Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 4 by Lindsay Dong and Version 3 by Vivi Li.
During our research we have observed that Ly6c, which is traditionally only used as a classic and non-classical monocyte / macrophage differentiating antigen, can be used as a new specific marker of the mouse vasculature and to assess qualitatively and quantitatively vascular changes in health and disease. Researchers believe that this innovative application of Ly6c immunodetection, which has shown three advantages (brighter signal, homogeneous staining and greater selectivity) compared to traditional vessel markers such as IB4 isolectin, will be of high interest to researchers in this field.
- vessels
- cell extravasation
- vascular plexus
- ischemia
1. Introduction
One of the main fields of study within neuroscience deals with the relationship between the circulatory system and the central nervous system (CNS). The circulatory system is responsible for providing oxygen and nutrients to all cells in the body, including the brain, as well as for removing the CO2 discarded by them. Unlike what happens in other organs, the brain has almost zero capacity to store energy in the long term, and therefore requires a continuous flow of oxygen and nutrients. In fact, despite accounting for about 2% of the weight of an adult, the brain requires approximately 20% of the heart output [1]. Therefore, the physiological role of the circulatory system is crucial; proof of this is that most neurodegenerative diseases or brain illnesses are associated with blood supply disorders, such as brain stroke or edema [2][3].
The retina is metabolically very active and, like the brain, also requires a large supply of nutrients and oxygen. The blood supply of the mouse retina is established in two vascular systems: the choriocapillaris, which irrigates the pigment epithelium and the outer retina, and the central retinal artery, which irrigates the inner retina [4]. Once the central retinal artery enters the retina through the optic nerve, it divides into three plexuses: the superficial vascular plexus in the plane of the retinal ganglion cells (RGCs) and the nerve fiber layer, the intermediate vascular plexus above the inner nuclear layer, and the deep vascular plexus above the outer nuclear layer [4].
Retinal vasculature alterations can have important effects on the visual system. A correlation between inner vascular deficits and RGC loss observed after the induction of ocular hypertension has been shown [5]. Similar to cerebral infarction, retinal ischemia is a serious problem that can lead to a severe loss of vision and even blindness, especially among the elderly population [6]. Glaucoma is a group of eye diseases that damage the optic nerve, the health of which is vital for good eyesight [7]. Although there are several risk factors for glaucoma, such as age or genetics, the main one is a high intraocular pressure, which is believed to cause a vascular and RGC axonal impairment [8][9][10].
Understanding the physiology and anatomy of the well-organized retinal vascular system is essential to monitor visual health and detect abnormalities associated with pathologies, such as glaucoma or ischemia.
There are several markers and techniques to identify the mouse blood vessels, summarized in Table 1.
Table 1. Markers to identify mouse blood vessels.
| Marker | Target | Characteristics | References |
|---|---|---|---|
| Isolectin-IB4 | Terminal α-d-galactosyl residues | Binds to blood vessels and to activated microglial cells | [5][11][12][13][14][15][16] |
| CD31 (cluster of differentiation 31) also known as PECAM-1 (platelet endothelial cell adhesion molecule) | Adhesion molecule that constitutes a large part of the intercellular junctions of endothelial cells | It is found on endothelial cells, platelets, Kupffer cells, macrophages, granulocytes, lymphocytes, megakaryocytes, osteoclasts, and in certain tumors | [12][16][17][18] |
| ICAM2 (intercellular adhesion molecule 2) also known as CD102 (cluster of differentiation 102) | Type I transmembrane glycoprotein present in the apical/luminal endothelial cell membrane | ICAM2 masks highlight vessel segments undergoing remodeling. It mediates adhesive interactions important for antigen-specific immune response | [11][18][19] |
| CLDN5 (claudin 5) | It is one of the six high abundant tight junction proteins in the blood–brain barrier in vivo and the dominant one in vitro | Transiently expressed in the retinal pigment epithelium (RPE) during development, where its expression correlates with permeability changes in the developing RPE | [12][20] |
| ColIV or Col4 (collagen IV) | One of the main components of the basement membrane (BM), a specialized extracellular matrix that compartmentalizes tissues, provides structural support, and influences cell behavior and signaling | Collagen IV is the most abundant structural BM component and is essential for BM integrity but not initial BM formation | [11][19][21] |
