Taro (Colocasia esculenta) jeist ważnym źródłem węglowodanów jako źródło an important source of carbohydrates as an energii i jest używany jako podstawowe pożywienie na całym świecie.y source and is used as a staple food throughout the world. It is rich in mucilage and Jest bogaty w śluz i arch granulki skrobi, dzięki czemu jest składnikiem wysoce przyswajalnymes, making it a highly digestible ingredient. ŚMuciluz może działać jako matryca i środek zagęszczający, wiążącyage can act as a matrix and a thickening, binding, emulgujący lub spieniający w żywności, farmacji i kilku innych dziedzinach badańsifying, or foaming agent in food, pharmaceutical, and several other fields of research. CMoreo więcej, śluz może być ekstrahowany z kilku żywych ver, mucilage can be extracted from several living organizmów i ma doskonałe właściwości funkcjonalne, takie jak zdolność zatrzymywania wody, zatrzymywania oleju i pęcznieniasms and has excellent functional properties, such as water-holding, oil-holding, and swelling capacities. DlatTherego te niezwykłe właściwości funkcjonalne sprawiają, że śluz jest obiecującym składnikiem o możliwych zastosowaniach przemysłowych.fore, these remarkable functional properties make mucilage a promising Poinadto kilka technik ekstrakcji, w tym metody ekstrakcji wspomaganejgredient with possible industrial applications. Furthermore, several extraction techniques, including enzymamie-assisted, ultradźwiękowej, wspomaganej mikrofalami, wodnej i rozpuszczalnikowej,sonication, microwave-assisted, aquatic, and solvent extraction methods, are usłużą do uzyskania ilościowych ilości śluzu taro.ed to obtain quantitative amounts of taro mucilage. Coldwater Eksextrakcję zimną wodą z wytrącaniem etanolem można uznać za skuteczną i opłacalną technikę uzyskiwania wysokiej jakości śluzu przy odpowiednich zastosowaniach przemysłowych, podczas gdy metoda ultradźwiękowa jest droższa, ale skutkuje większą ilością śluzu niż inne nowe techniki.ction with ethanol precipitation can be considered an effective and cost-effective technique to obtain high-quality mucilage with suitable industrial applications, whereas the ultrasonication method is more expensive but results in a higher amount of mucilage than other emerging techniques. Mucilage can also be used as a fat replacer or reducer, dye remover, coating agent, and antioxidating agent
- mucilage
- biopolymer
- polysaccharide
1. Proces ekstrakcji i właściwości składowe śluzu Taro
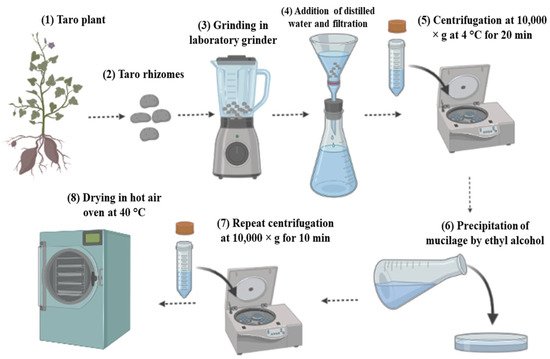
1. Extraction Process and Compositional Properties of Taro Mucilage
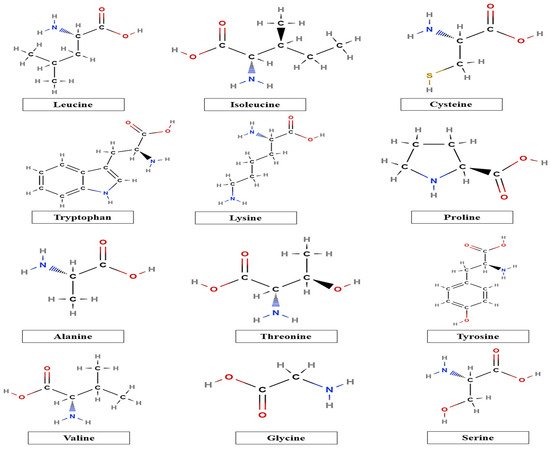
Furthermore, the chemical composition, including monosaccharide units and amino acids, and proximate composition of taro mucilage are also dependent upon the extraction techniques of mucilage. Taro mucilage consists of carbohydrates (arabinose, rhamnose, arabinose, fructose, mannose, galactose, fructose, and glucose) and also contains various amino acids, as shown in Figure 2. The high glucose content present in taro mucilage is due to the presence of starch during the extraction. In addition, taro mucilage is a rich source of high amounts of arabinogalactan-protein (AGP) (93–98%) and provides a high amount of AGP, which is responsible for the improvement of the emulsifying properties of mucilage due to the presence of hydrophilic and hydrophobic amino acids, as stated by Andrade et al. [12] and Njintang et al. [13]. Several phenolic compounds present in taro mucilage are esterified (bound phenolic compounds), which are interconnected to arabinose residues or carboxyl groups via ester bonds. These esterified compounds have great potential to inhibit enzymatic activity by polyphenol oxidases (PPO) such as laccase, which results in the improvement of functional properties.
