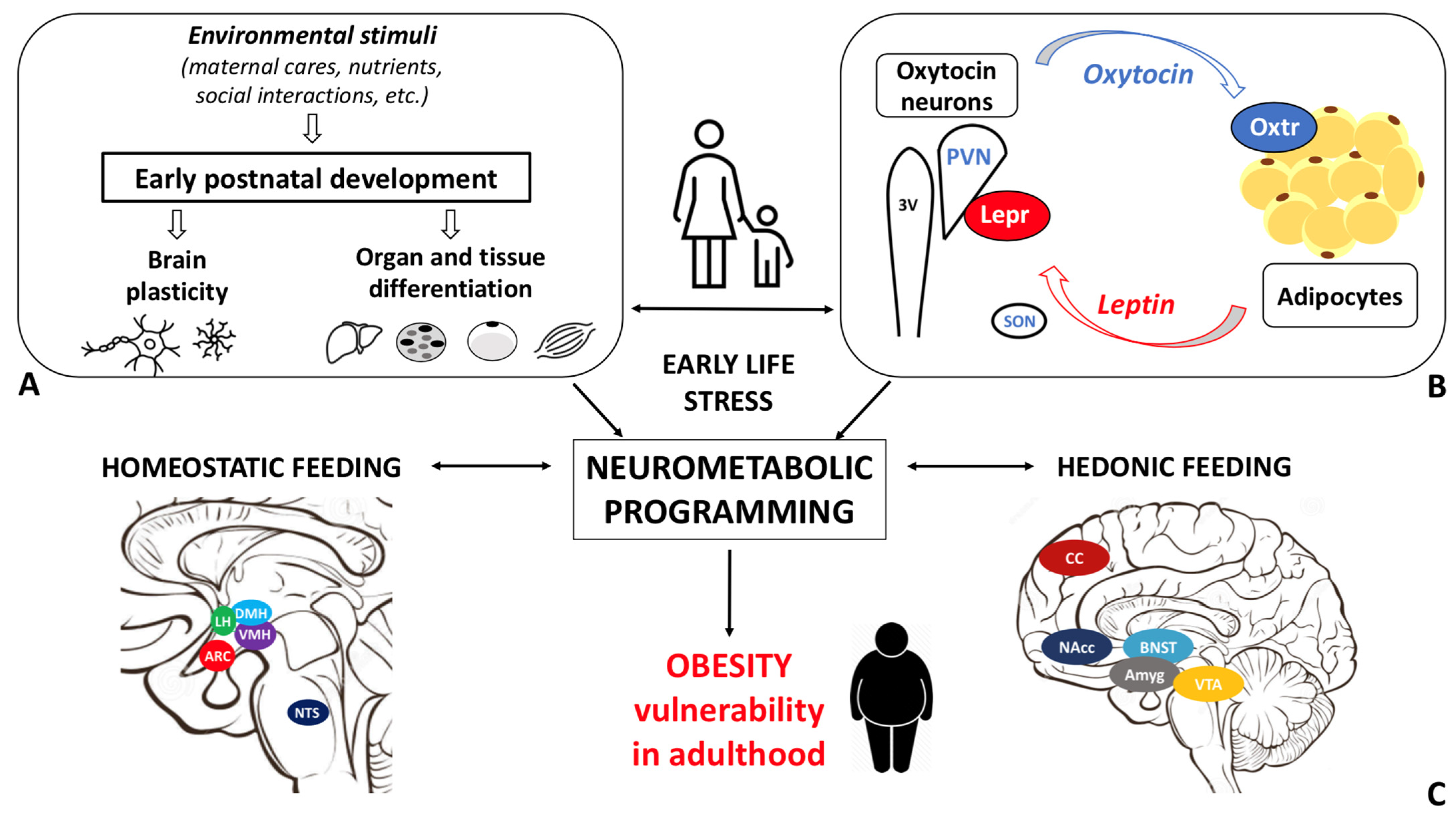
Obesity disease results from a dysfunctional modulation of the energy balance whose master regulator is the central nervous system. Consistently, the prevalence of obesity is higher among individuals who experienced early life stress (ELS). Oxytocin, a hypothalamic neurohormone, regulates the energy balance and modulates social, emotional, and eating behaviors, exerting both central and peripheral actions. Oxytocin closely cooperates with leptin in regulating energy homeostasis. Based on the available data, alterations in the oxytocin system may in part mediate the ELS-induced susceptibility to obesity.
- obesity
- early life stress (ELS)
- oxytocin
1. Obesity: Epidemiology, Etiopathophysiology, and Early Development
1. Obesity: epidemiology, etiopathophysiology and early development
The modulation of the energy balance occurs in the central nervous system (CNS)
. The master regulator is the hypothalamus where all signals from other brain areas and periphery are integrated and translated into specific behavioral, autonomic, and endocrine outputs [10][11][12][14]
. The crucial role of the CNS in obesity susceptibility is well documented by recent genome wide association studies that implicate pathways related to synaptic function, extracellular matrix composition, and glutamate signaling
, as well as brain G protein-coupled receptors, as primary in determining BMI variations
.
The maturation of the central neural circuitries involved in energy balance control is not completed at birth but occurs during early life. In mammals, postnatal ages are denoted by critical developmental periods during which organs and neural systems are highly plastic. In this timeframe, adverse nutritional, social, and environmental cues may program body metabolism to maximize energy accrual to face hostile conditions. Consistently, a higher prevalence of obesity among individuals exposed to early life stress (ELS) during both the pre- and postnatal periods is documented
.
