Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Urszula K. Komarnicka.
Reactive oxygen species (ROS) are involved in many biological and medical processes, ranging from neurodegenerative disorders and cancer to bacterial and viral diseases, and sometimes are of major commercial interest. They are important regulators of and secondary messengers in several cell-signaling pathways, including the reactive oxygen species-mediated death of different cells.
- copper
- peptide
- reactive oxygen species
- neurodegenerative diseases
- cancer
1. Introduction
Interestingly, reactive oxygen species can have both negative (dark side) and positive (bright side) effects on the human body. On the one hand, they can damage or destroy healthy cells leading to the initiation of many pathogenic processes; on the other hand, they play a crucial role as agents causing pathogen and cancer cell death [35][1].
Maintaining the balance between the overproduction of ROS and their elimination has a huge impact on the proper functioning of cells and redox processes [36][2]. However, if the balance is disturbed, e.g., in the case of inflammation, hyperoxia, or when the antioxidant defense fails, the excess of ROS contributes to the initiation of various pathological conditions. The most common of these include neurodegenerative diseases, cancer, and respiratory system diseases [37,38][3][4]. Various mechanisms of ROS action, leading to healthy cell destruction have been proposed. ROS can directly interact with lipids, nucleic acids, and proteins and cause their oxidation. During lipid peroxidation, malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) are formed [39,40][5][6]. Additionally, the interaction of MDA with DNA and/or proteins can lead to mutagenesis [37,41][3][7]. In turn, the contact of ROS with nucleic acid leads to DNA degradation by single and/or double DNA strand breakage, damage to deoxyribose, and modification of DNA bases. This action results in dysfunctional or maladaptive apoptosis and/or necrosis of healthy cells [37,42,43][3][8][9].
The above mentioned typical biochemical changes in the disease sites were inspiration to exploit unbalanced ROS levels for new drug development [6,7][10][11]. In general, low or moderate levels of ROS induce the non-specific damage of proteins, lipids and DNA and destroy bacteria, viruses, cancer, and fungus cells [44][12]. Sometimes, photosensitizes can be used simultaneously for synergistic therapeutic efficacy, which are one of the representative exogenous ROS sources and have the ability to excite oxygen to its singlet state by using exogenous light energy [45,46][13][14]. Other beneficial effects of ROS involve their physiological roles in the functioning of a number of cellular signaling systems [47][15]. Their production by non-phagocytic NADPH oxidase isoforms plays a key role in the regulation of intracellular signaling cascades in various types of non-phagocytic cells including fibroblasts, endothelial cells, vascular smooth muscle cells, cardiac myocytes, and thyroid tissue [48,49][16][17].
2. Bright Side of Copper–Peptide Complexes
2.1. Cancer Treatment
2.1.1. Copper Complexes with Amino Acids and Peptide
Copper(II) complexes with amino acids (AC) are a group of chemicals extensively studied with respect to various cancer cells. The liIterature is dominated by many examples of Cu(II) heteroleptic inorganic compounds especially with lysine, arginine (Figure 13A,C), and phenanthroline derivatives. Good examples are those with lysine and ornithine (Figure 13B); phenanthroline and bipyridine as the N,N′ coordinating bases give the classical complexes [Cu(Lys)(phen)(H2O)]2+ and [Cu(Orn)(bipy)(H2O)]2+ having potential anticancer properties.
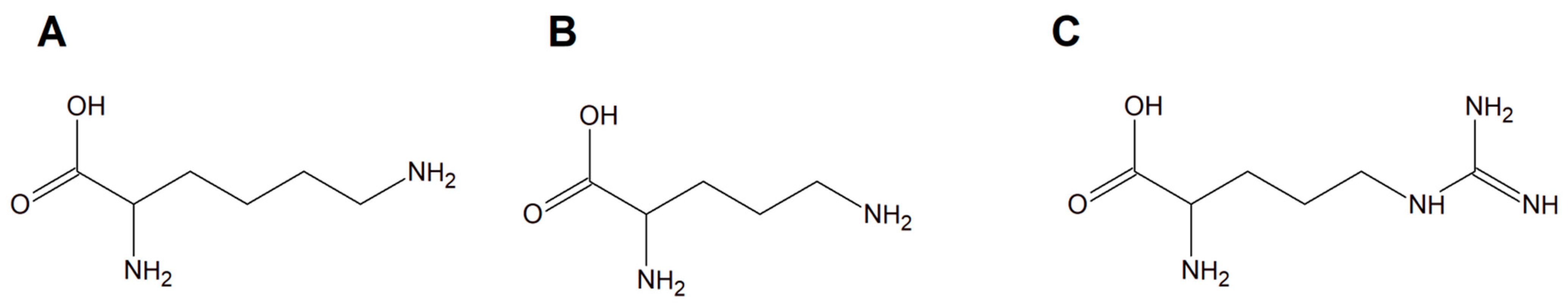

Figure 13.
Schematic structures of amino acids (
A
) lysine, (
B
) ornithine, and (
C
) arginine.
It was proven that some of these inorganic compounds are able to interact with deoxyribonucleic acid molecules by releasing their ligands, leading to Cu(II)/Cu(I) redox processes that initiate the formation of reactive oxygen species (ROS) in vivo, especially hydroxyl radicals [110,111][18][19]. This phenomenon suggested that these compounds may act as prodrugs having significant potential towards various cancer cell types [111][19]. Among the copper(II) complexes, a group with L−arginine (L−Arg; [Cu(L−Arg)2](NO3)2 and [Cu(L−Arg)(B)Cl]Cl⋅2.5H2O complexes (B = heterocyclic base)) was a remarkable idea for potential anticancer drugs [112,113][20][21]. Moreover, these compounds exhibited interesting antibacterial and antifungal activity. Importantly, they were found to act as strong minor groove binder agents causing plasmid DNA damage via a ROS-dependent action mode [113,114,115,116,117][21][22][23][24][25].
Interestingly, studies on the biological activity of different L−arginine copper(II) complexes with addition of phenanthroline molecules into the coordination sphere i.e., ([Cu(L-Arg)2(μ-4,4′-bpy)]Cl2⋅3H2O}(4,4′−bpy = 4,4′−bipyridine), [CuCl(L-Arg)(phen)]Cl·2H2O (phen = 1,10−phenanthroline) and [Cu(L-Arg)2(H2O)]C2O4·6H2O (C2O42--oxalate counter ion) have shown that these complexes were inactive towards cancer cells but exhibited antimicrobial activity by increasing oxidative stress [112,118,119][20][26][27]. Different groups of L−arginine copper(II) complexes ([Cu(L−Arg)2(NCS)](NCS)·H2O and [Cu(L−Arg)(NCS)2]) were intensively examined due to their therapeutic potential and they exhibited strong anticancer properties towards the A549 cell line (human lung epithelial carcinoma). The authors proved that [Cu(L−Arg)2(NCS)]+ is the only species in aqua solution (pH around 7.0) of the investigated inorganic compound [Cu(L−Arg)2(NCS)](NCS)·H2O. Importantly, it was proven that these species most probably are minor groove-binding agents. Additionally, they are able, in the presence of H2O2, to facilitate reactive oxygen species (ROS) generation and consequently damage of plasmid DNA. ROS are well recognized as mediators of DNA damage. They can cause double strand breaks (DSBs) of DNA through direct high-energy damage to the sugar backbone of DNA, and also through free radicals generated in cells—mostly ●OH [120][28]. Chemotherapeutics increase the ROS levels, which contributes to their genotoxicity [116,117][24][25]. ROS have also been reported to directly induce other forms of deoxyribonucleic acid damage through oxidation of nucleoside bases (e.g., 8-oxo guanine can be formed) [121][29]. Most probably these processes are the main ones involved in the anticancer mode of action of these copper complexes [122,123,124][30][31][32].