| Endoglin (ENG) also known as CD105 | Transmembrane glycoprotein that functions as a coreceptor for ligands of the transforming growth factor-β superfamily. It is predominantly expressed by activated endothelial cells | It is a facilitator of ligand binding and has a crucial role in angiogenesis. It is also a marker of mesenchymal stem cells and it is expressed in progenitor cells involved in vascular remodeling in animal models | [22][23][24] |
| ZO-1 (zonula occludens-1) also known as TJP1 (tight junction protein-1) | One of the proteins that create intercellular boundaries between the plasma membrane domains of epithelial and endothelial cells (endothelial cell–cell junctions) | Is thought to have both structural and signaling roles. It can also associate with claudin, occludin, and F-actin, at tight junction stands, where it provides a linkage between the actin cytoskeleton and the tight junction | [11][16][25] |
| CDH5 (cadherin 5) also known as VE-cadherin | It is a strictly endothelial specific adhesion molecule located at junctions between endothelial cells and promotes homotypic cell-to-cell interaction | It is vital for the maintenance and control of endothelial cell contacts. It is relevant for the control of vascular permeability and leukocyte extravasation and regulates various cellular processes such as cell proliferation and apoptosis and modulates vascular endothelial growth factor receptor functions. It is essential during embryonic angiogenesis | [11][16] |
| Erg (ETS-related gene) | It is expressed in the nuclei of endothelial cells | It is a transcription factor that has been linked to angiogenesis and to the promotion of vascular stability | [11][26] |
| Dextran-fluorophore (complex branched glucan labelled with a fluorophore) | Blood vessel lumen | Used for intravascular perfusion. High-molecular-weight FITC-dextran remains in the vasculature without diffusion | [27][28] |
| gel-BSA-FITC (gel-bovine serum albumin-fluorescein isothiocyanate) | Blood vessel lumen | Used for intravascular perfusion. The high molecular weight of albumin prevents the marker from crossing blood vessel walls, which ensures the confinement of the fluorescent signal within the blood vessels | [29] |
Among them, the isolectin IB4, which binds to the sugar residues of the glycocalyx present on the surface of the blood vessels, is one of the most used, especially in the retina [5][11][12][13][14][15][16]. It has been reported that it could be used as a premature indicator of endothelial regression, i.e., the pruning of superfluous connections by regression and the polarized migration of endothelial cells, since its lack of expression precedes that of other markers such as collagen IV [11]. However, in the CNS, IB4 also binds to activated microglia, impairing in some cases the visualization of the blood network [30]. Antibodies against ZO-1, cadherin, CD31, ICAM-2, and claudin-5 recognize the endothelial cell–cell junctions giving rise to a brighter signal where the cells attach to each other and a lower signal in the cytosol. The intravenous injection of fluorophore-coupled substances (e.g., dextran or gel-BSA) results in the fluorescent labelling of the whole space inside blood vessels and can be used to study vessel permeability [31]. Each marker has its advantages and disadvantages, but in mice none is as precise as the best marker for endothelial cells in rats, the rat endothelial cell antigen 1 (RECA1), which shows vascular endothelium cell-specificity both in vitro and in vivo [32].
Ly6c (lymphocyte antigen 6 complex, locus C1) is a monocyte/macrophage cell differentiation antigen commonly used to differentiate classical monocytes (Ly6chigh) from non-classical ones (Ly6clow) [33][34]. Alliot et al., in 1998 [30], showed that Ly6c was expressed in the blood vessels of the mouse brain but not in microglial cells, and researchers wondered whether the same was true for the healthy and diseased retina. Thus, using Ly6c immunodetection and IB4 as the positive control, researchers have characterized in depth the three retinal vascular plexuses in healthy retinas and in both retinas after the unilateral induction of retinal ischemia using the acute ocular hypertension model (AOHT) [35][36][37].
2. Determination of the Ly6c Value as a Vasculature Marker
Ly6c and IB4, as the control of blood vessel staining, were detected in several mouse organs and tissues. Ly6c was expressed in the vasculature of all analyzed samples (Figure 1A), including the brain [30]. In the intestine, Ly6c is expressed in blood vessels, and by the macrophages lining the villi [38] (arrow and asterisks, Figure 1A). To ascertain whether Ly6c in the retina, labelled macrophages/monocytes associated with blood vessels, researchers immunodetected Ly6c and CD115 in perfused and non-perfused retinas. CD115 (also known as receptor for macrophage colony stimulating factor [39]) is expressed by monocytes, macrophages, osteoclasts, and some epithelial cells. The Ly6c and CD115 signal in non-perfused tissue was similar, evidencing the blood vessels. However, in the perfused retinas, while Ly6c expression in the vessels remained, the CD115 signal disappeared (Figure 1B), indicating that Ly6c does not label perivascular macrophages/monocytes.