2. Early Life Stress
2. Early life stress
3. Oxytocin: The Neuroendocrine Hub of Social Bonding, Stress, Eating Behavior, and Metabolic HealthOxytocin: the neuroendocrine hub of social bonding, stress, eating behavior, and metabolic health
In the study of early adversity determined by abnormal infant care, particularly pertinent is the research on the neurohormone Oxt. Produced by neurons located in the hypothalamic paraventricular (PVN) and supraoptic (SON) nuclei (Figure 1Figure 1 A-E), Oxt plays a pivotal role in the regulation of a variety of behaviors including social, emotional, sexual, eating, and addiction behaviors [2114]. Oxt is produced by both magnocellular and parvocellular neurons: the formers are contained in PVN and SON and mainly project to the neurohypophysis where Oxt is released into the circulation, while the latters, mainly contained in the PVN but also scattered in other hypothalamic and extrahypothalamic areas, project to different hindbrain regions e.g., solitary tract nucleus [3932][4033]. Interestingly, specialized PVN and SON magnocellular Oxt neurons develop axon collaterals projecting to forebrain limbic regions (e.g., prefrontal cortex, nucleus accumbens, anterior and central amygdala, bed nucleus of the stria terminalis (BNST)-BNST-, hippocampus) (Figure 2Figure 2 A–-F). This, a finding hasrevealed only been described in advanced vertebrates and is believed thought to have developed together with the social and emotional behavioral complexity of species.
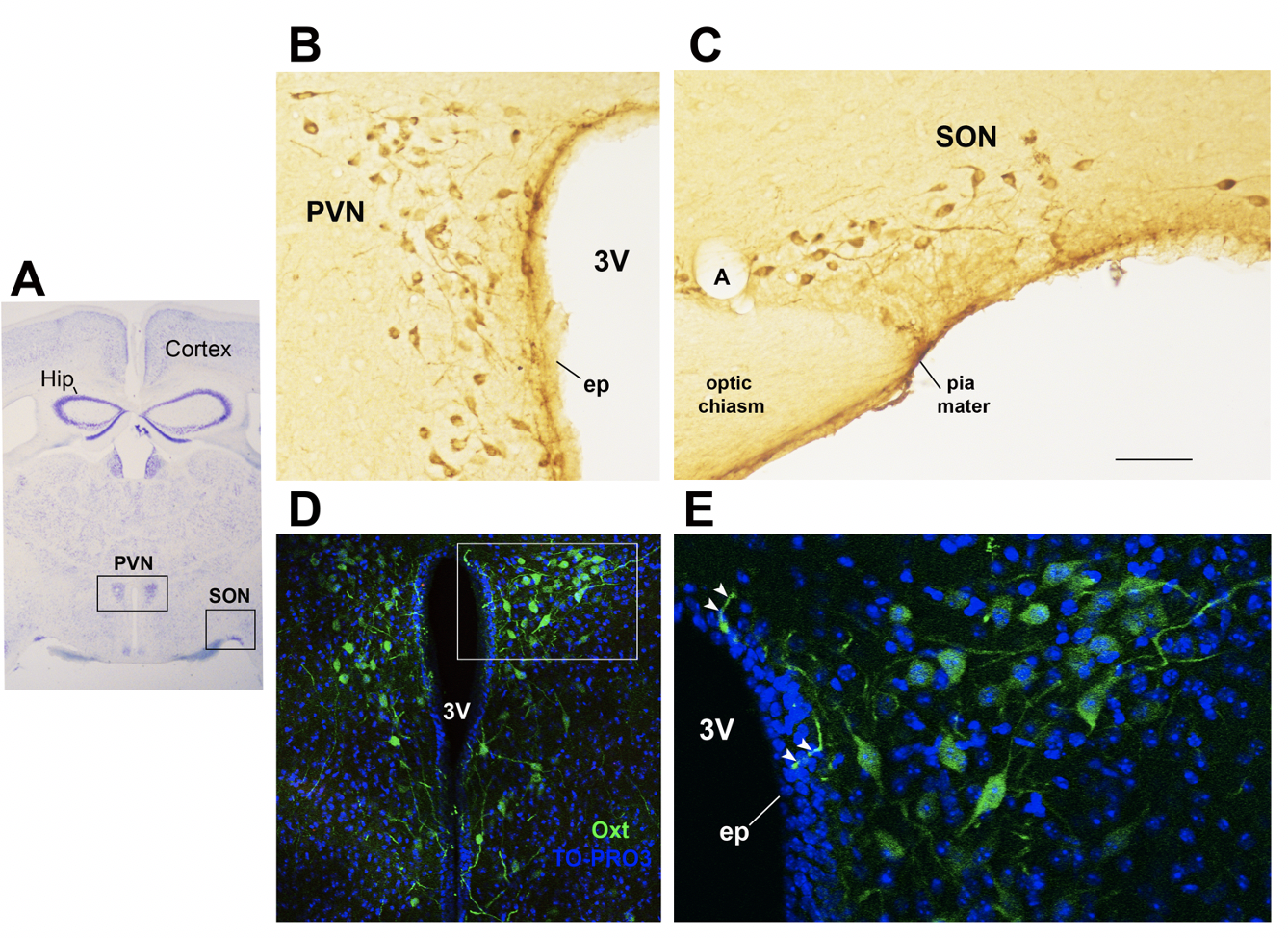
Figure 1. Oxytocinergic neurons in the paraventricular (PVN) and supraoptic (SON) hypothalamic nuclei. (A) Light microscopy (LM): Nissl-stained brain coronal section of the region corresponding to bregma −0.94 mm; PVN: hypothalamic paraventricular nucleus; SON: hypothalamic supraoptic nucleus; Hip: hippocampus. (B) LM: peroxidase immunohistochemistry of oxytocin (Oxt)-positive neurons in proximity of the ependymal layer (ep) of the third ventricle (3 V) in the PVN. (C) Peroxidase immunohistochemistry of Oxt neurons in the SON; A: artery. (D) Double-label confocal microscopy of Oxt neurons (green) and cell nuclei (blue TO-PRO3 staining) in the PVN. Panel (E) is an enlargement of the area framed in (D), showing Oxt-positive neurons and their projections (arrowheads) reaching and contacting the ependymal cells and the cerebrospinal fluid of the third ventricle (3 V). All figures refer to a 6-month-old male C57BL/6 mouse. Bregma reference sections from “The Mouse Brain Atlas”, Paxinos and Franklin (2001). The scale bar is included in C only and corresponds to different μm in each figure as follows: in (A): 1500 μm; in (B,C): 45 μm; in (D): 120 μm; in (E): 40 μm. All figures are original.