A copper–peptide complex consisting of a domain GGH (Gly-Gly-His) able to efficiently bind copper ions and the peptide (MPP) FrFKFrFK-CONH2 (Phe-r-Phe-Lys-Phe-r-Phe-Lys-CONH2, where r = D-arginine) was designed. It turned out to be well known for its mitochondria-penetrating properties (Figure 142). This inorganic compound is highly active towards various cancer cell lines, especially HeLa cells. It is worth noting that these cells are able to facilitate the selective intracellular uptake of Cu(II) ions. When the Cu–peptide compound reaches the mitochondria, the Fenton reaction is induced. As a result of this reaction, the reactive ●OH radical is produced that can induce cancer cell apoptosis [125][33].
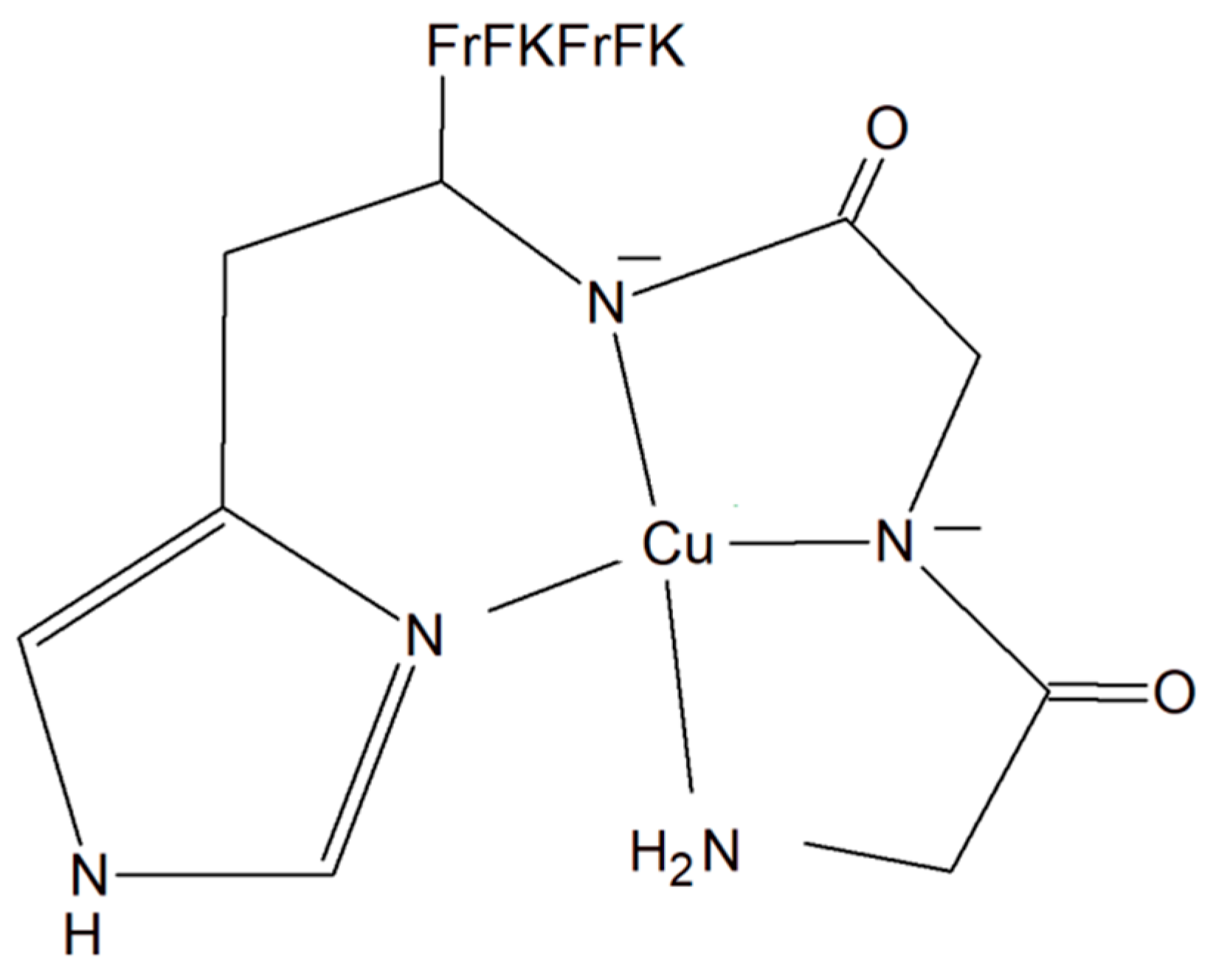

Figure 142. Copper–peptide complex consisting of GGH (Gly-Gly-His) and mitochondria-penetrating peptide (MPP) FrFKFrFK-CONH2 (Phe-r-Phe-Lys-Phe-r-Phe-Lys-CONH2, where r = D-arginine).
In modern medicine, especially in the field of novel broad-spectrum anticancer drugs, host defense peptides (HDPs) are attracting great attention. Metallating HDPs with Cu2+ is an effective, smart and novel synthetic strategy to increase the cytotoxicity of copper complexes against cancerous cells. It was found that peptidic (piscidins 1 (P1) and 3 (P3)) Cu(II) complexes not only physically but also chemically, with the help of reactive oxygen species, damage lipid membranes [126,127,128,129,130,131][34][35][36][37][38][39]. Interestingly, P1 seems to be a much more potent antimicrobial agent [127,130,132][35][38][40]. Additionally, this fragment is also active towards viruses such as HIV-160, coronaviruses [133][41], and pseudorabies [134][42]. However, some cytotoxic properties of both peptide fragments P1 and P3 against several cancerous cell lines were reported by Lin et al. (2012) [134][42]. The authors found that P1 can induce apoptosis in HT1080 cells supported by radical generation [126][34].
2.1.2. Copper Complexes with Peptide and Diimines
A new large group of Cu complexes with anticancer properties have been reported as a series of Cu(L-dipeptide)]·nH2O (L-dipeptide: L-Gly-Val, L-Gly-Leu, L-Gly-Phe, L-Ala-Gly, L-Ala-Phe, L-Val-Phe, L-Phe-Ala, L-Phe-Phe) [135[43][44][45],136,137], Cu(II)–L-dipeptide–phen (phen = 1,10-phenanthroline, L-dipeptide: Ala-Phe, Phe-Ala, Phe-Val and Phe-Phe [138[46][47][48][49],139,140,141], and Cu(II)–L-dipeptide–5-NO2-phen (where 5-NO2-phen = 5-NO2-1,10-phenanthroline; L-dipeptide: where L-dipeptide: Ala-Phe, Phe-Ala, Phe-Val and Phe-Phe) (Figure 153) [142][50]. Heteroleptic inorganic compounds Cu(II)–L-dipeptide–phen are much more active than Cu–phen or Cu–L-dipeptide complexes separately [135,136,137,138,139,140,141,142,143,144,145,146,147,148][43][44][45][46][47][48][49][50][51][52][53][54][55][56]. Their mode of cytotoxic action most probably includes DNA binding via phenanthroline ligand (intercalation processes) and also oxidative damage of this acid. The authors obtained similar results while investigating Cu(II)–L-dipeptide–5-NO2-phen.