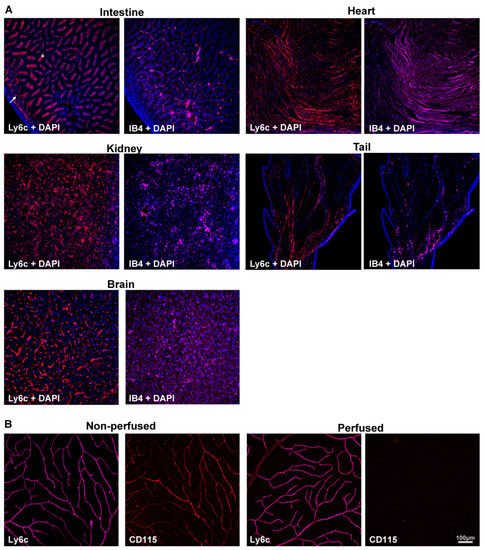

Figure 1. Ly6c is expressed in mouse blood vessels. (A) Ly6c (red) and IB4 (purple) detection in several tissues showing Ly6c signal in the brain, intestine, kidney, heart, and tail. The arrow points to blood vessels, and the asterisk to macrophages in the intestinal villi. Nuclei are counterstained with DAPI (blue). (B) Ly6c (purple) and CD115 (red) double immunodetection in non-perfused and perfused retinas. Scale bar is the same for all panels.
3. Retinal Vasculature Visualized with Ly6c
Next, researchers investigated Ly6C expression in the retina, using IB4 as the positive control. Ly6c was strongly expressed in arteries, veins, and capillaries (Figure 2). In the central retina, Ly6c identified the 12 main superficial retinal vessels: 6 veins and 6 arteries arranged alternately while the IB4 signal was seen in only half of them, the arteries. In the retinal periphery, with thinner and smaller vessels, the IB4 signal was weaker and more difficult to visualize and image than the Ly6c signal (Figure 2, compare panels F and G).
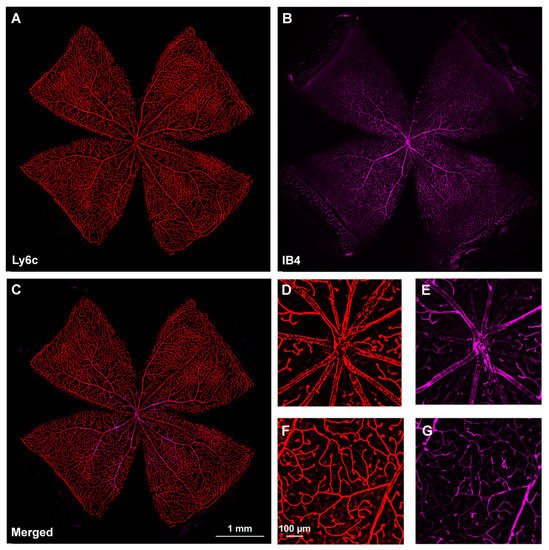

Figure 2. Comparison of Ly6c and IB4 vascular staining in the retina. (A–C) Photomontage of a representative intact retina showing Ly6C ((A), red), IB4 ((B), purple) and both signals (C) in the ganglion cell layer. (D–G) Magnifications from the central (D,F) and peripheral (E,G) retina are from the yellow squares in (C). Scale bar in (C) for (A–C) images, and in (F) for (D–G) panels.
In the three retinal plexuses (P1, superficial; P2, intermediate; and P3, deep), the Ly6c signal was bright and clear (Figure 3A). Flat mounts show the spatial anatomy of each plexus distinctly (Figure 3B). The inner or superficial plexus, P1, is the more ramified, and the capillaries are capped with an engrossment, while P3 capillaries are saccular, with fewer free ends. P2 has an intermediate morphology. Finally, researchers measured the area of the retina occupied by the vasculature in each of the plexuses. In intact healthy retinas, 15 ± 2.2, 24 ± 7, and 31 ± 1.4% of the retinal area was occupied by P1, P2, and P3, respectively (graph in Figure 4).