Oxt stimulates maternal care, maternal–-infant attachment, and social bonding and can is capable of attenuate theing stress response to stress, anxiety, and depression [21][28]. It , displaying anxiolytic and also has a crucial role in the regulation of the stress system by reducing HPA activity and by supporting the parasympathetic nervoutidepressant properties system [2814][4121][42]. Recent works on the neurobiological basis of attachment, coupled with studies on children adopted from orphanages, suggest that there may be a sensitive period for the development of Ooxt–ytocin-dopamine connections (particularly in the nucleus accumbes of the striatum), which exerts enduring effects on the neurobiology of social relationships [4134], for instance, by strengthencluding their ability to physiologically buffer stress [4235].
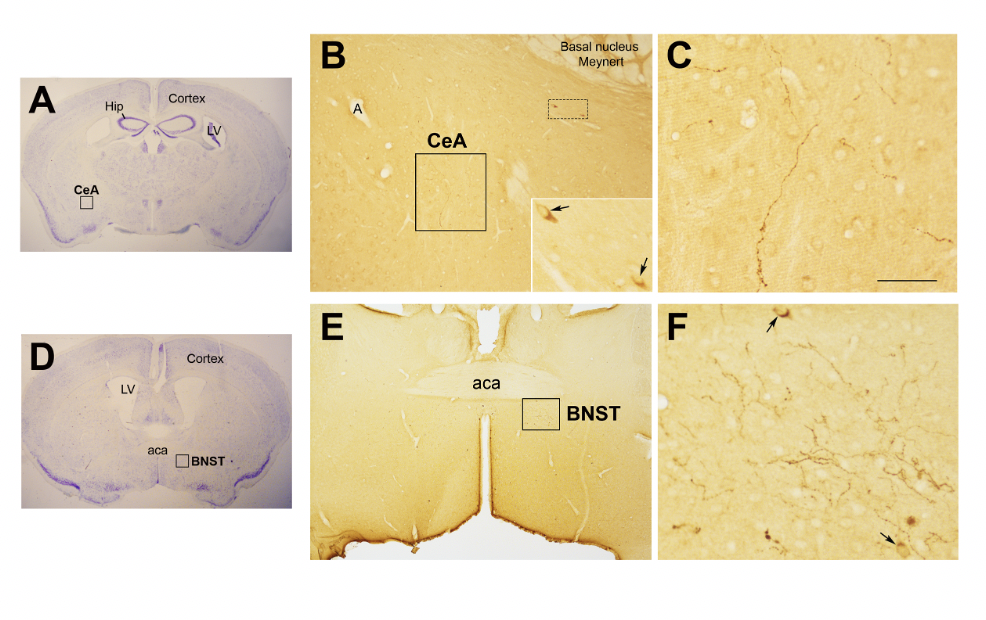
Figure 2. Oxytocinergic projections in the central nuclei of the amygdala (CeA) and in the bed nucleus of the stria terminalis (BNST). (A) Light microscopy (LM): Nissl-stained brain coronal section of bregma −0.94 mm; Hip: hippocampus; LV: lateral ventricle; CeA: central nuclei of the amygdala. (B) LM: peroxidase immunohistochemistry of oxytocin (Oxt), showing oxytocinergic fibers (framed area) and two parvocellular Oxt neurons (dotted framed area) in the CeA. Inset: enlargement of the dotted framed area, where Oxt-positive neurons are indicated by arrows. (C) Enlargement of the area framed in (B), rich in Oxt-positive fibers. (D) LM: Nissl-stained brain coronal section of the bregma 0.02 mm; BNST: bed nucleus of the stria terminalis; aca, anterior commissure. (E) LM: peroxidase immunohistochemistry of oxytocinergic projections in the BNST. (F) Enlargement of the area framed in (E) showing Oxt neurons (arrows) and Oxt projections in the BNST. All figures relate to a 6-month-old male C57BL/6 mouse. Bregma reference sections from “The Mouse Brain Atlas”, Paxinos and Franklin (2001). The scale bar is only specified in C and corresponds to different μm in each figure, as follows: in (A,D): 2300 μm; (B): 200 μm, inset 50 μm; in (C): 50 μm; in (E): 300 μm; in (F): 50 μm. All figures are original.
Interestingly, Oxt also attenuates addictive behaviors and inhibits appetite [4134]. Consistent with these data, there is a mounting body of evidence pointing at Oxt role in promoting weight loss and ameliorating obesity-related metabolic dysfunctions [2922][4336][4437][4538]. Intranasal Oxt administration is currently being tested for the treatment of obesity as this route facilitates an increase in the central concentrations of the nonapeptide through channels surrounding the trigeminal and olfactory nerve fibers [4639][4740][4841]. Considering the complex and multiple functions of Oxt, which affects aspects as diverse as mother–infant bonding, eating behavior, and stress response, its potential role in determining the impact of ELS on eating behavior and metabolic health deserves further investigation.