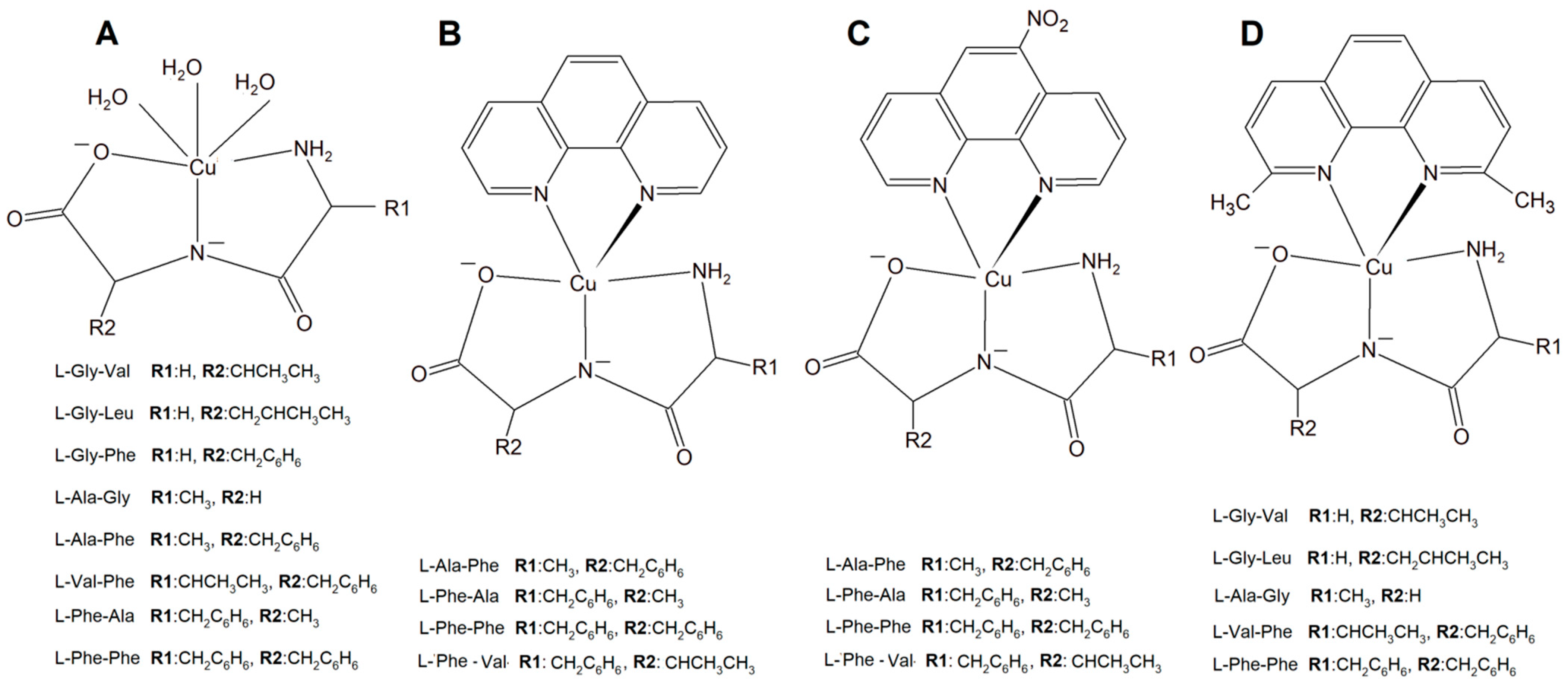

Figure 153. Copper(II) complexes (A) Cu–L-dipeptide, (B) Cu(II)–L-dipeptide–phen (phen = 1,10-phenanthroline), (C) Cu(II)–L-dipeptide–5-NO2-phen (5-NO2-phen = 5-NO2-1,10-phenanthroline), and (D) Cu(II)–L-dipeptide–dmp (dmp = 2,9-dimethyl-1,10-phenanthroline).
Another significant group of copper complexes [Cu(L-dipeptide)(dmp)]·nH2O bearing the dipeptide and neocuproine (dmp) ligand ([Cu(Gly-Val)(dmp)]·3H2O (Figure 153), [Cu(Gly-Leu)(dmp)]·H2O, [Cu(Ala-Gly)(dmp)]·4H2O, [Cu(Val-Phe)dmp)]·4.5H2O, and [Cu(Phe-Phe)(dmp)]·3H2O) were studied using various cancer lines [8][57]. It was proven that they possess better anticancer activity than those with phenanthroline ligands (Figure 153).
The synthesized inorganic compounds exhibited a high cytotoxic effect especially towards a few cancer cell lines: MDA-MB-231, MCF-7 (human metastatic breast adenocarcinomas, the first triple negative), MCF-10A (human normal breast cells), A549 (human lung epithelial carcinoma), and MRC-5 (human lung epithelial cells). Mechanistic studies revealed that the mode of action is mainly connected with DNA partial intercalation causing double strain damage, but also with an increase in the intracellular oxidative stress level [8,149][57][58].
2.1.3. Copper Complexes with Peptide and Imidazole
A group of Cu(II)–dipeptide complexes of 2-(4′-thiazolyl)benzimidazole, [Cu(Gly-Gly)(TBZ)(Cl)]·4H2O and [Cu(Gly-L-Leu)(TBZ)(Cl)]·H2O (Gly-Gly = glycyl-glycine, Gly-L-Leu = glycyl-L-leucine, and TBZ = 2-(4′-thiazolyl)benzimidazole) were synthesized and fully characterized (Figure 163). It was proved that all compounds partially intercalate to calf thymus DNA causing degradation. Interestingly, these complexes in the presence of ascorbic acid (AA), induced ●OH production. The presence of reactive oxygen species can cause peroxidation of lipid and cellular DNA leading to cancer cell death. Moreover, the activity of the complexes described above was tested in vitro towards a few human carcinoma cell lines (HeLa, A549 and HepG2). The analysis of the results showed that the complexes exhibited a significant cytotoxic effect (IC50 from 33.17 to 100 μM) toward HeLa cancer cells. These phenomena undoubtedly indicate that these inorganic compounds have the potential to be effective metallo–peptide anticancer agents [150][59].
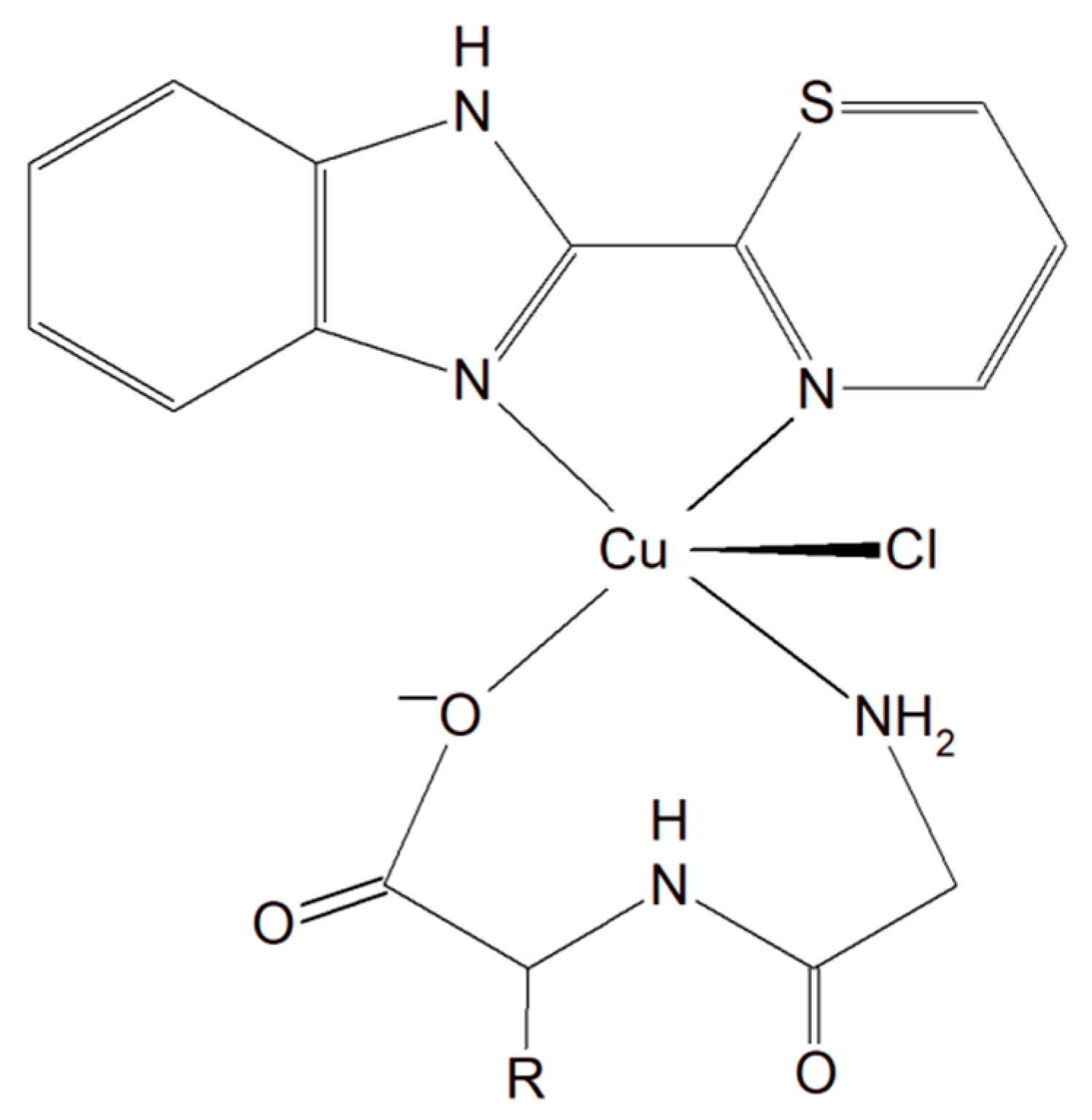

Figure 163.