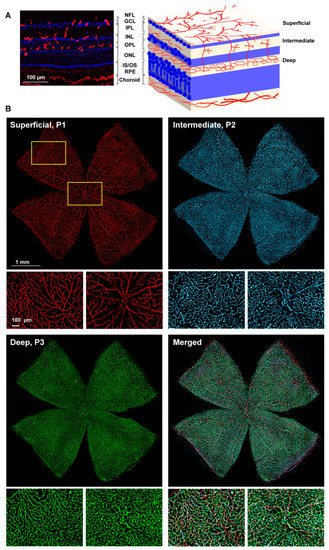
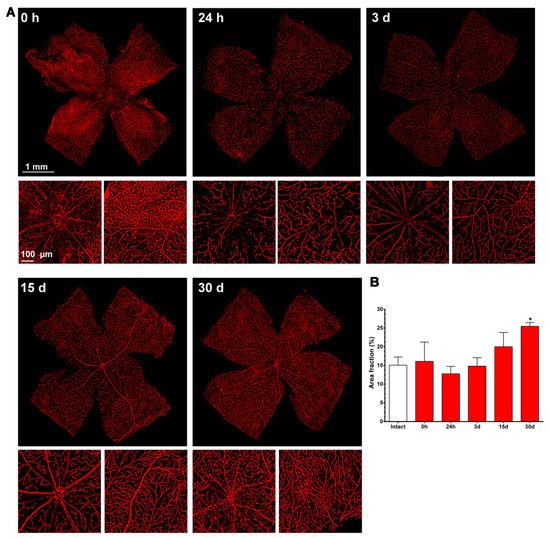

Figure 3. Retinal vascular plexuses. (A) Left: Ly6c immunodetection (red) in a retinal cross-section counterstained with DAPI (blue). Right: drawing depicting the retinal layers and the position of each vascular plexus. RGCL: retinal ganglion cell layer, IPL: inner plexiform layer, INL: inner nuclear layer, OPL: outer plexiform layer, ONL: outer nuclear layer. (B) Whole-mounted retina showing Ly6c staining in the three retinal vascular plexuses. Below each one there are magnifications from the two regions framed in yellow. 1 mm and 100 µm scale bars are for photomontages and magnifications, respectively.

Figure 4. Changes in the retinal vasculature after transient ischemia. (A) Ly6c immunodetection showing the superficial plexus (P1) of ischemic retinas analyzed from 0 h (immediately after) to 30 days after the induction of the ischemia. Below each photomontage are shown magnifications of the optic nerve head or retinal periphery. (B) Bar graphs showing the area fraction of the retina covered by P1 ± standard deviation in intact and contralateral retinas. * Significant compared to intact retinas (p < 0.05, Kruskal–Wallis, Dunn’s multiple comparisons test). 1 mm and 100 µm scale bars are for photomontages and magnifications, respectively.
4. Ly6c Identifies Vascular Changes in a Model of Retinal Ischemia
Once Ly6c expression in intact retinas was determined, researchers decided to validate its use in disease, using a model of retinal ischemia induced by increasing intra-ocular pressure by saline injection into the anterior chamber of the eye [36][37]. Researchers only analyzed P1, because P2 and P3 could not be imaged due to retinal thinning (see below).
4.1. Injured Retinas
As shown in Figure 4, immediately after the ischemia retinas were quite damaged, but they recovered progressively. During the first 3 days, the expression of Ly6c around the optic nerve head decreased, and some major vessels showed a discontinuous Ly6c expression which could mean vessel rupture or down-regulation of this protein. At 24 h and 3 days, classically activated monocyte/macrophages (Ly6chigh) were observed extravasated around the optic nerve head and in the periphery (Figure 5).
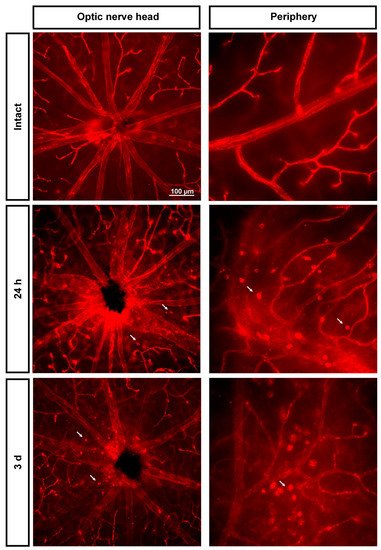

Figure 5. Extravasation of classically activated monocyte/macrophages early after the induction of transient ischemia. Ly6c expression in the superficial plexus around the optic nerve head and in the periphery of intact retinas and retinas analyzed 24 h and 3 days after the ischemia. Arrows point to extravasated classically activated monocytes/macrophages.
From 7 to 15 days there were no clear anatomical changes, although at 15 days there seemed to be more capillaries, with a tortuous morphology, in the periphery. At 30 days, the neo-vascularization was clearer and observed in the optic nerve head and the retinal periphery. Accordingly, the vascular fraction area of the retina increased significantly compared to that of the intact retinas (25 ± 1% vs. 15 ± 2%, graph in Figure 4).
To study P2 and P3, researchers resorted to confocal imaging; this approach also allowed the changes in retinal thickness to be assessed. As seen in Figure 6, there was a progressive retinal thinning, as previously reported [37], and, as a result, P2 and P3 were practically indistinguishable at 30 days.