5. Oxytocin System: Early Development and Impact of Early Life Stress
4. Oxytocin system: early development and impact of ELS
Studies conducted on rodents demonstrated that early adverse experiences could have a profound impact on the way the oxytocin system is shaped. Oxt neurons progressively increase from PND 2 to 21, reaching maturation by the second postnatal week
. On the other side, oxytocin neuron axons reach their targets only at early adulthood, when the Oxt receptor (Oxtr) is already widely expressed in different regions (detectable from embryonic day 14 in females and at PND 2 in males)
A. Importantly, Oxt synthesizing neurons play a fundamental role in teredheir own maturation: local Oxt release activates Oxtr expression and binding ied on Oxt neurons resulting in further Oxt discharge, a phenomenon that evidences how variation in Oxt levels during this period can deeply impact these neurons maturation [14][43][42]. It is possible that postnatal environmesponse to changes in maternal cantal stimuli affects Oxt production, hence the development of the Oxt system, with the final objective to retain only functional pathways necessary to face external conditions. This phenomenon may also explain the interindividual variability in Oxt neurons projection revealed by studies mapping the Oxt system [44]. In addition, different were spatio-temporal Oxtr expression patterns during development have been described in several males and females, suggesting a distinct and sex-specific sensitivity to Oxt levels during critical periods [33].
Alteration in Oxtr expression and binding have been detected in several brain regions in response to variation in maternal care in different animal models [3629][5245]. Maternal high licking and grooming (LG) result in increased Oxt expression at PND13, while low LG leads to reduced Oxtr protein levels and receptor binding in several females’’s brain regions (e.g., PVN, central nucleus of the amygdala) [3629][5246][5345]. FurAlthermore, while one study detecough, Tsuda et al., documented a higher number of Oxt- positive cells in the PVN of adult male mice exposed to MS [5447], other investigations with a similar protocol rudies reported a lower number of these cells in the SON and PVN [42][55][56]. In addfollowition, higher Oxtr expression and a higher number of Oxt projectionsg a similar protocol [48][35][49]. Oxto the basolateral amygdala were described in male mice exposed to MS [56]. E changes in response to early adversity usuare generally results in reducin the direction of blunted circulating Oxt levels [4235]. However, Oxt levels may actually risce in response to a prolonged exposure to adverse stimuli, possibly to protect the system from the harmfuldeleterious effects of stress [4235].
A meta-analyses performed by Ellis and colleagues documented lower plasma OXT levels and reduced or negative response to OXT intranasal administration among individuals who experienced childhood adversity [5750]. In addition, an unsecure attachment style was associated with lower OXTR expression in the peripheral blood mononuclear cells of women [51]. Based on this evidence, early experience shapes the adult Oxt system and manipulation of maternal care from infancy confers enduring changes in the Oxtr expression profile, a phenomenon whose mechanisms and implications are not fully elucidated. In addition, early stress in childhood may be produced by overexposure to blue light of digital device screens affecting sleep patterns in children as young as 2 years old, as discussed recently in [56].
Recent studies documented the association between the alteration in the Oxt system induced by ELS and the development of cardiovascular diseases [52][53][54]. However, little is known about the link between Oxt, ELS, and metabolic health. While data on the relationship between ELS and Oxt and between ELS and metabolic health are scanty, evidence linking ELS to metabolic health, focusing on the Oxt system, is not documented in the literature. Specifically, it is unclear how ELS induces changes in the action of and sensitivity to Oxt and how these changes influence adipose tissue development, energy balance, metabolic health, and feeding behaviors in adulthood. The sex-dependent differences in the Oxt system may affect the adipose proliferative niches (hence, adipose tissue development) and may partly contribute to the distinctive fat mass distribution and susceptibility to obesity comorbidities described in males and females. Furthermore, while the effect of Oxt manipulation in early development has been widely investigated [42], little is known about the function of endogenous Oxt at such a time [23]. Several lines of research have demonstrated how spatio-temporal Oxtr expression varies in response to environmental cues, especially during critical periods. It is widely accepted that Oxtr may be a developmental plasticity gene that acts as a transducer of the social environment to finetune the experience-dependent plasticity of the social brain [33]. Therefore, Oxtr expression and distribution, as opposed to circulating Oxt levels per se, may be of particular significance. As noted above, Oxt influences reward-related behaviors in different ways: while it reduces motivated behavior toward palatable food, it modulates social rewards and attention-orienting responses to external social cues [55]. It is reasonable to hypothesize that dysfunctional social attachment to the parental figure during early development (MS or LN) alters the oxytocinergic system maturation such that the reward response to feeding prevails on the reward response to social cues. This paradigmatic model may be employed not only to study obesity vulnerability, but also psychiatric diseases, such as eating disorders, depression, and addiction. Indeed, the limitations related to the use of animal models for the study of such complex diseases should be acknowledged. Growth in an unpredictable environment, where maternal care (feeding) is not constantly ensured, may result in a neurometabolic programming that maximizes energy accrual and minimizes its waste. As a result, besides the enhanced reward value of food, the ability to store energy to cope with an adverse environment may also be increased. Deeper investigation of the biological underpinnings of ELS-induced metabolic and eating behavior abnormalities is warranted to characterize unexplored mechanisms responsible for obesity and to identify novel preventive and/or therapeutic strategies.
5. Early Life StressS, Oxytocin, Eating Behavior, and Metabolic Health: Future Research Directions
| 1 | Do the Oxt and Leptin systems influence each other’s development? |
| 2 | Is the Oxt–Lep systems interaction impacted by obesity? |
| 3 | Is Oxt the mediator of ELS-induced changes in metabolic health? |
| 4 | What are the consequences of ELS on short- and long-term eating behaviors, such as chow vs. palatable food consumption? |
| 5 | What are the consequences of ELS on total weight changes and metabolic health, such as adipose tissue development and resting energy expenditure? |
| 6 | What are the consequences of ELS on the vulnerability to an obesogenic environment in adulthood, such as adipose tissue dysfunction and metabolic abnormalities? |
References
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. Wigger, D.C.; Groger, N.; Lesse, A.; Krause, S.; Merz, T.; Gundel, H.; Braun, K.; McCook, O.; Radermacher, P.; Bock, J.; et al. Maternal Separation Induces Long-Term Alterations in the Cardiac Oxytocin Receptor and Cystathionine gamma-Lyase Expression in Mice. Oxid. Med. Cell Longev. 2020, 2020, 4309605.