Cu(II)–dipeptide complexes of 2-(4′-thiazolyl)benzimidazole.
Two different mononuclear peptide–copper(II) complexes with imidazole derivatives, [Cu(Gly-L-val)(HPB)(H2O)]·ClO4·1.5H2O (Figure 174) and [Cu(Gly-L-val)(PBT)(H2O)]·ClO4 (Gly-L-val = glycyl-L-valine, HPB = 2-(2′-pyridyl)benzimidazole, PBT = 2-(2′-pyridyl)benzothiazole), were described. It was found that this type of complex, with imidazole derivatives, can bind to calf thymus DNA through hydrophobic interactions. Importantly, the inorganic compounds displayed, in the presence of ascorbic acid, induced oxidative cleavage of plasmid DNA. This process resulted in reactive oxygen species, especially ●OH production. The cytotoxic study of the Cu(II) complexes against A549, HeLa, and PC-3 tumor cell lines and NIH3T3 (non-tumor cell line) revealed that [Cu(Gly-L-Val)(HPB)(H2O)]·ClO4·1.5H2O exhibited better cytotoxicity towards A549 and PC-3 than [Cu(Gly-L-val)(PBT)(H2O)]·ClO4 and the widely used drug cisplatin [151][60].
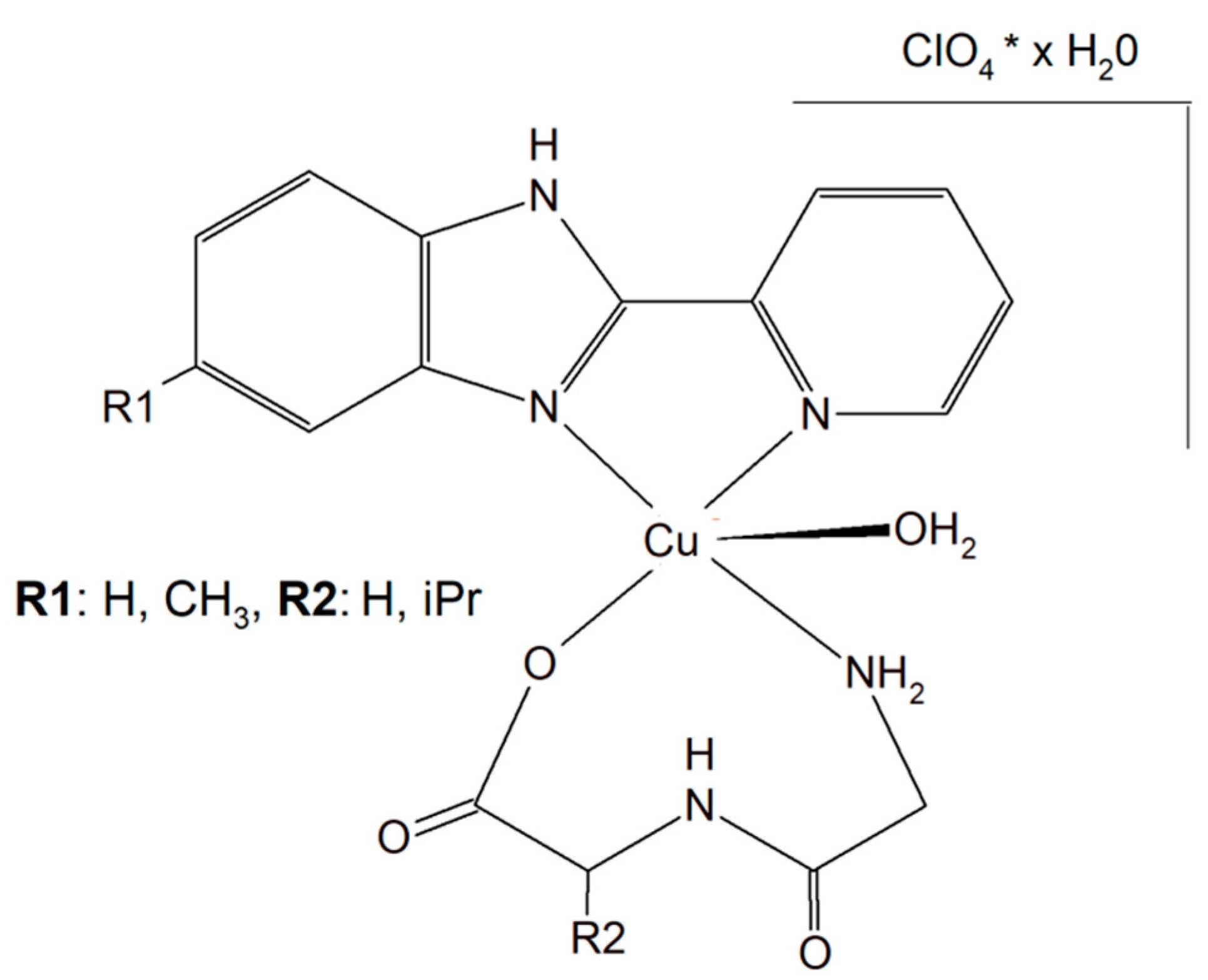

Figure 174. Mononuclear peptide–copper(II) complexes with imidazole derivatives, [Cu(Gly-L-val)(HPB)(H2O)]·ClO4·1.5H2O and Cu(II) complex of 5-methyl-2-(2′-pyridyl)benzimidazole (HPBM; [Cu(Gly-gly)(HPBM)(H2O)]ClO4·0.5H2O).
The search for better and more selective anticancer drugs led to the synthesis of the Cu(II) complex of 5-methyl-2-(2′-pyridyl)benzimidazole (HPBM; [Cu(Gly-gly)(HPBM)(H2O)]ClO4·0.5H2O (Figure 174) and [Cu(Gly-L-leu)(HPBM)(H2O)]ClO4, where Gly-Gly = Glycyl-glycine, Gly-L-leu = Glycyl-L-leucine). All the complexes turned out to be highly active towards a few types of cancer cells (A549, HeLa, and PC-3). It has been discovered that the possible action mode is related to intracellular reactive oxygen species (ROS) generation with damage of mitochondria and DNA. The results clearly prove that the complexes could induce HeLa cell apoptosis via a ROS-mediated mitochondrial pathway [152][61].
2.1.4. Copper Complexes with Phosphines
To improve the selectivity of copper(I) complexes, a simple dipeptide motif (sarcosyl-glycine SarGly) was attached to the inorganic compound ([CuI(dmp)(P(Ph)2CH2-SarGly-OH)], where dmp stands for 2,9-dimethyl-1,10-phenanthroline) (Figure 185). The cytotoxic effect of the compounds and cisplatin was tested towards cancer cell lines: mouse colon carcinoma (CT26; 1IC50 = 3.12 ± 0.1 µM), human lung adenocarcinoma (A549; IC50 = 2.01 ± 0.2 µM), and human breast adenocarcinoma (MCF7; IC50 = 0.98 ± 0.2 µM) as well as against a primary line of human pulmonary fibroblasts (MRC-5; IC50 = 78.56 ± 1.1 µM).