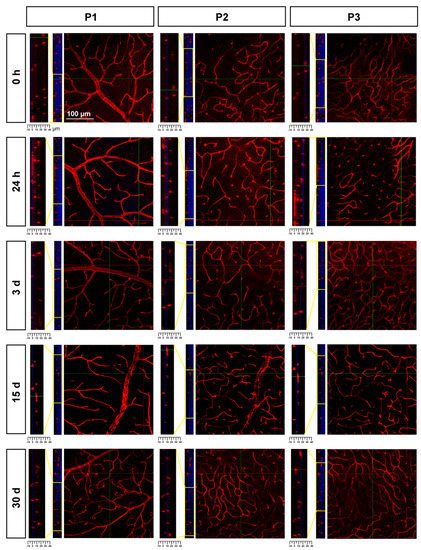

Figure 6. Retinal thinning after ischemia. Confocal images showing Ly6c signal in the three plexuses from 0 h to 30 days after the induction of the ischemia. To the left of each magnification is shown the z-stack spanning the retinal thickness (DAPI in blue) and a magnification of the z-stack (yellow square) where the ganglion cell layer is positioned a 0 µm in all images.
4.2. Contralateral Retinas
The retinal area fraction of the superficial plexus (P1) in the contralateral retinas diminished gradually, reaching significance compared to the intact retinas at 30 days (15 ± 2% vs. 12 ± 0.7, Figure 7).
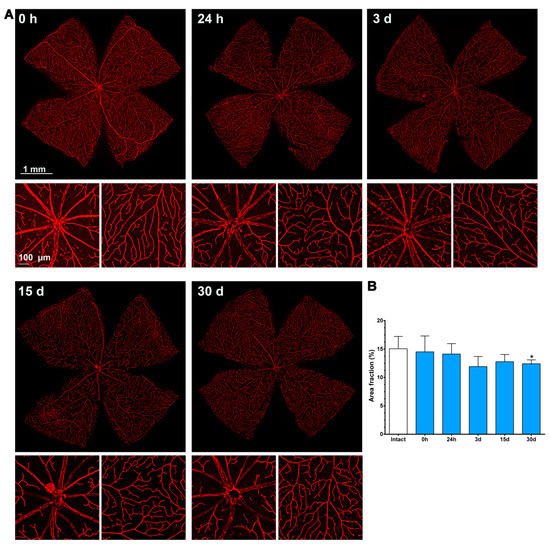

Figure 7. Changes in the retinal vasculature of the contralateral uninjured retinas. (A) Ly6c immunodetection showing the superficial plexus (P1) of contralateral retinas analyzed from 0 h (immediately after) to 30 days after the induction of the ischemia. Below each photomontage are shown magnifications of the optic nerve head or retinal periphery. (B) Bar graphs showing the area fraction of the retina covered by P1 ± standard deviation in intact and contralateral retinas. * Significant compared to intact retinas (p < 0.05, Kruskal–Wallis, Dunn’s multiple comparisons test). 1 mm and 100 µm scale bars are for photomontages and magnifications, respectively.
In P2 and P3, there was a gradual loss of Ly6c expression (Figure 8) until it became almost undetectable at 30 days. To elucidate whether this reflected a loss of P2 and P3 vessels or a down-regulation of Ly6c expression, a double staining with IB4 was performed. As the positive control researchers used injured retinas (Figure 9). In the injured retinas, Ly6c marked the three vascular plexuses, while IB4 staining revealed abundant activated microglial cells in P1 and P2 around the vessels, masking them. In P3, the IB4 and Ly6c signal was circumscribed to the vessels. In the contralateral retinas 30 days after the ischemic insult, Ly6c expression in P2 and P3 had disappeared but not the vascular plexuses, which were clearly observed with IB4. These results indicate that the loss of the Ly6c signal in the contralateral P2 and P3 plexuses was due to a down-regulation of Ly6c rather that to a regression of the vasculature.