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019, 381, 2440–2450. McCook, O.; Denoix, N.; Radermacher, P.; Waller, C.; Merz, T. H2S and Oxytocin Systems in Early Life Stress and Cardiovascular Disease. J. Clin. Med. 2021, 10, 3484.
- EASO. Eauropean Association for the Study of Obesity. Obesity Statistics. 2020. Available online: https://www.karger.com/Article/FullText/508082 (accessed on 24 January 2022).Szczepanska-Sadowska, E.; Wsol, A.; Cudnoch-Jedrzejewska, A.; Zera, T. Complementary Role of Oxytocin and Vasopressin in Cardiovascular Regulation. Int. J. Mol. Sci. 2021, 22, 11465.
- WHO. WHO European Childhood Obesity Surveillance Initiative (COSI). Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/activities/who-european-childhood-obesity-surveillance-initiative-cosi (accessed on 24 January 2022).Yamamoto, Y.; Cushing, B.S.; Kramer, K.M.; Epperson, P.D.; Hoffman, G.E.; Carter, C.S. Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender-specific manner. Neuroscience 2004, 125, 947–955.
- Geserick, M.; Vogel, M.; Gausche, R.; Lipek, T.; Spielau, U.; Keller, E.; Pfaffle, R.; Kiess, W.; Korner, A. Acceleration of BMI in Early Childhood and Risk of Sustained Obesity. N. Engl. J. Med. 2018, 379, 1303–1312. Baracz, S.J.; Everett, N.A.; Cornish, J.L. The impact of early life stress on the central oxytocin system and susceptibility for drug addiction: Applicability of oxytocin as a pharmacotherapy. Neurosci. Biobehav. Rev. 2020, 110, 114–132.
- WHO. Obesity Report. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 25 January 2021).Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908.
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H.; World Obesity, F. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. Liu, C.M.; Hsu, T.M.; Suarez, A.N.; Subramanian, K.S.; Fatemi, R.A.; Cortella, A.M.; Noble, E.E.; Roitman, M.F.; Kanoski, S.E. Central oxytocin signaling inhibits food reward-motivated behaviors and VTA dopamine responses to food-predictive cues in male rats. Horm. Behav. 2020, 126, 104855.
- Colleluori, G.; Perugini, J.; Barbatelli, G.; Cinti, S. Mammary gland adipocytes in lactation cycle, obesity and breast cancer. Rev. Endocr. Metab. Disord. 2021, 22, 241–255.
- US Government Office for Science. Tackling Obesities: Future Choices—Project Report. 2007. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/287937/07-1184x-tackling-obesities-future-choices-report.pdf (accessed on 7 February 2022).
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. Giordano, A.; Nisoli, E. Neuroendocrinology of Energy Balance. Obesity, Endocrinology. 2018. Available online: https://link.springer.com/referenceworkentry/10.1007/978-3-319-47685-8_4-1?noAccess=true (accessed on 10 February 2022).
- Severi, I.; Fosca, M.; Colleluori, G.; Marini, F.; Imperatori, L.; Senzacqua, M.; Di Vincenzo, A.; Barbatelli, G.; Fiori, F.; Rau, J.V.; et al. High-Fat Diet Impairs Mouse Median Eminence: A Study by Transmission and Scanning Electron Microscopy Coupled with Raman Spectroscopy. Int. J. Mol. Sci. 2021, 22, 8049. Leng, G.; Adan, R.A.H.; Belot, M.; Brunstrom, J.M.; de Graaf, K.; Dickson, S.L.; Hare, T.; Maier, S.; Menzies, J.; Preissl, H.; et al. The determinants of food choice. Proc. Nutr. Soc. 2017, 76, 316–327.
- Colleluori, G.; Villareal, D.T. Aging, obesity, sarcopenia and the effect of diet and exercise intervention. Exp. Gerontol. 2021, 155, 111561. Andermann, M.L.; Lowell, B.B. Toward a Wiring Diagram Understanding of Appetite Control. Neuron 2017, 95, 757–778.
- Giordano, A.; Murano, I.; Mondini, E.; Perugini, J.; Smorlesi, A.; Severi, I.; Barazzoni, R.; Scherer, P.E.; Cinti, S. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J. Lipid Res. 2013, 54, 2423–2436. Myers, M.G., Jr.; Affinati, A.H.; Richardson, N.; Schwartz, M.W. Central nervous system regulation of organismal energy and glucose homeostasis. Nat. Metab. 2021, 3, 737–750.
- Wu, H.; Ballantyne, C.M. Skeletal muscle inflammation and insulin resistance in obesity. J. Clin. Investig. 2017, 127, 43–54. Leng, G. The Heart of the Brain: The Hypothalamus and Its Hormones; The MIT Press: Cambridge, MA, USA, 2018.
- Lee, J.S.; Kim, S.H.; Jun, D.W.; Han, J.H.; Jang, E.C.; Park, J.Y.; Son, B.K.; Kim, S.H.; Jo, Y.J.; Park, Y.S.; et al. Clinical implications of fatty pancreas: Correlations between fatty pancreas and metabolic syndrome. World J. Gastroenterol. 2009, 15, 1869–1875. Waterson, M.J.; Horvath, T.L. Neuronal Regulation of Energy Homeostasis: Beyond the Hypothalamus and Feeding. Cell Metab. 2015, 22, 962–970.
- Zhang, X.; Zhang, G.; Zhang, H.; Karin, M.; Bai, H.; Cai, D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 2008, 135, 61–73. Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206.