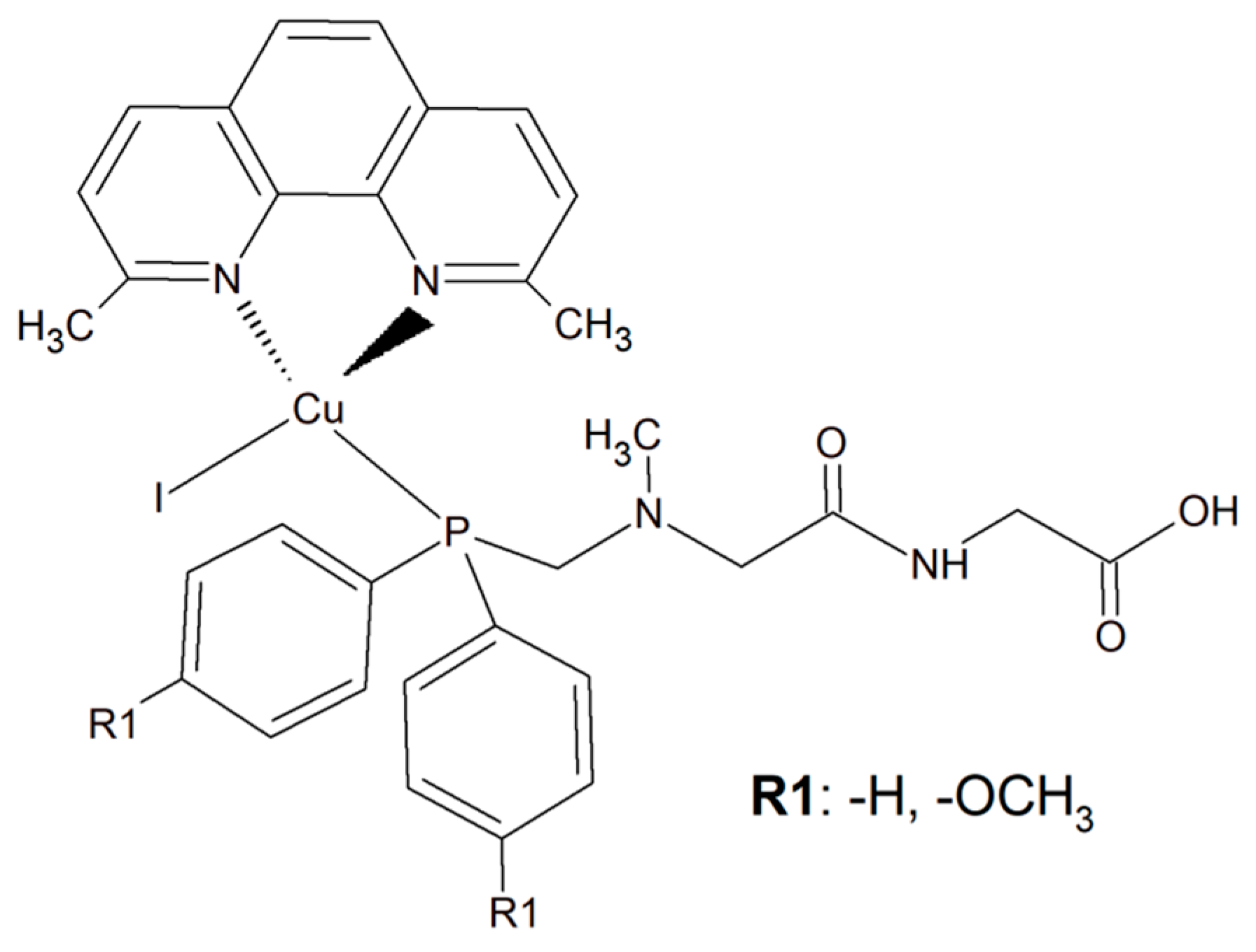

Figure 185. The inorganic compounds [CuI(dmp)(P(Ph)2CH2-Sar-Gly-OH)] and [Cu(I)(dmp)(P(p-OCH3-Ph)2CH2SarGly)].
Attachment of the peptide motif SarGly to the cytotoxic Cu(I) complex via phosphine motif significantly enhanced the selectivity of this inorganic compound toward the cancerous cells. Insight into the mode of action of this Cu(I) complex showed that it led to apoptotic cell MCF7 death. What is more, a decrease in mitochondrial membrane potential and increase in caspase-9 and -3 activities were observed. Importantly, the investigated compound was able to generate a high level of ROS. Most probably these radicals were the reason for oxidative damage of the sugar–phosphate backbone of DNA [153][62].
An analogous copper(I) complex [Cu(I)(dmp)(P(p-OCH3-Ph)2CH2SarGly)] was synthesized but with methoxy groups introduced on the phosphine phenyl rings (Figure 185). The cytotoxicity of this inorganic compound was checked in vitro towards colon, lung, breast, pancreatic, and prostate tumor cell lines, as well as towards non-tumor cell lines: lung, kidney, and keratinocyte. The Cu(I) complex turned out to be significantly more effective than cisplatin towards all tested cancerous cell lines. The addition of the methoxy group onto the phenyl rings of the phosphine ligand caused increased cytotoxic activity resulting in damage to breast, pancreatic and prostate tumor cell lines in vitro. Importantly, after the connection of the peptide motif to the metal ion via the phosphine ligand, a significant increase in the selectivity towards cancer cells was observed. Additionally, the described metal complexes were found to be redox active and reactive oxygen species generation was detected [154][63].
2.2. Neurodegenerative Diseases
Despite the rapid growth of scientific publications describing neurodegenerative disorders, especially Alzheimer’s disease (AD), the exact etiology of this of type issue is still not well understood as we described above in the previous section (Section 2.1). Moreover, there is still no good therapeutic opportunity accessible for this disorder [155][64]. While there is no treatment, there are five FDA-approved medicines to manage the symptoms of AD. However, they can only prevent the disease from getting much worse with time [156][65]. It has been proven that in vitro, Cu2+ removal from amyloids prevents its accumulation leading to the inhibition of hydroxyl radical (●OH) production. For the reasons mentioned above, it is suggested that a potential therapy for AD is metal chelation therapy [155,156][64][65].
Substances characterized by antioxidant and anti-inflammatory activity and compounds capable of restoring copper balance can prevent age-associated cognitive decline and eliminate, delay, and even cure many common neurodegenerative conditions. The human tripeptide GHK (glycyl-L-histidyl-L-lysine) (Figure 196) was discovered in 1973 as one of the human blood proteins. This peptidic motif is able to form copper inorganic compounds (GHK-Cu). Importantly, the tripeptide GHK is characterized by plenty of different properties such as regenerative and protective actions including antioxidant, anti-inflammatory, and wound healing properties. Current research showed that GHK tripeptide is a very promising agent for the treatment of age-associated neurodegeneration and cognitive decline [157][66].