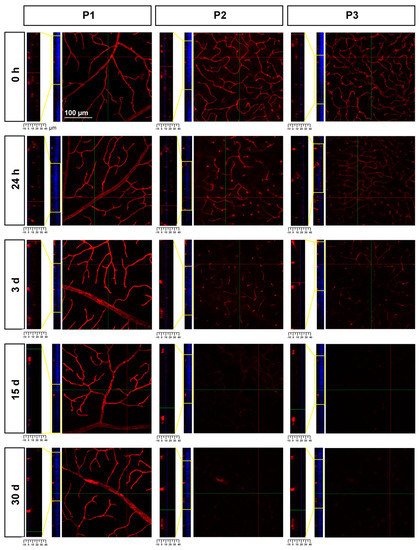
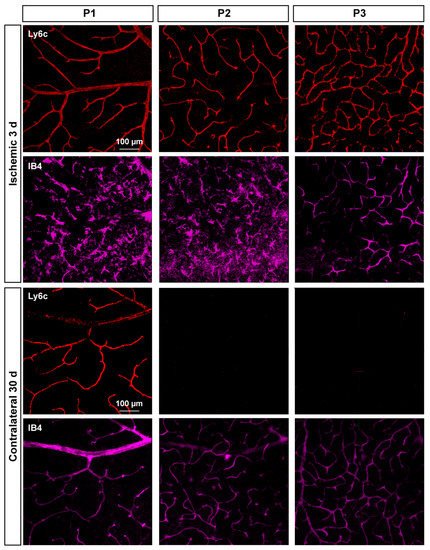

Figure 8. Loss of Ly6c expression in the intermediate and deep vascular plexuses of the contralateral uninjured retinas. Confocal images showing Ly6c signal in the three plexuses from 0 h to 30 days after the induction of the ischemia. To the left of each magnification is shown the z-stack spanning the retinal thickness (DAPI in blue) and a magnification of the z-stack (yellow square) where the ganglion cell layer is positioned a 0 µm in all images.

Figure 9. Microglial activation in the injured retinas and down-regulation of Ly6c in the contralateral ones. Confocal images showing Ly6c and IB4 staining in the three vascular plexuses of injured and contralateral retinas analyzed at 3 and 30 days after the induction of the ischemia, respectively.
References
- Lendahl, U.; Nilsson, P.; Betsholtz, C. Emerging Links between Cerebrovascular and Neurodegenerative Diseases-a Special Role for Pericytes. EMBO Rep. 2019, 20, e48070.
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The Role of Brain Vasculature in Neurodegenerative Disorders. Nat. Neurosci. 2018, 21, 1318–1331.
- Cortes-Canteli, M.; Iadecola, C. Alzheimer’s Disease and Vascular Aging: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 942–951.
- Sun, Y.; Smith, L.E.H. Retinal Vasculature in Development and Diseases. Annu. Rev. Vis. Sci. 2018, 4, 101–122.
- Almasieh, M.; MacIntyre, J.N.; Pouliot, M.; Casanova, C.; Vaucher, E.; Kelly, M.E.M.; Di Polo, A. Acetylcholinesterase Inhibition Promotes Retinal Vasoprotection and Increases Ocular Blood Flow in Experimental Glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3171–3183.
- Colucciello, M. Retinal Vascular Disease in Hypertension. Postgrad. Med. 2005, 117, 33–42.
- Schuster, A.K.; Erb, C.; Hoffmann, E.M.; Dietlein, T.; Pfeiffer, N. The Diagnosis and Treatment of Glaucoma. Dtsch. Arztebl. Int. 2020, 117, 225–234.
- Kimura, A.; Namekata, K.; Guo, X.; Noro, T.; Harada, C.; Harada, T. Targeting Oxidative Stress for Treatment of Glaucoma and Optic Neuritis. Oxid. Med. Cell. Longev. 2017, 2017, 2817252.
- Harder, J.M.; Williams, P.A.; Braine, C.E.; Yang, H.S.; Thomas, J.M.; Foxworth, N.E.; John, S.W.M.; Howell, G.R. Complement Peptide C3a Receptor 1 Promotes Optic Nerve Degeneration in DBA/2J Mice. J. Neuroinflamm. 2020, 17, 336.
- He, Z.; Vingrys, A.J.; Armitage, J.A.; Bui, B.V. The Role of Blood Pressure in Glaucoma. Clin. Exp. Optom. 2011, 94, 133–149.
- Franco, C.A.; Jones, M.L.; Bernabeu, M.O.; Geudens, I.; Mathivet, T.; Rosa, A.; Lopes, F.M.; Lima, A.P.; Ragab, A.; Collins, R.T.; et al. Dynamic Endothelial Cell Rearrangements Drive Developmental Vessel Regression. PLoS Biol. 2015, 13, e1002125.
- Rust, R.; Grönnert, L.; Dogançay, B.; Schwab, M.E. A Revised View on Growth and Remodeling in the Retinal Vasculature. Sci. Rep. 2019, 9, 1–9.