2. Techno-Functional Property of Taro Mucilage
3. Application of Taro Mucilage
3.1. Application of Mucilage as an Emulsifying Agent
3.2. Application of Taro Mucilage as a Fat Replacer
3.3. Antioxidant Activity of Taro Mucilage
3.4. Application of Taro Mucilage as Dye Remover
References
- Rodríguez-González, S.; Martínez-Flores, H.E.; Chávez-Moreno, C.K.; Macías-Rodríguez, L.I.; Zavala-Mendoza, E.; Garnica-Romo, M.G.; Chacón-García, L. Extraction and characterization of mucilage from wild species of O puntia. J. Food Process Eng. 2014, 37, 285–292.
- Chiang, C.F.; Lai, L.S. Effect of enzyme-assisted extraction on the physicochemical properties of mucilage from the fronds of Asplenium australasicum (J. Sm.) Hook. Int. J. Biol. Macromol. 2019, 124, 346–353.
- Gheribi, R.; Khwaldia, K. Cactus mucilage for food packaging applications. Coatings 2019, 9, 655.
- Zhao, X.; Qiao, L.; Wu, A.M. Effective extraction of Arabidopsis adherent seed mucilage by ultrasonic treatment. Sci. Rep. 2017, 7, 1–8.
- Moreno, L.; Medina, O.; Rojas, A.L. Mucilage and cellulosic derivatives as clarifiers for the improvement of the non-centrifugal sugar production process. Food Chem. 2022, 367, 130657.
- Rostami, H.; Gharibzahedi, S.M.T. Mathematical modeling of mucilage extraction kinetic from the waste hydrolysates of fruiting bodies of Zizyphus jujuba mill. J. Food Processing Preserv. 2017, 41, e13064.
- Tavares, S.A.; Pereira, J.; Guerreiro, M.C.; Pimenta, C.J.; Pereira, L.; Missagia, S.V. Physical and chemical characteristics of the mucilage of lyophilized yam. Ciência E Agrotecnologia 2011, 35, 973–979.
- Bayar, N.; Bouallegue, T.; Achour, M.; Kriaa, M.; Bougatef, A.; Kammoun, R. Ultrasonic extraction of pectin from Opuntia ficus indica cladodes after mucilage removal: Optimization of experimental conditions and evaluation of chemical and functional properties. Food Chem. 2017, 235, 275–282.
- Lin, H.; Huang, A.S. Chemical composition and some physical properties of a water-soluble gum in taro (Colocasia esculenta). Food Chem. 1993, 48, 403–409.
- Andrade, L.A.; de Oliveira Silva, D.A.; Nunes, C.A.; Pereira, J. Experimental techniques for the extraction of taro mucilage with enhanced emulsifier properties using chemical characterization. Food Chem. 2020, 327, 127095.
- Tosif, M.M.; Najda, A.; Bains, A.; Kaushik, R.; Dhull, S.B.; Chawla, P.; Walasek-Janusz, M. A comprehensive review on plant-derived mucilage: Characterization, functional properties, applications, and its utilization for nanocarrier fabrication. Polymers 2021, 13, 1066.
- Andrade, L.A.; Nunes, C.A.; Pereira, J. Relationship between the chemical components of taro rhizome mucilage and its emulsifying property. Food Chem. 2015, 178, 331–338.
- Njintang, N.Y.; Boudjeko, T.; Tatsadjieu, L.N.; Nguema-Ona, E.; Scher, J.; Mbofung, C.M. Compositional, spectroscopic and rheological analyses of mucilage isolated from taro (Colocasia esculenta L. Schott) corms. J. Food Sci. Technol. 2014, 51, 900–907.
- Manhivi, V.E.; Venter, S.; Amonsou, E.O.; Kudanga, T. Composition, thermal and rheological properties of polysaccharides from amadumbe (Colocasia esculenta) and cactus (Opuntia spp.). Carbohydr. Polym. 2018, 195, 163–169.
- Charles, A.L.; Huang, T.C.; Chang, Y.H. Structural analysis and characterization of a mucopolysaccharide isolated from roots of cassava (Manihot esculenta Crantz L.). Food Hydrocoll. 2008, 22, 184–191.