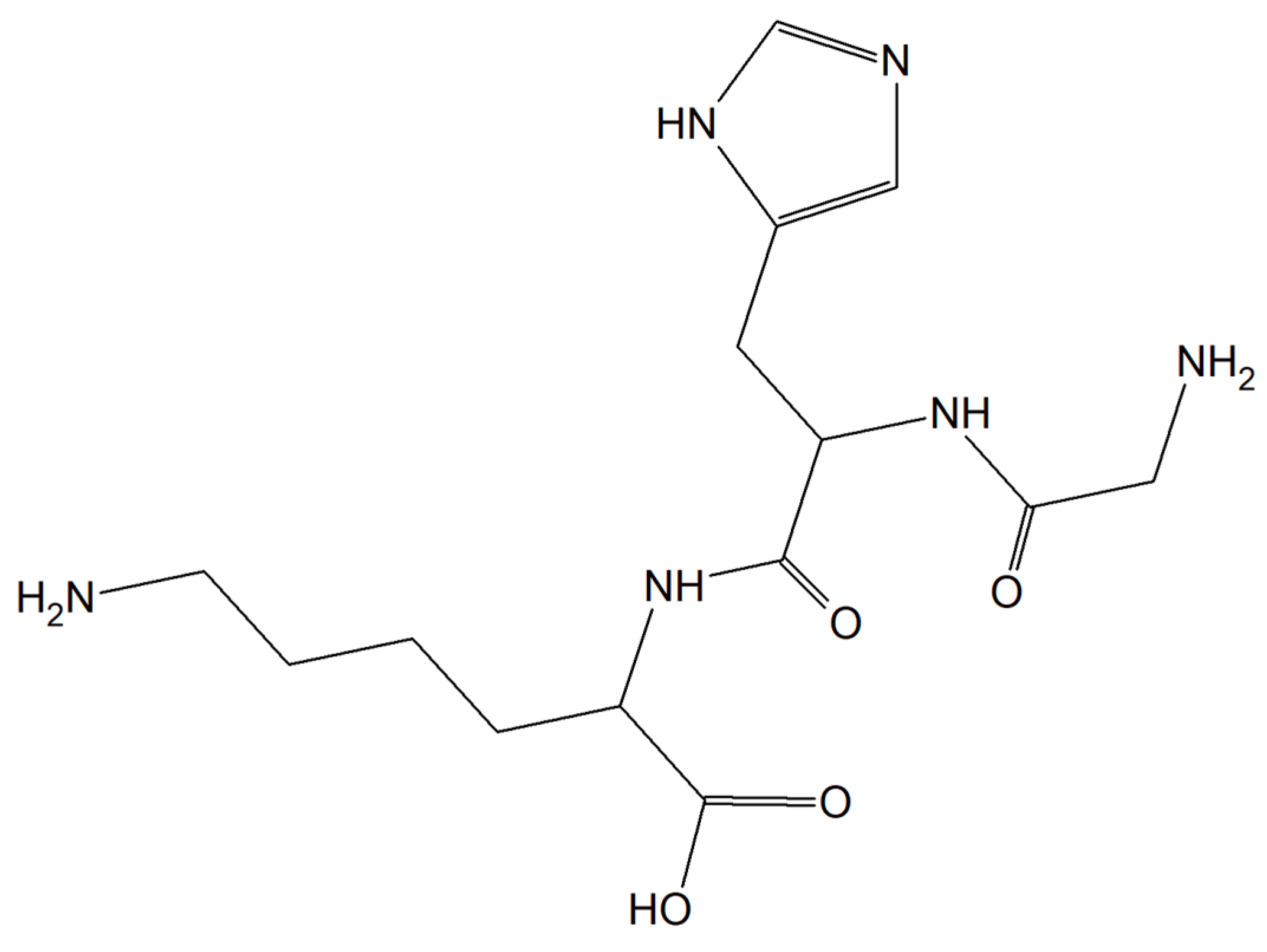

Figure 196.
The human tripeptide GHK (glycyl-L-histidyl-L-lysine).
2.3. Antiviral Properties
Viral infections occur all over the world and have a significant impact on people’s health, finances and quality of life. Currently, short antiviral peptides are a perfect example of a therapeutic agent that binds various metal ions such as Cu [158][67]. A great example of such a peptide is 3,4-dihydroxyphenylalanine (DOPA) with a peptide motif with adhesive properties (a diphenylalanine motif induces peptide self-assembly; sequence DOPA-(Phe)2-(His)6 is responsible for metal binding, Figure 207) [159][68].

Figure 207.
The metal-binding hexahistidine sequence DOPA-(Phe)
2
-(His)
6
.
The authors proved that this compound is able to release monovalent copper ions that interact with hydrogen peroxide leading to ROS generation, giving the compound significant antiviral properties. Additionally, the relief of Cu(I) and H2O2 from the old PCN-coated surface, as well as the probability of renewing these surfaces, enhances the potential of this coating [158][67].
It is worth emphasizing that copper ions alone exhibited antiviral properties. Interesting research was performed by a few independent scientific groups [160,161,162][69][70][71] showing that copper ions can inactivate viruses (e.g., herpes simplex virus (HSV), feline calicivirus (FCV), bronchitis virus, poliovirus, and human immunodeficiency virus type 1 (HIV-1)) since copper ions can disrupt the activity of certain proteins and are responsible for hydroxyl radical production [162][71]. However, when the concentration of copper ions was too high, the resulting toxicity was fatal because copper chloride can inactivate specific proteins and produce a large number of reactive oxygen species in cells inducing apoptosis [162][71].
References
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Harmful and Beneficial Role of ROS. Oxid Med. Cell Longev. 2016, 2016, 7909186.
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017, 44, 532–553.
- Auten, R.L.; Davis, J.M. Oxygen toxicity and reactive oxygen species: The devil is in the details. Pediatr Res. 2009, 66, 121–127.
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477.
- Barrera, G.; Pizzimenti, S.; Daga, M.; Dianzani, C.; Arcaro, A.; Cetrangolo, G.P.; Giordano, G.; Cucci, M.A.; Graf, M.; Gentile, F. Lipid Peroxidation-Derived Aldehydes, 4-Hydroxynonenal and Malondialdehyde in Aging-Related Disorders. Antioxidants 2018, 7, 102.
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642.
- Saieva, C.; Peluso, M.; Palli, D.; Cellai, F.; Ceroti, M.; Selvi, V.; Bendinelli, B.; Assedi, M.; Munnia, A.; Masala, G. Dietary and lifestyle determinants of malondialdehyde DNA adducts in a representative sample of the Florence City population. Mutagenesis 2016, 31, 475–480.
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; TsouhFokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front Physiol. 2020, 11, 694.
- Hawkins, C.L.; Davies, M.J. Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 2019, 294, 19683–19708.
- Finkel, T. Radical medicine: Treating ageing to cure disease. Nat. Rev. Mol. Cell Biol. 2005, 6, 971–976.
- Tao, W.; He, Z. ROS-responsive drug delivery systems for biomedical. Asian J. Pharm. Sci. 2018, 13, 101–112.
- Schieber, M.; Chande, N.S. Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 19, 453–462.
- Sai, D.L.; Lee, J.; Nguyen, D.L.; Kim, Y.-P. Tailoring photosensitive ROS for advanced photodynamic therapy. Exp. Mol. Med. 2021, 52, 495–504.
- Escudero, A. Photodynamic therapy: Photosensitizers and nanostructures. Mater. Chem. Front. 2021, 5, 3788–3812.
- Milkovic, L. Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells 2019, 8, 793.
- Forte, M. Targeting Nitric Oxide with Natural Derived Compounds as a Therapeutic Strategy in Vascular Diseases. Oxid. Med. Cell Longev. 2016, 1155, 7364138.
- Lourenço, F.C. Nitric Oxide Pathways in Neurovascular Coupling Under Normal and Stress Conditions in the Brain: Strategies to Rescue Aberrant Coupling and Improve Cerebral Blood Flow. Front. Physiol. 2021, 12, 729201.
- Le, M.; Rathje, O.; Levina, A.; Lay, P.A. High cytotoxicity of vanadium(IV) complexes with 1,10-phenanthroline and related ligands is due to decomposition in cell culture medium. Biol. Inorg. Chem. 2017, 22, 663–672.
- Martínez-Valencia, B.; Corona-Motolinia, N.D.; Sánchez-Laraa, E.; Norieg, L.; Sánchez-Gaytán, B.L.; Castro, M.E.; Meléndez-Bustamante, F.; González-Vergara, E. Cyclo-tetravanadate bridged copper complexes as potential double bullet pro-metallodrugs for cancer treatment. J. Inorg. Biochem. 2020, 208, 111081–111092.
- Wojciechowska, A.; Szuster-Ciesielska, A.; Sztandera, M.; Bregier-Jarzebowska, R.; Jarzab, A.; Rojek, T.; Komarnicka, U.K.; Bojarska-Junak, T.; Jezierska, J. L-argininatocopper(II) complexes in solution exert significant selective anticancer and antimicrobial activities. Appl. Organomet. Chem. 2020, 34, 5698–5706.
