed at improving plant growth. Curr. Microbiol. 2011, 62, 173–181.
- Albacete, A.; Ghanem, M.E.; Martínez-Andújar, C.; Acosta, M.; Sánchez-Bravo, J.; Martínez, V.; Lutts, S.; Dodd, I.C.; Pérez-Alfocea, F. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 2008, 59, 4119–4131.
- Perez-Alfocea, F.; Albacete, A.; Ghanem, M.E.; Dodd, I.C. Hormonal regulation of source-sink relations to maintain crop productivity under salinity: A case study of root-to-shoot signalling in tomato. Funct. Plant Biol. 2010, 37, 592–603.
- Jha, C.K.; Saraf, M. Plant growth promoting rhizobacteria (PGPR): A review. J. Agril. Res. Dev. 2015, 5, 108–119.
- Ahmad, F.; Ahmad, I.; Khan, M.S. Indole acetic acid production by the indigenous isolates of azotobacter and fluorescent pseudomonas in the presence and absence of tryptophan. Turk. J. Biol. 2005, 29, 29–34.
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signalling. FEMS Microbiol. Rev. 2007, 31, 425–448.
- Bhattacharyya, P.; Jha, D. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350.
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246.
- Siddikee, M.A.; Glick, B.R.; Chauhan, P.S.; Jong-Yim, W.; Sa, T. Enhancement of growth and salt tolerance of red pepper seedlings (Capsicum annuum L.) by regulating stress ethylene synthesis with halotolerant bacteria containing 1-aminocyclopropane-1-carboxylic acid deaminase activity. Plant Physiol. Biochem. 2011, 49, 427–434.
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39.
- Poli, A.; Anzelmo, G.; Nicolaus, B. Bacterial exopolysaccharides from extreme marine habitats: Production, characterization and biological activities. Mar. Drugs 2010, 8, 1779–1802.
- Upadhyay, S.K.; Singh, J.S.; Singh, D.P. Exopolysaccharide-producing plant growth promoting rhizobacteria under salinity condition. Pedosphere 2011, 21, 214–222.
- Naseem, H.; Ahsan, M.; Shahid, M.A.; Khan, N. Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J. Basic Microbiol. 2018, 58, 1009–1022.
- Costa, O.Y.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1636.
- Nunkaew, T.; Kantachote, D.; Nitoda, T.; Kanzaki, H.; Ritchie, R.J. Characterization of exopolymeric substances from selected Rhodopseudomonas palustris strains and their ability to adsorb sodium ions. Carbohydr. Polym. 2015, 115, 334–341.
- Ashraf, M.; Hasnain, S.; Berge, O.; Mahmood, T. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol. Fertil. Soils 2004, 40, 157–162.
- Hichem, H.; Mounir, D.; Naceur, E.A. Differential responses of two maize (Zea mays L.) varieties to salt stress: Changes on polyphenols composition of foliage and oxidative damages. Ind. Crop Prod. 2009, 30, 144–151.
- Scandalios, J.G. The rise of ROS. Trends Biochem. Sci. 2002, 27, 483–486.
- Parvaiz, A.; Khalid, U.R.H.; Ashwani, K.; Muhammad, A.; Nudrat, A.A. Salt-induced changes in photosynthetic activity and oxidative defence system of three cultivars of mustard (Brassica juncea L.). Afr. J. Biotechnol. 2012, 11, 2694–2703.
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and anti-oxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037.
- Rasool, S.; Ahmad, A.; Siddiqi, T.O.; Ahmad, P. Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol. Plant. 2013, 35, 1039–1050.
- Kim, S.Y.; Lim, J.H.; Park, M.R.; Kim, Y.J.; Park, T.I.; Seo, Y.W.; Choi, K.G.; Yun, S.G. Enhanced antioxidant enzymes are associated with reduced hydrogen peroxide in barley roots under saline stress. BMB Rep. 2005, 38, 218–224.
- Kearl, J.; McNary, C.; Lowman, J.S.; Mei, C.; Aanderud, Z.T.; Smith, S.T.; West, J.; Colton, E.; Hamson, M.; Nielsen, B.L. Salt-tolerant halophyte rhizosphere bacteria stimulate growth of alfalfa in salty soil. Front. Microbiol. 2019, 10, 1849.
- Kamilova, F.; Okon, Y.; de Weert, S.; Hora, K. Commercialization of microbes: Manufacturing, inoculation, best practice for objective field testing, and registration. In Principles of Plant-Microbe Interactions; Springer: Berlin/Heidelberg, Germany, 2015; Volume 33, pp. 319–327.
- Paul, D.; Nair, S. Stress adaptations in a plant growth promoting rhizobacterium (PGPR) with increasing salinity in the coastal agricultural soils. J. Basic Microbiol. 2018, 48, 378–384.
- Bueno Batista, M.; Dixon, R. Manipulating nitrogen regulation in diazotrophic bacteria for agronomic benefit. Biochem. Soc. Trans. 2019, 47, 603–614.
More