- Wu, Y.; Eskin, N.A.M.; Cui, W.; Pokharel, B. Emulsifying properties of water soluble yellow mustard mucilage: A comparative study with gum Arabic and citrus pectin. Food Hydrocoll. 2015, 47, 191–196.
- Dereje, B. Composition, morphology and physicochemical properties of starches derived from indigenous Ethiopian tuber crops: A review. Int. J. Biol. Macromol. 2021, 187, 911–921.
- Priyadarshi, R.; Kumar, B.; Rhim, J.W. Green and facile synthesis of carboxymethylcellulose/ZnO nanocomposite hydrogels crosslinked with Zn2+ ions. Int. J. Biol. Macromol. 2020, 162, 229–235.
- Alpizar-Reyes, E.; Carrillo-Navas, H.; Gallardo-Rivera, R.; Varela-Guerrero, V.; Alvarez-Ramirez, J.; Pérez-Alonso, C. Functional properties and physicochemical characteristics of tamarind (Tamarindus indica L.) seed mucilage powder as a novel hydrocolloid. J. Food Eng. 2009, 2017, 68–75.
- Keshani-Dokht, S.; Emam-Djomeh, Z.; Yarmand, M.S.; Fathi, M. Extraction, chemical composition, rheological behavior, antioxidant activity and functional properties of Cordia myxa mucilage. Int. J. Biol. Macromol. 2018, 118, 485–493.
- Singh, G.; Singh, S.; Kumar, B.; Gaikwad, K.K. Active barrier chitosan films containing gallic acid based oxygen scavenger. J. Food Meas. Charact. 2021, 15, 585–593.
- Kumar, B.; Negi, Y.S. Water absorption and viscosity behaviour of thermally stable novel graft copolymer of carboxymethyl cellulose and poly (sodium 1-hydroxy acrylate). Carbohydr. Polym. 2018, 181, 862–870.
- Kumar, B.; Priyadarshi, R.; Deeba, F.; Kulshreshtha, A.; Gaikwad, K.K.; Kim, J.; Kumar, A.; Negi, Y.S. Nanoporous sodium carboxymethyl cellulose-g-poly (Sodium acrylate)/fecl3 hydrogel beads: Synthesis and characterization. Gels 2020, 6, 49.
- Jones, B.O.; John, O.O.; Luke, C.; Ochieng, A.; Bassey, B.J. Application of mucilage from Dicerocaryum eriocarpum plant as biosorption medium in the removal of selected heavy metal ions. J. Environ. Manag. 2016, 177, 365–372.
- Soukoulis, C.; Gaiani, C.; Hoffmann, L. Plant seed mucilage as emerging biopolymer in food industry applications. Curr. Opin. Food Sci. 2018, 22, 28–42.
- Prajapati, V.D.; Maheriya, P.M.; Jani, G.K.; Patil, P.D.; Patel, B.N. Lepidium sativum Linn.: A current addition to the family of mucilage and its applications. Int. J. Biol. Macromol. 2014, 65, 72–80.
- Raj, V.; Shim, J.J.; Lee, J. Grafting modification of okra mucilage: Recent findings, applications, and future directions. Carbohydr. Polym. 2020, 246, 116653.
- Contreras-Padilla, M.; Rodríguez-García, M.E.; Gutiérrez-Cortez, E.; del Carmen Valderrama-Bravo, M.; Rojas-Molina, J.I.; Rivera-Muñoz, E.M. Physicochemical and rheological characterization of Opuntia ficus mucilage at three different maturity stages of cladode. Eur. Polym. J. 2016, 78, 226–234.
- Medina-Torres, L.; Núñez-Ramírez, D.M.; Calderas, F.; González-Laredo, R.F.; Minjares-Fuentes, R.; Valadez-García, M.A.; Bernad-Bernad, M.J.; Manero, O. Microencapsulation of gallic acid by spray drying with aloe vera mucilage (aloe barbadensis miller) as wall material. Ind. Crops Prod. 2019, 138, 111461.
- Doost, A.S.; Nasrabadi, M.N.; Goli, S.A.H.; Van Troys, M.; Dubruel, P.; De Neve, N.; Van der Meeren, P. Maillard conjugation of whey protein isolate with water-soluble fraction of almond gum or flaxseed mucilage by dry heat treatment. Food Res. Int. 2020, 128, 108779.
- Drozłowska, E.; Bartkowiak, A.; Łopusiewicz, Ł. Characterization of flaxseed oil bimodal emulsions prepared with flaxseed oil cake extract applied as a natural emulsifying agent. Polymers 2020, 12, 2207.