- Wojciechowska, A.; Gągor, A.; Zierkiewicz, W.; Jarząb, A.; Dylonga, A.; Duczmala, M. Metal–organic framework in an l-arginine copper(ii) ion polymer: Structure, properties, theoretical studies and microbiological activity. RSC Adv. 2015, 5, 36295–36307.
- Badetti, E.; Calgaro, L.; Falchi, L.; Bonetto, A.; Bettiol, C.; Leonetti, B.; Ambrosi, E.; Zendri, E.; Marcomini, P. Interaction between Copper Oxide Nanoparticles and Amino Acids: Influence on the Antibacterial Activity. Nanomaterials 2019, 9, 792.
- Van Hecke, K.; Nam, P.C.; Nguyen, M.T.; Van Meervelt, L. Netropsin interactions in the minor groove of d(GGCCAATTGG) studied by a combination of resolution enhancement and ab initio calculations. FEBS J. 2005, 272, 3531–3539.
- Mashima, T.; Nishikawa, F.; Kamatari, Y.O.; Fujiwara, H.; Saimura, M.; Nagata, T.; Kodaki, T.; Nishikawa, S.; Kuwata, K.; Katahira, M. Anti-prion activity of an RNA aptamer and its structural basis. Nucleic Acids Res. 2013, 41, 1355–1364.
- Macmaster, R.; Sedelnikova, S.; Baker, P.J.; Bolt, E.L.; Lloyd, R.G.; Rafferty, J.B. RusA Holliday junction resolvase: DNA complex structure—Insights into selectivity and specificity. Nucleic Acids Res. 2006, 34, 5577–5582.
- Liao, S.R.; Le, X.Y.; Feng, X.L. Syntheses, characterizations and SOD-like activities of ternary copper(II) complexes with 1,10-phenanthroline and L-α-amino acids. J. Coord. Chem. 2008, 61, 847–854.
- Harada, K.; Franke, A.D. Identification of two novel arginine binding DNAs. EMBO 1995, 14, 5798–5809.
- Conklin, K.A. Chemotherapy-associated oxidative stress: Impact on chemotherapeutic effectiveness. Integr. Cancer Ther. 2004, 3, 294–300.
- Srinivasa, S.U.; Bryce, W.Q.T.; Vellayappan, B.V.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084.
- Wojciechowska, A.; Rojek, T.; Gorzsas, A.; Malik-Gajewska, M.; Duczmal, M. Isothiocyanate controlled architecture, spectroscopic, and magnetic behavior of copper(II) l–arginine complexes. J. Coord. Chem. 2019, 72, 1358–1362.
- Hosseininezhad, S.M.; Hosseinali, R. An analysis of the reactions of L-arginine with Cu(II), Co(II), Fe(III), Zn(II), and Cr(III). Adv. Environ. Biol. 2014, 7, 315.
- Wojciechowska, A.; Bregier, R.; Komarnicka, U.K.; Kozieł, S.; Szuster, A.; Sztandera, M.; Jarząb, A.; Staszak, Z.; Witkowska, D.; Bojarska, A.; et al. Isothiocyanate l−argininato copper(II) complexes—Solution structure, DNA interaction, anticancer and antimicrobial activity. Chem. Biol. Interact. 2021, 348, 109636–109641.
- Li, X.; Hao, S.; Han, A.; Yang, Y.; Fang, G.; Wang, S. Intracellular Fenton reaction based on mitochondria-targeted copper(II)–peptide complex for induced apoptosis. J. Mater. Chem. 2019, 7, 4008–4016.
- Chekmenev, E.Y.; Vollmar, B.S.; Forseth, K.T.; Manion, M.C.; Jones, S.M.; Wagner, T.J.; Endicott, R.M.; Kyriss, B.P.; Homem, L.M.; Pate, M.; et al. Investigating molecular recognition and biological function at interfaces using piscidins, antimicrobial peptides from fish. Biochim. Biophys. Acta 2006, 1758, 1359–1372.
- Chekmenev, E.Y.; Jones, S.M.; Nikolayeva, Y.M.; Vollmar, B.S.; Wagner, T.J.; Gor’kov, P.L.; Brey, W.W.; Manion, M.N.; Cotton, D.M. High-field NMR studies of molecular recognition and structure-function relationships in antimicrobial piscidins at the water-lipid bilayer interface. J. Am. Chem. Soc. 2006, 128, 5308–5309.
- Comert, F.; Heinrich, F.; Chowdhury, A.; Schoeneck, M.; Darling, D.; Anderson, K.W.; Daben, M.; Angeles-Boza, A.M.; Silin, V.; Cotton, M.L.; et al. Copper-binding anticancer peptides from the piscidin family: An expanded mechanism that encompasses physical and chemical bilayer disruption. Sci. Rep. 2021, 11, 12620–12631.
- Mihailescu, M.; Sorci, M.; Seckute, J.; Silin, V.I.; Hammer, J.; Perrin, P.S., Jr.; Hernandez, J.I.; Smajic, N.; Shrestha, A.; Bogardus, K.A.; et al. Structure and function in antimicrobial piscidins: Histidine position, directionality of membrane insertion, and pH-dependent permeabilization. J. Am. Chem. Soc. 2019, 141, 9837–9853.
- Comert, F.; Greenwood, A.; Maramba, J.; Acevedo, R.; Lucas, L.; Kulasinghe, T.; Cairns, L.S.; Wen, Y.; Fu, R.; Hammer, J.; et al. The host-defense peptide piscidin P1 reorganizes lipid domains in membranes and decreases activation energiesin mechanosensitive ion channels. J. Biol. Chem. 2019, 294, 18557–18570.
- Libardo, M.D.J.; Bahar, A.A.; Ma, B.; Fu, R.; McCormick, L.E.; Zhao, J.; McCallum, S.A.; Nussinov, R.; Ren, D.; Angeles-Boza, A.M.; et al. Nuclease activity gives an edge to host-defense peptide piscidin 3 over piscidin 1, rendering it more effective against persisters and biofilms. FEBS J. 2017, 284, 3662–3683.
- Lei, Z.; Liu, Q.; Zhu, Q.; Yang, B.; Khaliq, H.; Sun, A.; Qi, Y.; Moku, G.K.; Su, Y.; Wang, J.; et al. Comparative pharmacokinetics and preliminary pharmacodynamics evaluation of piscidin 1 against PRV and PEDVin rats. Front Chem. 2018, 6, 244.
- Hu, H.; Guo, N.; Chen, S.; Wang, Y.; Liu, B.; He, O. Antiviral activity of piscidin 1 against pseudorabies virus both in vitro and in vivo. Virol. J. 2019, 16, 95.
- Lin, H.-J.; Huang, T.-C.; Muthusamy, S.; Lee, J.-F.; Duann, Y.-F.; Lin, C.-H. Piscidin-1, an antimicrobial peptide from fish (Hybrid Striped Bass Moronesaxatilis x M. chrysops), induces apoptotic and necrotic activity in HT1080 cells. Zool. Sci. 2012, 29, 327–332.
- Facchin, G.; Torre, M.H.; Kremer, E.; Piro, O.E.; Castellano, E.E.; Baran, E.J. Synthesis and characterization of three new Cu(II)-dipeptide complexes. J. Inorg. Biochem. 2002, 89, 174–180.
- Facchin, G.; Torre, M.E. Cu (II) complexation with His–Gly and His–Ala. X-ray structure of ·6H2O. Inorg. Chim. Acta 2003, 355, 408–413.
- Sanchiz, J.; Kremer, C. Magnetic properties of copper (II) complexes containing peptides. Crystal structure of . J. Mol. Struct. 2006, 797, 179–183.
- Facchin, G.; Kremer, E. Interaction of Cu-dipeptide complexes with calf thymus DNA and antiproliferative activity of in osteosarcoma-derived cells. Polyhedron 2009, 28, 2329–2334.