- Giordano, A.; Nisoli, E. Neuroendocrinology of Energy Balance. Obesity, Endocrinology. 2018. Available online: https://link.springer.com/referenceworkentry/10.1007/978-3-319-47685-8_4-1?noAccess=true (accessed on 10 February 2022). Akbari, P.; Gilani, A.; Sosina, O.; Kosmicki, J.A.; Khrimian, L.; Fang, Y.Y.; Persaud, T.; Garcia, V.; Sun, D.; Li, A.; et al. Sequencing of 640,000 exomes identifies GPR75 variants associated with protection from obesity. Science 2021, 373, 8683.
- Leng, G.; Adan, R.A.H.; Belot, M.; Brunstrom, J.M.; de Graaf, K.; Dickson, S.L.; Hare, T.; Maier, S.; Menzies, J.; Preissl, H.; et al. The determinants of food choice. Proc. Nutr. Soc. 2017, 76, 316–327. Moreno-Mendez, E.; Quintero-Fabian, S.; Fernandez-Mejia, C.; Lazo-de-la-Vega-Monroy, M. Early-life programming of adipose tissue. Nutr. Res. Rev. 2020, 33, 244–259.
- Andermann, M.L.; Lowell, B.B. Toward a Wiring Diagram Understanding of Appetite Control. Neuron 2017, 95, 757–778. Miller, G.E.; Chen, E.; Parker, K.J. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol. Bull. 2011, 137, 959–997.
- Myers, M.G., Jr.; Affinati, A.H.; Richardson, N.; Schwartz, M.W. Central nervous system regulation of organismal energy and glucose homeostasis. Nat. Metab. 2021, 3, 737–750. Miller, A.L.; Lumeng, J.C. Pathways of Association from Stress to Obesity in Early Childhood. Obesity 2018, 26, 1117–1124.
- Leng, G. The Heart of the Brain: The Hypothalamus and Its Hormones; The MIT Press: Cambridge, MA, USA, 2018. Carter, C.S. The Role of Oxytocin and Vasopressin in Attachment. Psychodyn. Psychiatry 2017, 45, 499–517.
- Waterson, M.J.; Horvath, T.L. Neuronal Regulation of Energy Homeostasis: Beyond the Hypothalamus and Feeding. Cell Metab. 2015, 22, 962–970. Lawson, E.A.; Olszewski, P.K.; Weller, A.; Blevins, J.E. The role of oxytocin in regulation of appetitive behaviour, body weight and glucose homeostasis. J. Neuroendocrinol. 2020, 32, e12805.
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. Baracz, S.J.; Everett, N.A.; Cornish, J.L. The impact of early life stress on the central oxytocin system and susceptibility for drug addiction: Applicability of oxytocin as a pharmacotherapy. Neurosci. Biobehav. Rev. 2020, 110, 114–132.
- Akbari, P.; Gilani, A.; Sosina, O.; Kosmicki, J.A.; Khrimian, L.; Fang, Y.Y.; Persaud, T.; Garcia, V.; Sun, D.; Li, A.; et al. Sequencing of 640,000 exomes identifies GPR75 variants associated with protection from obesity. Science 2021, 373, 8683. Bowlby, J. Attachment and Loss, 2nd ed.; Basic Books: New York, NY, USA, 1969.
- Moreno-Mendez, E.; Quintero-Fabian, S.; Fernandez-Mejia, C.; Lazo-de-la-Vega-Monroy, M. Early-life programming of adipose tissue. Nutr. Res. Rev. 2020, 33, 244–259. Winnicott, D.W. Mother and Child: A Primer of First Relationships; Basic Books: New York, NY, USA, 1957.
- Miller, G.E.; Chen, E.; Parker, K.J. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol. Bull. 2011, 137, 959–997. Nelson, C.A., 3rd; Gabard-Durnam, L.J. Early Adversity and Critical Periods: Neurodevelopmental Consequences of Violating the Expectable Environment. Trends Neurosci. 2020, 43, 133–143.
- Miller, A.L.; Lumeng, J.C. Pathways of Association from Stress to Obesity in Early Childhood. Obesity 2018, 26, 1117–1124. Rice, C.J.; Sandman, C.A.; Lenjavi, M.R.; Baram, T.Z. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 2008, 149, 4892–4900.
- Carter, C.S. The Role of Oxytocin and Vasopressin in Attachment. Psychodyn. Psychiatry 2017, 45, 499–517. Molet, J.; Maras, P.M.; Avishai-Eliner, S.; Baram, T.Z. Naturalistic rodent models of chronic early-life stress. Dev. Psychobiol. 2014, 56, 1675–1688.
- Lawson, E.A.; Olszewski, P.K.; Weller, A.; Blevins, J.E. The role of oxytocin in regulation of appetitive behaviour, body weight and glucose homeostasis. J. Neuroendocrinol. 2020, 32, e12805. Curley, J.P.; Champagne, F.A. Influence of maternal care on the developing brain: Mechanisms, temporal dynamics and sensitive periods. Front. Neuroendocrinol. 2016, 40, 52–66.
- Baracz, S.J.; Everett, N.A.; Cornish, J.L. The impact of early life stress on the central oxytocin system and susceptibility for drug addiction: Applicability of oxytocin as a pharmacotherapy. Neurosci. Biobehav. Rev. 2020, 110, 114–132. Garcia-Caceres, C.; Balland, E.; Prevot, V.; Luquet, S.; Woods, S.C.; Koch, M.; Horvath, T.L.; Yi, C.X.; Chowen, J.A.; Verkhratsky, A.; et al. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat. Neurosci. 2019, 22, 7–14.
- Bowlby, J. Attachment and Loss, 2nd ed.; Basic Books: New York, NY, USA, 1969. Abbink, M.R.; van Deijk, A.F.; Heine, V.M.; Verheijen, M.H.; Korosi, A. The involvement of astrocytes in early-life adversity induced programming of the brain. Glia 2019, 67, 1637–1653.
- Winnicott, D.W. Mother and Child: A Primer of First Relationships; Basic Books: New York, NY, USA, 1957. Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436.