- Dehghani, S.; Noshad, M.; Rastegarzadeh, S.; Hojjati, M.; Fazlara, A. Electrospun chia seed mucilage/PVA encapsulated with green cardamonmum essential oils: Antioxidant and antibacterial property. Int. J. Biol. Macromol. 2020, 161, 1–9.
- Naji-Tabasi, S.; Razavi, S.M.A. Functional properties and applications of basil seed gum: An overview. Food Hydrocoll. 2017, 73, 313–325.
- Li, J.M.; Nie, S.P. The functional and nutritional aspects of hydrocolloids in foods. Food Hydrocoll. 2016, 53, 46–61.
- Salehi, F. Effect of common and new gums on the quality, physical, and textural properties of bakery products: A review. J. Texture Stud. 2016, 51, 361–370.
- Amiri Aghdaei, S.S.; Aalami, M.; Babaei Geefan, S.; Ranjbar, A. Application of Isfarzeh seed (Plantago ovate L.) mucilage as a fat mimetic in mayonnaise. J. Food Sci. Technol. 2014, 51, 2748–2754.
- Saengphol, E.; Pirak, T. Hoary basil seed mucilage as fat replacer and its effect on quality characteristics of chicken meat model. Agric. Nat. Resour. 2014, 52, 382–387.
- Chiang, J.H.; Ong, D.S.M.; Ng, F.S.K.; Hua, X.Y.; Tay, W.L.W.; Henry, C.J. Application of chia (Salvia hispanica) mucilage as an ingredient replacer in foods. Trends Food Sci. Technol. 2021, 115, 105–116.
- Felisberto, M.H.F.; Wahanik, A.L.; Gomes-Ruffi, C.R.; Clerici, M.T.P.S.; Chang, Y.K.; Steel, C.J. Use of chia (Salvia hispanica L.) mucilage gel to reduce fat in pound cakes. LWT-Food Sci. Technol. 2015, 63, 1049–1055.
- Nagata, C.L.P.; Andrade, L.A.; Pereira, J. Optimization of taro mucilage and fat levels in sliced breads. J. Food Sci. Technol. 2015, 52, 5890–5897.
- Wu, Y.; Hui, D.; Eskin, N.A.M.; Cui, S.W. Water-soluble yellow mustard mucilage: A novel ingredient with potent antioxidant properties. Int. J. Biol. Macromol. 2016, 91, 710–715.
- Beigomi, M.; Mohsenzadeh, M.; Salari, A. Characterization of a novel biodegradable edible film obtained from Dracocephalum moldavica seed mucilage. Int. J. Biol. Macromol. 2018, 108, 874–883.
- Liguori, G.; Gentile, C.; Gaglio, R.; Perrone, A.; Guarcello, R.; Francesca, N.; Fretto, S.; Inglese, P.; Settanni, L. Effect of addition of Opuntia ficus-indica mucilage on the biological leavening, physical, nutritional, antioxidant and sensory aspects of bread. J. Biosci. Bioeng. 2020, 129, 184–191.
- Behbahani, B.A.; Fooladi, A.A.I. Shirazi balangu (Lallemantia royleana) seed mucilage: Chemical composition, molecular weight, biological activity and its evaluation as edible coating on beefs. Int. J. Biol. Macromol. 2018, 114, 882–889.
- Mousavi, S.R.; Rahmati-Joneidabad, M.; Noshad, M. Effect of chia seed mucilage/bacterial cellulose edible coating on bioactive compounds and antioxidant activity of strawberries during cold storage. Int. J. Biol. Macromol. 2021, 190, 618–623.
- Nguimbou, R.M.; Boudjeko, T.; Njintang, N.Y.; Himeda, M.; Scher, J.; Mbofung, C.M. Mucilage chemical profile and antioxidant properties of giant swamp taro tubers. J. Food Sci. Technol. 2014, 51, 3559–3567.
- Mohite, A.M. Formulation of edible films from fenugreek mucilage and taro starch. SN Appl. Sci. 2020, 2, 1900.
- Moghaddas, S.M.T.H.; Elahi, B.; Javanbakht, V. Biosynthesis of pure zinc oxide nanoparticles using Quince seed mucilage for photocatalytic dye degradation. J. Alloy. Compd. 2020, 821, 153519.
- Mirzaei, S.; Javanbakht, V. Dye removal from aqueous solution by a novel dual cross-linked biocomposite obtained from mucilage of Plantago Psyllium and eggshell membrane. Int. J. Biol. Macromol. 2019, 134, 1187–1204.
- Hosseinzadeh, H.; Mohammadi, S. Quince seed mucilage magnetic nanocomposites as novel bioadsorbents for efficient removal of cationic dyes from aqueous solutions. Carbohydr. Polym. 2015, 134, 213–221.