- Iglesias, S.; Alvarez, N.; Ribeiro, R.R.; Barroso, R.P.; Costa-Filho, A.J.; Kramer, M.G.; Facchin, G. Synthesis, structural characterization and cytotoxic activity of ternary copper (II)–dipeptide–phenanthroline complexes. A step towards the development of new copper compounds for the treatment of cancer. J. Inorg. Biochem. 2014, 139, 117–123.
- Lim, M.; Sinn, E.; Martin, R.B. Crystal structure of a mixed-ligand complex of copper (II), 1, 10-phenanthroline, and glycylglycinedianion: Glycylglycinato (1, 10-phenanthroline) copper (II) trihydrate. Inorg. Chem. 1976, 15, 807–811.
- Bhirud, R.G.; Srivastava, T.S. Synthesis, characterization and superoxide dismutase activity of some ternary copper (II) dipeptide-2,2′-bipyridine, 1,10-phenanthroline and 2,9-dimethyl-1,10-phenanthroline complexes. Inorg. Chim. Acta 1991, 179, 125–131.
- Alvarez, S.N.; Kramer, M.; Torre, M.H.; Kremer, E.; Ellena, J.; Filho, A.; Facchin, G. Structural characterization and cytotoxic activity of heteroleptic copper (II) complexes with L-dipeptides and 5-NO2-phenanthroline. Crystal structure of ·4H2O. Struct. Chem. Crystallogr. Commun. 2015, 1, 1–7.
- Vieira, E.D.; Casado, N.M. Weak exchange interaction supported by a biologically relevant long chemical bridge in a Cu-peptide model compound. Inorg. Chem. 2006, 45, 2942–2947.
- Ng, C.; Kong, S.M.; Tiong, Y.L.; Maah, M.J.; Sukram, N.; Ahmad, M.; Khoo, B.A. Selective anticancer copper(II)-mixed ligand complexes: Targeting of ROS and proteasomes. Metallomics 2014, 6, 892–906.
- Facchin, G.; Kremer, E. Structural characterization of a series of new Cu-dipeptide complexes in solid state and in solution. Polyhedron 2006, 25, 2597–2604.
- Facchin, G.; Veiga, N.; Kramer, M.G.; Batista, A.A.; Várnagy, K.; Farkas, E.; Moreno, V.; Torre, M.H. Experimental and theoretical studies of copper complexes with isomeric dipeptides as novel candidates against breast cancer. J. Inorg. Biochem. 2016, 162, 52–61.
- Alvarez, N.; Noble, C.; Torre, M.H.; Kremer, E.; Costa-Filhod, A.J.; Mendesd, L.F.; Kramere, M.G.; Facchina, G. Synthesis, structural characterization and cytotoxic activity against tumor cells of heteroleptic copper (I) complexes with aromatic diimines and phosphines. Inorg. Chim. Acta 2017, 466, 559–564.
- Sugimori, T.; Shibakawa, K.; Sugimori, T.; Shibakawa, K.; Masuda, H.; Odani, A.; Yamauchi, O. Ternary metal (II) complexes with tyrosine-containing dipeptides. Structures of copper (II) and palladium (II) complexes involving L-tyrosylglycine and stabilization of copper (II) complexes due to intramolecular aromatic ring stacking. Inorg. Chem. 1993, 32, 4951–4959.
- Alvarez, N.D.; Diana Viña, D.; Leite, C.M.; Mendes; Batista, A.A.; Ellena, J.; Costa-Filho, A.J.; Facchin, G. Synthesis and structural characterization of a series of ternary copper(II)-L-dipeptide-neocuproine complexes. Study of their cytotoxicity against cancer cells including MDA-MB-231, triple negative breast cancer cells. J. Inorg. Biochem. 2020, 203, 110930–110941.
- Gaála, A.; Mihucz, V.G.; Bősze, S.; Szabó, I.; Baranyi, M.; Horváth, P.; Streli, C.; Szoboszla, N. Comparative in vitro investigation of anticancer copper chelating agents. Microchem. J. 2018, 136, 227–235.
- Xia-Bing, F.; Zhang, J.-J.; Liu, D.-D.; Gan, Q.; Gao, H.-W.; Mao, Z.-W.; Le, X.-Y. Cu(II)–dipeptide complexes of 2-(4′-thiazolyl)benzimidazole: Synthesis, DNA oxidative damage, antioxidant and in vitro antitumor activity. J. Inorg. Biochem. 2015, 143, 77–87.
- Gana, Q.; Zhang, C.-L.; Wang, B.-F.; Xiong, Y.-H.; Fu, Y.-L.; Maoac, Z.-W.; Le, X.-Y. Two new mixed copper(II)–dipeptide complexes of N,N-donor heterocycle ligands: Studies on their non-covalent DNA binding, chemical nuclease, antioxidant and anticancer activities. RSC Adv. 2016, 6, 35952–35965.
- Qi, Y.Y.; Gan, Q.; Liu, Y.X.; Xiong, Y.H.; Mao, Z.W.; Le, X.Y. Two new Cu(II) dipeptide complexes based on 5-methyl-2-(2′-pyridyl)benzimidazole as potential antimicrobial and anticancer drugs: Special exploration of their possible anticancer mechanism. Eur. J. Med. Chem. 2018, 154, 220–232.
- Komarnicka, U.K.; Kozieł, S.; Starosta, R.; Kyzioł, A. Selective Cu(I) complex with phosphine-peptide (SarGly) conjugate contra breast cancer: Synthesis, spectroscopic characterization and insight into cytotoxic action. J. Inorg. Biochem. 2018, 186, 162–175.
- Komarnicka, U.K.; Kozieł, S.; Zabierowski, P.; Kruszyński, R.; Lesiówa, M.K.; Tisato, F.; Porchia, M.; Kyzioł, A. Copper(I) complexes with phosphines P(p-OCH3-Ph)2CH2OH and P(p-OCH3-Ph)2CH2SarGly. Synthesis, multimodal DNA interactions, and prooxidative and in vitro antiproliferative activity. J. Inorg. Biochem. 2020, 203, 110926.
- Hegde, M.L.; Bharathi, P.; Suram, A.; Venugopal, C.; Jagannathan, R.; Poddar, P.; Srinivas, P.; Sambamurti, K.; Rao, K.J.; Scancar, J.; et al. Challenges Associated with Metal Chelation Therapy in Alzheimer’s Disease. J. Alzheimers Dis. 2009, 17, 457–468.
- Szeto, J.Y.Y.; Lewis, S.J.G. Current Treatment Options for Alzheimer’s Disease and Parkinson’s Disease Dementia. Curr. Neuropharmacol. 2016, 14, 326–338.
- Pickart, L.; Michelle, J.; Soltero, V.; Margolina, A. The Human Tripeptide GHK-Cu in Prevention of Oxidative Stress and Degenerative Conditions of Aging:Implications for Cognitive Health. Oxidative Med. Cell. Longev. 2012, 5, 8.
- Boas, D. A Novel Copper-Binding Peptide That Self-Assembles Into a Transparent Antibacterial and Antiviral Coating Front. Bioeng. Biotechnol. 2021, 20, 11–19.
- Andersen, A.; Chen, Y.; Birkedal, H. Bioinspired Metal–Polyphenol Materials: Self-Healing and Beyond. Biomimetics 2019, 4, 30.
- Sagripanti, J.L.; Routson, L.B.; Bonifacino, A.C.; Lytle, C.D. Mechanism of coppermediatedinactivation of herpes simplex virus. Antimicrob. Agents Chemother. 1997, 41, 812–817.
- Betanzos-Cabrera, G.; Rez, F.J.R.; Oz, J.L.M.; Barrn, B.L.; Maldonado, R. Inactivation ofHSV-2 by ascorbate-Cu (II) and its protecting evaluation in CF-1 miceagainst encephalitis. J. Virol. Methods 2004, 120, 8.
- Guo, W.J.; Ye, S.S.; Cao, N.; Huang, J.; Gao, J.; Chen, Q.Y. ROS-mediatedautophagy was involved in cancer cell death induced by novel copper(II)complex. Exp. Toxicol. Pathol. 2010, 62, 577–582.
More