- Nelson, C.A., 3rd; Gabard-Durnam, L.J. Early Adversity and Critical Periods: Neurodevelopmental Consequences of Violating the Expectable Environment. Trends Neurosci. 2020, 43, 133–143. Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908.
- Rice, C.J.; Sandman, C.A.; Lenjavi, M.R.; Baram, T.Z. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 2008, 149, 4892–4900. Feldman, R. The Neurobiology of Human Attachments. Trends Cogn. Sci. 2017, 21, 80–99.
- Molet, J.; Maras, P.M.; Avishai-Eliner, S.; Baram, T.Z. Naturalistic rodent models of chronic early-life stress. Dev. Psychobiol. 2014, 56, 1675–1688. Kim, S.; Kwok, S.; Mayes, L.C.; Potenza, M.N.; Rutherford, H.J.V.; Strathearn, L. Early adverse experience and substance addiction: Dopamine, oxytocin, and glucocorticoid pathways. Ann. N. Y. Acad. Sci. 2017, 1394, 74–91.
- Curley, J.P.; Champagne, F.A. Influence of maternal care on the developing brain: Mechanisms, temporal dynamics and sensitive periods. Front. Neuroendocrinol. 2016, 40, 52–66. Lawson, E.A. The effects of oxytocin on eating behaviour and metabolism in humans. Nat. Rev. Endocrinol. 2017, 13, 700–709.
- Garcia-Caceres, C.; Balland, E.; Prevot, V.; Luquet, S.; Woods, S.C.; Koch, M.; Horvath, T.L.; Yi, C.X.; Chowen, J.A.; Verkhratsky, A.; et al. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat. Neurosci. 2019, 22, 7–14. Ding, C.; Leow, M.K.; Magkos, F. Oxytocin in metabolic homeostasis: Implications for obesity and diabetes management. Obes. Rev. 2019, 20, 22–40.
- Abbink, M.R.; van Deijk, A.F.; Heine, V.M.; Verheijen, M.H.; Korosi, A. The involvement of astrocytes in early-life adversity induced programming of the brain. Glia 2019, 67, 1637–1653. Zhang, H.; Wu, C.; Chen, Q.; Chen, X.; Xu, Z.; Wu, J.; Cai, D. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS ONE 2013, 8, e61477.
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. McCormack, S. Intranasal Oxytocin to Promote Weight Loss in Children, Adolescents, and Adults with Brain Tumors and Hypothalamic Obesity Syndrome. Recruiting. Available online: https://clinicaltrials.gov/ct2/show/NCT02849743 (accessed on 24 January 2022).
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. Lawson, E.A. A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Effects of Repeat Doses of Intranasal Oxytocin in Obese Adults. Completed. Available online: https://clinicaltrials.gov/ct2/show/NCT03043053 (accessed on 24 January 2022).
- Feldman, R. The Neurobiology of Human Attachments. Trends Cogn. Sci. 2017, 21, 80–99. Espinoza, S. The Physiologic Effects of Intranasal Oxytocin on Sarcopenic Obesity (INOSO). Completed. Available online: https://clinicaltrials.gov/ct2/show/NCT03119610 (accessed on 24 January 2022).
- Kim, S.; Kwok, S.; Mayes, L.C.; Potenza, M.N.; Rutherford, H.J.V.; Strathearn, L. Early adverse experience and substance addiction: Dopamine, oxytocin, and glucocorticoid pathways. Ann. N. Y. Acad. Sci. 2017, 1394, 74–91. Yamamoto, Y.; Cushing, B.S.; Kramer, K.M.; Epperson, P.D.; Hoffman, G.E.; Carter, C.S. Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender-specific manner. Neuroscience 2004, 125, 947–955.
- Lawson, E.A. The effects of oxytocin on eating behaviour and metabolism in humans. Nat. Rev. Endocrinol. 2017, 13, 700–709. Leng, G.; Caquineau, C.; Sabatier, N. Regulation of oxytocin secretion. Vitam. Horm. 2005, 71, 27–58.
- Ding, C.; Leow, M.K.; Magkos, F. Oxytocin in metabolic homeostasis: Implications for obesity and diabetes management. Obes. Rev. 2019, 20, 22–40. Liao, P.Y.; Chiu, Y.M.; Yu, J.H.; Chen, S.K. Mapping Central Projection of Oxytocin Neurons in Unmated Mice Using Cre and Alkaline Phosphatase Reporter. Front. Neuroanat. 2020, 14, 559402.
- Zhang, H.; Wu, C.; Chen, Q.; Chen, X.; Xu, Z.; Wu, J.; Cai, D. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS ONE 2013, 8, e61477. Francis, D.D.; Champagne, F.C.; Meaney, M.J. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J. Neuroendocrinol. 2000, 12, 1145–1148.
- McCormack, S. Intranasal Oxytocin to Promote Weight Loss in Children, Adolescents, and Adults with Brain Tumors and Hypothalamic Obesity Syndrome. Recruiting. Available online: https://clinicaltrials.gov/ct2/show/NCT02849743 (accessed on 24 January 2022).Francis, D.D.; Young, L.J.; Meaney, M.J.; Insel, T.R. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: Gender differences. J. Neuroendocrinol. 2002, 14, 349–353.
- Lawson, E.A. A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Effects of Repeat Doses of Intranasal Oxytocin in Obese Adults. Completed. Available online: https://clinicaltrials.gov/ct2/show/NCT03043053 (accessed on 24 January 2022).Tsuda, M.C.; Yamaguchi, N.; Ogawa, S. Early life stress disrupts peripubertal development of aggression in male mice. Neuroreport 2011, 22, 259–263.
- Espinoza, S. The Physiologic Effects of Intranasal Oxytocin on Sarcopenic Obesity (INOSO). Completed. Available online: https://clinicaltrials.gov/ct2/show/NCT03119610 (accessed on 24 January 2022).Veenema, A.H.; Bredewold, R.; Neumann, I.D. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: Link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology 2007, 32, 437–450.