- Fu, Z.; Löfqvist, C.A.; Liegl, R.; Wang, Z.; Sun, Y.; Gong, Y.; Liu, C.; Meng, S.S.; Burnim, S.B.; Arellano, I.; et al. Photoreceptor Glucose Metabolism Determines Normal Retinal Vascular Growth. EMBO Mol. Med. 2018, 10, 76–90.
- Dudiki, T.; Meller, J.; Mahajan, G.; Liu, H.; Zhevlakova, I.; Stefl, S.; Witherow, C.; Podrez, E.; Kothapalli, C.R.; Byzova, T.V. Microglia Control Vascular Architecture via a TGFβ1 Dependent Paracrine Mechanism Linked to Tissue Mechanics. Nat. Commun. 2020, 11, 1–16.
- Shen, W.; Fruttiger, M.; Zhu, L.; Chung, S.H.; Barnett, N.L.; Kirk, J.K.; Lee, S.R.; Coorey, N.J.; Killingsworth, M.; Sherman, L.S.; et al. Conditional Müller Cell Ablation Causes Independent Neuronal and Vascular Pathologies in a Novel Transgenic Model. J. Neurosci. 2012, 32, 15715–15727.
- Franco, C.A.; Blanc, J.; Parlakian, A.; Blanco, R.; Aspalter, I.M.; Kazakova, N.; Diguet, N.; Mylonas, E.; Gao-Li, J.; Vaahtokari, A.; et al. SRF Selectively Controls Tip Cell Invasive Behavior in Angiogenesis. Development 2013, 140, 2321–2333.
- Kim, S.J.; Kim, S.A.; Choi, Y.A.; Park, D.Y.; Lee, J. Alpha-Smooth Muscle Actin-Positive Perivascular Cells in Diabetic Retina and Choroid. Int. J. Mol. Sci. 2020, 21, 2158.
- Halai, K.; Whiteford, J.; Ma, B.; Nourshargh, S.; Woodfin, A. ICAM-2 Facilitates Luminal Interactions between Neutrophils and Endothelial Cells in Vivo. J. Cell Sci. 2014, 127, 620–629.
- Zhou, Q.; Perovic, T.; Fechner, I.; Edgar, L.T.; Hoskins, P.R.; Gerhardt, H.; Krüger, T.; Bernabeu, M.O. Association between Erythrocyte Dynamics and Vessel Remodelling in Developmental Vascular Networks. J. R. Soc. Interface 2021, 18, 20210113.
- Berndt, P.; Winkler, L.; Cording, J.; Breitkreuz-Korff, O.; Rex, A.; Dithmer, S.; Rausch, V.; Blasig, R.; Richter, M.; Sporbert, A.; et al. Tight Junction Proteins at the Blood–Brain Barrier: Far More than Claudin-5. Cell. Mol. Life Sci. 2019, 76, 1987–2002.
- Scott, A.; Powner, M.B.; Fruttiger, M. Quantification of Vascular Tortuosity as an Early Outcome Measure in Oxygen Induced Retinopathy (OIR). Exp. Eye Res. 2014, 120, 55–60.
- Tual-chalot, S.; Mahmoud, M.; Allinson, K.R.; Redgrave, R.E.; Zhai, Z.; Oh, S.P.; Fruttiger, M.; Arthur, H.M. Endothelial Depletion of Acvrl1 in Mice Leads to Arteriovenous Malformations Associated with Reduced Endoglin Expression. PLoS ONE 2014, 9, e98646.
- Rabelink, T.J.; Lebrin, F. Thresholds of Endoglin Expression in Endothelial Cells Explains Vascular Etiology in Hereditary Hemorrhagic Telangiectasia Type 1. Int. J. Mol. Sci. 2021, 22, 8948.
- Barnett, J.M.; Suarez, S.; McCollum, G.W.; Penn, J.S. Endoglin Promotes Angiogenesis in Cell- and Animal-Based Models of Retinal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6490–6498.
- Tornavaca, O.; Chia, M.; Dufton, N.; Almagro, L.O.; Conway, D.E.; Randi, A.M.; Schwartz, M.A.; Matter, K.; Balda, M.S. ZO-1 Controls Endothelial Adherens Junctions, Cell–Cell Tension, Angiogenesis, and Barrier Formation. J. Cell Biol. 2015, 208, 821–838.
- Shah, A.V.; Birdsey, G.M.; Peghaire, C.; Pitulescu, M.E.; Dufton, N.P.; Yang, Y.; Weinberg, I.; Osuna Almagro, L.; Payne, L.; Mason, J.C.; et al. The Endothelial Transcription Factor ERG Mediates Angiopoietin-1-Dependent Control of Notch Signalling and Vascular Stability. Nat. Commun. 2017, 8, 16002.