- Yamamoto, Y.; Cushing, B.S.; Kramer, K.M.; Epperson, P.D.; Hoffman, G.E.; Carter, C.S. Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender-specific manner. Neuroscience 2004, 125, 947–955. Wei, F.; Li, W.; Ma, B.; Deng, X.; Zhang, L.; Zhao, L.; Zheng, T.; Jing, Y. Experiences affect social behaviors via altering neuronal morphology and oxytocin system. Psychoneuroendocrinology 2021, 129, 105247.
- Leng, G.; Caquineau, C.; Sabatier, N. Regulation of oxytocin secretion. Vitam. Horm. 2005, 71, 27–58. Ellis, B.J.; Horn, A.J.; Carter, C.S.; van IJzendoorn, M.H.; Bakermans-Kranenburg, M.J. Developmental programming of oxytocin through variation in early-life stress: Four meta-analyses and a theoretical reinterpretation. Clin. Psychol. Rev. 2021, 86, 101985.
- Liao, P.Y.; Chiu, Y.M.; Yu, J.H.; Chen, S.K. Mapping Central Projection of Oxytocin Neurons in Unmated Mice Using Cre and Alkaline Phosphatase Reporter. Front. Neuroanat. 2020, 14, 559402. Krause, S.; Boeck, C.; Gumpp, A.M.; Rottler, E.; Schury, K.; Karabatsiakis, A.; Buchheim, A.; Gundel, H.; Kolassa, I.T.; Waller, C. Child Maltreatment Is Associated with a Reduction of the Oxytocin Receptor in Peripheral Blood Mononuclear Cells. Front. Psychol. 2018, 9, 173.
- Francis, D.D.; Champagne, F.C.; Meaney, M.J. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J. Neuroendocrinol. 2000, 12, 1145–1148. Wigger, D.C.; Groger, N.; Lesse, A.; Krause, S.; Merz, T.; Gundel, H.; Braun, K.; McCook, O.; Radermacher, P.; Bock, J.; et al. Maternal Separation Induces Long-Term Alterations in the Cardiac Oxytocin Receptor and Cystathionine gamma-Lyase Expression in Mice. Oxid. Med. Cell Longev. 2020, 2020, 4309605.
- Francis, D.D.; Young, L.J.; Meaney, M.J.; Insel, T.R. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: Gender differences. J. Neuroendocrinol. 2002, 14, 349–353. McCook, O.; Denoix, N.; Radermacher, P.; Waller, C.; Merz, T. H2S and Oxytocin Systems in Early Life Stress and Cardiovascular Disease. J. Clin. Med. 2021, 10, 3484.
- Tsuda, M.C.; Yamaguchi, N.; Ogawa, S. Early life stress disrupts peripubertal development of aggression in male mice. Neuroreport 2011, 22, 259–263. Szczepanska-Sadowska, E.; Wsol, A.; Cudnoch-Jedrzejewska, A.; Zera, T. Complementary Role of Oxytocin and Vasopressin in Cardiovascular Regulation. Int. J. Mol. Sci. 2021, 22, 11465.
- Veenema, A.H.; Bredewold, R.; Neumann, I.D. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: Link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology 2007, 32, 437–450. Liu, C.M.; Hsu, T.M.; Suarez, A.N.; Subramanian, K.S.; Fatemi, R.A.; Cortella, A.M.; Noble, E.E.; Roitman, M.F.; Kanoski, S.E. Central oxytocin signaling inhibits food reward-motivated behaviors and VTA dopamine responses to food-predictive cues in male rats. Horm. Behav. 2020, 126, 104855. < >56. Dresp-Langley, B. Children's health in the digital age. Int. J. Environ. Res. Public Health 2020, 17, 3240. https://doi.org/10.3390/ijerph17093240
- Wei, F.; Li, W.; Ma, B.; Deng, X.; Zhang, L.; Zhao, L.; Zheng, T.; Jing, Y. Experiences affect social behaviors via altering neuronal morphology and oxytocin system. Psychoneuroendocrinology 2021, 129, 105247.
- Krause, S.; Boeck, C.; Gumpp, A.M.; Rottler, E.; Schury, K.; Karabatsiakis, A.; Buchheim, A.; Gundel, H.; Kolassa, I.T.; Waller, C. Child Maltreatment Is Associated with a Reduction of the Oxytocin Receptor in Peripheral Blood Mononuclear Cells. Front. Psychol. 2018, 9, 173.
- Wigger, D.C.; Groger, N.; Lesse, A.; Krause, S.; Merz, T.; Gundel, H.; Braun, K.; McCook, O.; Radermacher, P.; Bock, J.; et al. Maternal Separation Induces Long-Term Alterations in the Cardiac Oxytocin Receptor and Cystathionine gamma-Lyase Expression in Mice. Oxid. Med. Cell Longev. 2020, 2020, 4309605.
- McCook, O.; Denoix, N.; Radermacher, P.; Waller, C.; Merz, T. H2S and Oxytocin Systems in Early Life Stress and Cardiovascular Disease. J. Clin. Med. 2021, 10, 3484.
- Szczepanska-Sadowska, E.; Wsol, A.; Cudnoch-Jedrzejewska, A.; Zera, T. Complementary Role of Oxytocin and Vasopressin in Cardiovascular Regulation. Int. J. Mol. Sci. 2021, 22, 11465.
- Liu, C.M.; Hsu, T.M.; Suarez, A.N.; Subramanian, K.S.; Fatemi, R.A.; Cortella, A.M.; Noble, E.E.; Roitman, M.F.; Kanoski, S.E. Central oxytocin signaling inhibits food reward-motivated behaviors and VTA dopamine responses to food-predictive cues in male rats. Horm. Behav. 2020, 126, 104855.