- Li, S.; Li, T.; Luo, Y.; Yu, H.; Sun, Y.; Zhou, H.; Liang, X.; Huang, J.; Tang, S. Retro-Orbital Injection of FITC-Dextran Is an Effective and Economical Method for Observing Mouse Retinal Vessels. Mol. Vis. 2011, 17, 3566–3573.
- Auffray, C.; Fogg, D.; Garfa, M.; Elain, G.; Join-Lambert, O.; Kayal, S.; Sarnacki, S.; Cumano, A.; Lauvau, G.; Geissmann, F. Monitoring of Blood Vessels and Tissues by a Population of Monocytes with Patrolling Behavior. Science 2007, 317, 666–670.
- Di Giovanna, A.P.; Tibo, A.; Silvestri, L.; Müllenbroich, M.C.; Costantini, I.; Allegra Mascaro, A.L.; Sacconi, L.; Frasconi, P.; Pavone, F.S. Whole-Brain Vasculature Reconstruction at the Single Capillary Level. Sci. Rep. 2018, 8, 12573.
- Alliot, F.; Rutin, J.; Pessac, B. Ly-6C Is Expressed in Brain Vessels Endothelial Cells but Not in Microgila of the Mouse. Neurosci. Lett. 1998, 251, 37–40.
- Trost, A.; Motloch, K.; Bruckner, D.; Schroedl, F.; Bogner, B.; Kaser-Eichberger, A.; Runge, C.; Strohmaier, C.; Klein, B.; Aigner, L.; et al. Time-Dependent Retinal Ganglion Cell Loss, Microglial Activation and Blood-Retina-Barrier Tightness in an Acute Model of Ocular Hypertension. Exp. Eye Res. 2015, 136, 59–71.
- Duijvestijn, A.M.; van Goor, H.; Klatter, F.; Majoor, G.D.; van Bussel, E.; van Breda Vriesman, P.J. Antibodies Defining Rat Endothelial Cells: RECA-1, a Pan-Endothelial Cell-Specific Monoclonal Antibody. Lab. Investig. 1992, 66, 459–466.
- Alkhani, A.; Levy, C.S.; Tsui, M.; Rosenberg, K.A.; Polovina, K.; Mattis, A.N.; Mack, M.; Van Dyken, S.; Wang, B.M.; Maher, J.J.; et al. Ly6cLo Non-Classical Monocytes Promote Resolution of Rhesus Rotavirus-Mediated Perinatal Hepatic Inflammation. Sci. Rep. 2020, 10, 7165.
- Teh, Y.C.; Ding, J.L.; Ng, L.G.; Chong, S.Z. Capturing the Fantastic Voyage of Monocytes Through Time and Space. Front. Immunol. 2019, 10, 834.
- Rovere, G.; Nadal-Nicolás, F.M.; Wang, J.; Bernal-Garro, J.M.; García-Carrillo, N.; Villegas-Pérez, M.P.; Agudo-Barriuso, M.; Vidal-Sanz, M. Melanopsin-Containing or Non-Melanopsin–Containing Retinal Ganglion Cells Response to Acute Ocular Hypertension With or Without Brain-Derived Neurotrophic Factor Neuroprotection. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6652–6661.
- Wang, J.; Valiente-Soriano, F.J.; Nadal-Nicolás, F.M.; Rovere, G.; Chen, S.; Huang, W.; Agudo-Barriuso, M.; Jonas, J.B.; Vidal-Sanz, M.; Zhang, X. MicroRNA Regulation in an Animal Model of Acute Ocular Hypertension. Acta Ophthalmol. 2017, 95, e10–e21.
- Gallego-Ortega, A.; Norte-Muñoz, M.; Miralles de Imperial-Ollero, J.A.; Bernal-Garro, J.M.; Valiente-Soriano, F.J.; de la Villa Polo, P.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; Vidal-Sanz, M. Functional and Morphological Alterations in a Glaucoma Model of Acute Ocular Hypertension. Prog. Brain Res. 2020, 256, 1–29.
- Honda, M.; Surewaard, B.G.J.; Watanabe, M.; Hedrick, C.C.; Lee, W.-Y.; Brown, K.; McCoy, K.D.; Kubes, P. Perivascular Localization of Macrophages in the Intestinal Mucosa Is Regulated by Nr4a1 and the Microbiome. Nat. Commun. 2020, 11, 1329.
- Sherr, C.J.; Roussel, M.F.; Rettenmier, C.W. Colony-Stimulating Factor-1 Receptor (c-Fms). J. Cell. Biochem. 1988, 38, 179–187.
More
